Obtaining Vegetable Production Enriched with Minor Micronutrients Using Fullerene Derivatives
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Design
- Evaluation of the influence of introducing C60 fullerenol into the soil on the cucumber plant and the content of the main macro- and microelements in the soil and plants.
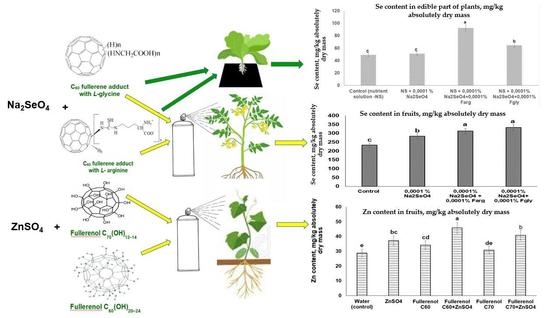
- Study of the influence of introducing C60-L-Gly or C60-L-Arg and Na2SeO4 compositions into the nutrient solution on Chinese cabbage and the selenium content in the plants.
- Study of the influence of foliar treatment with C60-L-Gly or C60-L-Arg and Na2SeO4 compositions on tomato and the selenium content in its production.
- Study of the influence of foliar treatment with compositions of the fullerenol C60(OH)22–24 or C70(OH)12–14 and ZnSO4 on cucumber and the zinc content in its production.
- Evaluation of the influence of introducing C60 fullerenol into the soil on the cucumber plant and the content of the main macro- and microelements in the soil and plants
2.2. Plants’ Biofortification with Se or Zn
- 2.
- Study of the influence of introducing C60-L-Gly or C60-L-Arg and Na2SeO4 compositions into the nutrient solution on Chinese cabbage and the selenium content in the plants
- 3.
- Study of the influence of foliar treatment with C60-L-Gly or C60-L-Arg and Na2SeO4 compositions on tomato and the selenium content in its production
- 4.
- Study of the influence of foliar treatment with compositions of the fullerenol C60(OH)22–24 or C70(OH)12–14 and ZnSO4 on cucumber and the zinc content in its production
2.3. Soil Analyses
2.4. Plant Analyses
2.4.1. Morphology Measurements
- Sleav.—leaves’ area;
- Scut—cut area;
- mcut—cut mass (average value);
- mleav.—raw mass of leaves from a plant.
2.4.2. Photosynthetic Pigment Analysis
2.4.3. Activity of Antioxidant Systems
2.4.4. Quality and Safety Indicators of Plant Production
2.5. Statistical Analysis
3. Results
3.1. The Influence of Fullerenol C60(OH)22–24 Introduction into the Soil on the Cucumber Plant and the Content of the Main Macro- and Microelements in the Soil and Plants
- -
- It mainly contributes in the form of a trend or a significant decrease (maximum change of 47%) in the activity of the oxidative enzyme peroxidase in leaves and roots. The exception is the roots in the variant with the introduction of fullerenol into the soil at a concentration of 100 mg/kg, where the activity of peroxidase was higher than that in the control by 14%;
- -
- A significant decrease (maximum change of 41%) in the intensity of lipid peroxidation;
- -
3.2. Enrichment of Plants with Selenium Anions
3.2.1. Influence of the C60-L-Gly or C60-L-Arg and Na2SeO4 Compositions Introduced into the Nutrient Solution on Chinese Cabbage and the Selenium Content in the Plants
3.2.2. Influence of Foliar Treatment with C60-L-Gly or C60-L-Arg and Na2SeO4 Compositions on Tomato and the Selenium Content in Its Production
3.2.3. Influence of Foliar Treatment with the Compositions of Fullerenol C60(OH)22–24 or C70(OH)12–14 and ZnSO4 on Cucumber and the Zinc Content in its Production
- -
- -
- An increase in the leaves’ area and, consequently, the assimilation surface for absorbing light and undergoing photosynthetic reactions with the formation and accumulation of plastic photosynthetic products, which is indirectly confirmed by an increase (tendency) in the values of the leaves’ raw and dry mass (Table 10 and Table S6);
- -
- Interconnected with the above-mentioned increase in the mass of the fruit, the number, and, as a result, the mass of fruits per plant.
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vergnaud, A.-C.; Romaguera, D.; Peeters, P.H.; van Gils, C.H.; Chan, D.S.; Romieu, I.; Freisling, H.; Ferrari, P.; Clavel-Chapelon, F.; Fagherazzi, G.; et al. Adherence to the World Cancer Research Fund/American Institute for Cancer Research guidelines and risk of death in Europe: Results from the European Prospective Investigation into Nutrition and Cancer cohort study. Am. J. Clin. Nutr. 2013, 97, 1107–1120. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Granato, D.; Barba, F.J.; Kovačević, D.B.; Lorenzo, J.M.; Cruz, A.G.; Putnik, P. Functional Foods: Product Development, Technological Trends, Efficacy Testing, and Safety. Annu. Rev. Food Sci. Technol. 2020, 11, 93–118. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, S.; Wang, P.; Yamaji, N.; Ma, J.F. Plant nutrition for human nutrition: Hints from rice research and future perspectives. Mol. Plant 2020, 13, 825–835. [Google Scholar] [CrossRef] [PubMed]
- Puzin, S.N.; Pogozheva, A.V.; Potapov, V.N. Optimizing nutrition of older people as a mean of preventing premature aging. Probl. Nutr. 2018, 87, 69–77. [Google Scholar] [CrossRef]
- Hurst, R.; Hooper, L.; Norat, T.; Lau, R.; Aune, D.; Greenwood, D.C.; Vieira, R.; Collings, R.; Harvey, L.J.; Sterne, J.A.; et al. Selenium and prostate cancer: Systematic review and meta-analysis. Am. J. Clin. Nutr. 2012, 96, 111–122. [Google Scholar] [CrossRef] [Green Version]
- Ibrahim, S.A.Z.; Kerkadi, A.; Agouni, A. Selenium and Health: An Update on the Situation in the Middle East and North Africa. Nutrients 2019, 11, 1457. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Costello, L.C.; Franklin, R.B. Zinc is decreased in prostate cancer: An established relationship of prostate cancer! J. Biol. Inorg. Chem. 2011, 16, 3–8. [Google Scholar] [CrossRef]
- Ho, E.; Song, Y. Zinc and prostatic cancer. Curr. Opin. Clin. Nutr. Metab. Care 2009, 12, 640–645. [Google Scholar] [CrossRef] [Green Version]
- Kieliszek, M.; Bła’zejak, S. Current Knowledge on the Importance of Selenium in Food for Living Organisms: A Review. Molecules 2016, 21, 609. [Google Scholar] [CrossRef] [Green Version]
- World Health Organization. Trace Elements in Human Nutrition and Health; World Health Organization: Geneva, Switzerland, 1996; 360p. [Google Scholar]
- Vitamin and Mineral Requirements in Human Nutrition: Report of a Joint FAO/WHO Expert Consultation. Bangkok, Thailand, 21–30 September 1998; World Health Organization and Food and Agriculture Organization of the United Nations: Geneva, Switzerland, 2004; 362p.
- Scherz, H.; Kirchhoff, E. Trace elements in foods: Zinc contents of raw foods—A comparison of data originating from different geographical regions of the world. J. Food Compos. Anal. 2006, 19, 420–433. [Google Scholar] [CrossRef]
- Praharaj, S.; Skalicky, M.; Maitra, S.; Bhadra, P.; Shankar, T.; Brestic, M.; Hossain, A. Zinc biofortification in food crops could alleviate the zinc malnutrition in human health. Molecules 2021, 26, 3509. [Google Scholar] [CrossRef] [PubMed]
- Ros, G.H.; van Rotterdam, A.M.D.; Bussink, D.W.; Bindraban, P.S. Selenium fertilization strategies for bio-fortification of food: An agro-ecosystem approach. Plant Soil 2016, 404, 99–112. [Google Scholar] [CrossRef]
- Franchini, A.; Quattrini, E.; Giuffrida, F.; Ferrante, A. Biofortification of fresh leafy vegetables using a nutrient solution containing selenium. J. Sci. Food Agric. 2023, 9, 5472–5480. [Google Scholar] [CrossRef]
- Perminova, I.V. From green chemistry and nature-like technologies towards ecoadaptive chemistry and technology. Pure Appl. Chem. 2019, 91, 851–864. [Google Scholar] [CrossRef]
- Folmanis, G.E.; Fedotov, M.A. Dispersion methods of preparation of nanosized biological agents for vegetable crops. J. Phys. Conf. Ser. 2020, 1, 012061. [Google Scholar] [CrossRef]
- Churilov, G.I.; Polischuk, S.D.; Kuznetsov, D.; Borychev, S.N.; Byshov, N.V.; Churilov, D.G. Agro ecological grounding for the application of metal nanopowders in agriculture. Int. J. Nanotechnol. 2018, 15, 258–279. [Google Scholar] [CrossRef]
- Zhou, H.; Yang, J.; Kronzuker, H.J.; Shi, W. Selenium biofortification and interactions with other elements in plants: A review. Front. Plant Sci. 2020, 11, 586421. [Google Scholar] [CrossRef]
- Alfthan, G. Effect of selenium fertilization on the human selenium status and the environment. Norw. J. Agric. Sci. 1993, 11, 175–181. [Google Scholar]
- Ermakov, V.V. Biogeochemistry of selenium and its importance in the prevention of endemic human diseases. In Bulletin of the Department of Earth Sciences; RAS: Moscow, Russia, 2004; Volume 1, pp. 1–17. (In Russian) [Google Scholar]
- Ekholm, P.; Reinivuo, H.; Mattila, P.; Pakkala, H.; Koronen, J.; Happonen, A.; Hellstrom, J.; Pvaskainen, M.L. Changes in the mineral and trace element contents of cereals, fruits and vegetables in Finland. J. Food. Comp. Anal. 2007, 20, 487–495. [Google Scholar] [CrossRef]
- Golubkina, N.; Moldovan, A.; Kekina, H.; Kharchenko, V.; Sekara, A.; Vasileva, V.; Caruso, G. Joint biofortification of plants with selenium and iodine: New field of discoveries. Plants 2021, 10, 1352. [Google Scholar] [CrossRef]
- Koivistoinnen, J.K.; Huttenen, J.K. Selenium in food and nutrition in Finland. An overview on research and action. Ann. Clin. Res. 1986, 18, 296–298. [Google Scholar]
- Varo, P.; Alftan, G.; Huttenen, J.; Aro, A. Nationwide selenium supplementation in Finland-effects on diet, blood and tissue levels, and health. In Selenium in Biology and Human Health; Burk, R.F., Ed.; Springer: New York, NY, USA, 1994; pp. 198–218. ISBN 978-1-4612-2592-8. [Google Scholar]
- Torshin, S.P.; Udelnova, T.M.; Yagodin, B.A. Biogeochemistry and agrochemistry of selenium and methods for eliminating selenium deficiency in food and feedstuffs. Agrochemistry 1996, 8, 127–145. (In Russian) [Google Scholar]
- Rak, M.V.; Safronovskaya, G.M.; Barashkova, E.N.; Tikhonovich, Z.N.; Mukovozchik, V.A. Method for Dressing Clover with Meadow Selenium. Russian Federation Patent 14128 C1, 28 February 2011. (In Russian). [Google Scholar]
- Pushkarev, A. Enrichment of broccoli with selenium using apiones. Veg. Farming Green Housekeep. 2012, 4, 4–7. (In Russian) [Google Scholar]
- Oliveira, V.C.D.; Faquin, V.; Andrade, F.R.; Carneiro, J.P.; da Silva Júnior, E.C.; de Souza, K.R.D.; Guilherme, L.R.G. Physiological and physicochemical responses of potato to selenium biofortification in tropical soil. Potato Res. 2019, 62, 315–331. [Google Scholar] [CrossRef]
- Thavaraja, D.; Abare, A.; Mapa, I.; Koyne, S.J.; Thavaraja, K.S. Lentil accession selection for global selenium biofortification. Plants 2017, 3, 34. [Google Scholar] [CrossRef] [Green Version]
- Oliveira, V.C.D.; Faquin, V.; Guimarães, K.C.; Andrade, F.R.; Pereira, J.; Guilherme, L.R.G. Agronomic biofortification of carrot with selenium. Cienc. Agrotecnol. 2018, 42, 138–147. [Google Scholar] [CrossRef]
- Germ, M.; Stibilj, V.; Šircelj, H.; Jerše, A.; Kroflič, A.; Golob, A.; Maršić, N.K. Biofortification of common buckwheat microgreens and seeds with different forms of selenium and iodine. J. Sci. Food Agric. 2019, 99, 4353–4362. [Google Scholar] [CrossRef]
- Ducsay, L.; Ložek, O.; Marček, M.; Varényiová, M.; Hozlár, P.; Lošák, T. Possibility of selenium biofortification of winter wheat grain. Plant Soil Environ. 2016, 62, 379–383. [Google Scholar] [CrossRef] [Green Version]
- Hawrylak-Nowak, B. Comparative effects of selenite and selenate on growth and selenium accumulation in lettuce plants under hydroponic conditions. Plant Growth Regul. 2013, 70, 149–157. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Wu, Y.; Li, B.; Yang, Y.; Yang, Y. Selenium accumulation characteristics and biofortification potentiality in turnip (Brassica rapa var. rapa) supplied with selenite or selenite. Front. Plant Sci. 2018, 8, 2207. [Google Scholar] [CrossRef] [Green Version]
- Lidon, F.C.; Oliveira, K.; Galhano, C.; Guerra, M.; Ribeiro, M.M.; Pelica, J.; Reboredo, F.H. Selenium biofortification of rice through foliar application with selenite and selenite. Exp. Agric. 2019, 55, 528–542. [Google Scholar] [CrossRef]
- Finley, J.W. Selenium accumulation in plant foods. Nutr. Rev. 2005, 63, 196–202. [Google Scholar] [CrossRef] [PubMed]
- Dumpis, M.A.; Nikolayev, D.N.; Litasova, E.V.; Iljin, V.V.; Brusina, M.A.; Piotrovsky, L.B. Biological activity of fullerenes-reality and prospects. Rev. Clin. Pharmacol. Drug Ther. 2018, 16, 4–20. [Google Scholar] [CrossRef]
- Semenov, K.N.; Charykov, N.A.; Keskinov, V.A. Fullerenol synthesis and identification. Properties of fullerenol water solutions. J. Chem. Eng. Data 2011, 56, 230–239. [Google Scholar] [CrossRef]
- Andreev, I.; Petrukhina, A.; Garmanova, A.; Babakhin, A.; Andreev, S.; Romanova, V.; Troshin, P.; Troshina, O.; Du Buske, L. Penetration of fullerene C60 derivatives through biological membranes. Fuller. Nanotub. Carbon Nanostructures 2008, 16, 89–102. [Google Scholar] [CrossRef]
- Gao, J.; Wang, Y.; Folta, K.M.; Krishna, V.; Bai, W.; Indeglia, P.; Georgieva, A.; Nakamura, H.; Koopman, B.; Moudgi, B. Polyhydroxy fullerenes (fullerols or fullerenols): Beneficial effects on growth and lifespan in diverse biological models. PLoS ONE 2011, 6, e19976. [Google Scholar] [CrossRef] [Green Version]
- Chen, R.; Ratnikova, T.A.; Stone, M.B.; Lin, S.; Lard, M.; Huang, G.; Hudson, J.S.; Ke, P.C. Differential uptake of carbon nanoparticles by plant and mammalian cells. Small 2010, 6, 612–617. [Google Scholar] [CrossRef]
- Kole, C.; Kole, P.; Randunu, K.M.; Choudhary, P.; Podila, R.; Ke, P.C.; Rao, A.M.; Marcus, R.K. Nanobiotechnology can boost crop production and quality: First evidence from increased plant biomass, fruit yield and phytomedicine content in bitter melon (Momordica charantia). BMC Biotechnol. 2013, 13, 37–58. [Google Scholar] [CrossRef] [Green Version]
- Lin, S.; Reppert, J.; Hu, Q.; Hudson, J.S.; Reid, M.L.; Ratnikova, T.A.; Rao, A.M.; Luo, H.; Ke, P.C. Uptake, translocation, and transmission of carbon nanomaterials in rice plants. Small 2009, 5, 1128–1132. [Google Scholar] [CrossRef]
- Avanasi, R.; Jackson, W.A.; Sherwin, B.; Mudge, J.F.; Anderson, T.A. C60 fullerene soil sorption, biodegradation, and plant uptake. Environ. Sci. Technol. 2014, 48, 2792–2797. [Google Scholar] [CrossRef]
- Liang, C.; Xiao, H.; Hu, Z.; Zhang, X.; Hu, J. Uptake, transportation, and accumulation of C60 fullerene and heavy metal ions (Cd, Cu, and Pb) in rice plants grown in an agricultural soil. Environ. Pollut. 2018, 235, 330–338. [Google Scholar] [CrossRef] [PubMed]
- Panova, G.G.; Ktitorova, I.N.; Skobeleva, O.V.; Sinjavina, N.G.; Charykov, N.A.; Semenov, K.N. Impact of polyhydroxy fullerene (fullerol or fullerenol) on growth and biophysical characteristics of barley seedlings in favourable and stressful conditions. Plant Growth Regul. 2016, 79, 309–317. [Google Scholar] [CrossRef]
- Semenov, K.N.; Meshcheriakov, A.A.; Charykov, N.A.; Dmitrenko, M.E.; Keskinov, V.A.; Murin, I.V.; Panova, G.G.; Sharoyko, V.V.; Kanash, E.V.; Khomyakov, Y.V. Physico-chemical and biological properties of C 60-l-hydroxyproline water solutions. RSC Adv. 2017, 7, 15189–15200. [Google Scholar] [CrossRef] [Green Version]
- Panova, G.G.; Kanash, E.V.; Khomyakov, Y.V.; Shpanev, A.M.; Serebryakov, E.B.; Semenov, K.N.; Shemchuk, O.S.; Andrusenko, E.V.; Podolsky, N.E.; Sharoyko, V.V.; et al. Bioactivity study of the C60-L-threonine derivative for potential application in agriculture. J. Nanomater. 2019, 2306518, 13. [Google Scholar] [CrossRef] [Green Version]
- Panova, G.G.; Zhuravleva, A.S.; Khomyakov, Y.V.; Vertebnyi, V.E.; Ageev, S.V.; Sharoyko, V.V.; Semenov, K.N.; Petrov, A.V.; Podolsky, N.E.; Morozova, E.I. Plant impact properties of carboxylated fullerene C60 [C (COOH)2]3. J. Mol. Struct. 2021, 1235, 130163. [Google Scholar] [CrossRef]
- Bityutskii, N.P.; Yakkonen, K.L.; Lukina, K.A.; Semenov, K.N.; Panova, G.G. Fullerenol can ameliorate iron deficiency in cucumber grown hydroponically. J. Plant Growth Regul. 2020, 40, 1017–1031. [Google Scholar] [CrossRef]
- Shpanev, A.M.; Denisyuk, E.S.; Shilova, O.A.; Semenov, K.N.; Panova, G.G. Carbon and silica nanostructures in the protection of spring barley from diseases in the North-West Russia. Bioelectrochemical systems based on the electroactivity of plants and microorganisms in the root environment (review). Sel'skokhozyaistvennaya Biol. 2022, 57, 441–459. [Google Scholar] [CrossRef]
- Panova, G.G.; Semenov, K.N.; Artemyeva, A.M.; Rogozhin, E.A.; Barashkova, A.S.; Kornyukhin, D.L.; Khomyakov, Y.V.; Balashov, E.V.; Galushko, A.S.; Vertebny, V.E.; et al. Influence of nanocompositions based on derivatives of light fullerenes on cultivated plants in favorable and stressful conditions of their habitat. J. Tech. Phys. 2022, 92, 1045–1059. [Google Scholar] [CrossRef]
- Samadi, S.; Lajayer, B.A.; Moghiseh, E.; Rodríguez-Couto, S. Effect of carbon nanomaterials on cell toxicity, biomass production, nutritional and active compound accumulation in plants. Environ. Technol. Innov. 2021, 21, 101323. [Google Scholar] [CrossRef]
- Kovac, T.; Marcek, T.; Šarkanj, B.; Borišev, I.; Ižakovic, M.; Jukic, K.; Krska, R.F. C60 (OH)24 Nanoparticles and drought impact on wheat (Triticum aestivum L.) during growth and infection with Aspergillus flavus. J. Fungi 2021, 7, 236. [Google Scholar] [CrossRef]
- Tai, F.; Wang, S.; Liang, B.; Li, Y.; Wu, J.; Fan, C.; Wang, W. Quaternary ammonium iminofullerenes improve root growth of oxidative-stress maize through ASA-GSH cycle modulating redox homeostasis of roots and ROS-mediated root-hair elongation. J. Nanobiotechnol. 2022, 20, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Xiong, J.L.; Li, J.; Wang, H.C.; Zhang, C.L.; Naeem, M.S. Fullerol improves seed germination, biomass accumulation, photosynthesis and antioxidant system in Brassica napus L. under water stress. Plant Physiol. Biochem. 2018, 129, 130–140. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Zhang, H.; Ruan, L.; Chen, L.; Li, H.; Chang, X.L.; Yang, S.T. Bioaccumulation of 13 C-fullerenol nanomaterials in wheat. Environ. Sci. Nano 2016, 3, 799–805. [Google Scholar] [CrossRef]
- Semenov, K.N.; Charykov, N.A.; Namazbaev, V.I.; Keskinov, V.A. Method of producing mixture of fullerenols. RU 2495821 C2, 20 October 2013. (In Russian). Available online: https://pubchem.ncbi.nlm.nih.gov/patent/RU-2495821-C2 (accessed on 28 May 2023).
- Semenov, K.N.; Charykov, N.A.; Pronskikh, A.E.; Keskinov, V.A. Fullerenol-70-d: Synthesis, identification, polythermal solubility and density of water solutions. Nanosyst. Phys. Chem. Math. 2012, 3, 146–156. [Google Scholar]
- Shestopalova, A.A.; Semenov, K.N.; Charykov, N.A.; Postnov, V.N.; Ivanova, N.M.; Sharoyko, V.V.; Keskinov, V.A.; Letenko, D.G.; Nikitin, V.A.; Klepikov, V.V.; et al. Physico-chemical properties of the C60-arginine water solutions. J. Mol. Liq. 2015, 211, 301–307. [Google Scholar] [CrossRef] [Green Version]
- Panova, G.G.; Chernousov, I.N.; Udalova, O.R.; Alexandrov, A.V.; Karmanov, I.V.; Anikina, L.M.; Sudakov, V.L.; Yakushev, V.P. Scientific basis for large year-round yields of high-quality crop products under artificial lighting. Russ. Agric. Sci. 2015, 41, 335–339. [Google Scholar] [CrossRef]
- Panova, G.G.; Udalova, O.R.; Kanash, E.V.; Galushko, A.S.; Kochetov, A.A.; Priyatkin, N.S.; Arkhypov, M.V.; Chernousov, I.N. Fundamentals of physical modeling of “ideal” agroecosystems. Tech. Phys. 2020, 65, 1563–1569. [Google Scholar] [CrossRef]
- State Standard of Russian Federation. Products of Fruits and Vegetables. Processing. Methods for Determination of Vitamin C; Standartinform Publishing: Moscow, Russia, 2003; pp. 24556–24589. (In Russian) [Google Scholar]
- Guidelines for the determination of nitrates and nitrites in crop production MU N. In USSR Minist. Health USSR State Agroprom; Publishing: Moscow, Russia, 1989; pp. 5048–5089. 52p. (In Russian)
- Interstate Standard GOST EN 14084–2014; Foodstuffs. Definition of trace elements. Determination of lead, cadmium, zinc, copper and iron content by atomic absorption spectrometry (AAS) after microwave digestion. In Eurasian Council for Standardization, Metrology and Certification; National Institute of Standards: Minsk, Belarus, 2014; 17p. (In Russian)
- Technical Regulation of the Customs Union “On Food Safety” (TR TS 021/2011) dated 09.12.2011 N021/2011 (as amended on 14 July 2021), Official Website of the Customs Union Commission. 173p. Available online: www.tsouz.ru (accessed on 15 December 2021).
- Assessment of the quality of food products and assessment of the population’s access to domestic food products, contributing to the elimination of macro- and micronutrient deficiencies: Guidelines. MP 2.3.7.0168-20. Bull. Regul. Methodol. Doc. State Sanit. Epidemiol. Superv. Russ. Fed. 2020, 2, 43–106. (In Russian)
- GOST R 53182–2008; Foodstuffs. Determination of Trace Elements. Determination of Total Arsenic and Selenium by Hydride Generation Atomic Absorption Spectrometry (HGAAS) Method after Pressure Digestion. Standartinform Publishing: Moscow, Russia, 2010; p. 12. (In Russian)
- ISO 10390; Soil, Treated Biowaste and Sludg–Determination of pH. ISO: Genewa, Switzerland, 2021.
- RF State Standard 26489-85; Soils. Determination of Exchangeable Ammonium by CINAO Method. Standards Publishing House: Moscow, Russia,, 1985. (In Russian)
- RF State Standard 26951-86; Soils. Determination of Nitrates by Ionometric Method. Standards Publishing House: Moscow, Russia, 1986. (In Russian)
- RF State Standard R 54650-2011; Soils. Determination of Mobile Compounds of Phosphorus and Potassium According to the Kirsanov Method in the Modification of CINAO. Federal Agency for Technical Regulation and Metrology on the Internet. Standartinform Publishing: Moscow, Russia, 2011. (In Russian)
- RF State Standard 26487-85; Soils. Determination of Exchangeable Calcium and Exchangeable (Mobile) Magnesium by CINAO Methods. Standards Publishing House: Moscow, Russia, 1985. (In Russian)
- Nichiporovich, A.A. Photosynthesis and the productive process. M Sci. 1988, 276. (In Russian) [Google Scholar]
- Trineeva, O.V.; Safonova, E.F.; Slivkin, A.I.; Voropaeva, S.V. Method of Spectrophotometric Quantitative Determination in the Leaves of Stinging Nettle in the Joint Presence of Chlorophyll, Carotenoids and Hydroxycinnamic Acids. Russian Federation Patent 2531940, 27 October 2014. (In Russian). [Google Scholar]
- Kumar, G.N.M.; Knowles, N.R. Changes in lipid peroxidation and lipolytic and freeradical scavenging enzyme activities during aging and sprouting of potato (Solanum tuberosum) seed-tubers. Plant. Physiol. 1993, 102, 115–124. [Google Scholar] [CrossRef] [Green Version]
- Lukatkin, A.S. Contribution of oxidative stress to development of cold-induced damage on leaves of chilling-sensitive plants: 1. Reactive oxygen species formation during plant during plant chilling. Russ. J. Plant Physiol. 2002, 49, 622–627. [Google Scholar] [CrossRef]
- Pochinok, H.N. Methods of Biochemical Analysis of Plants; Naukovo Dumka: Kyiv, Ukraine, 1976; 334p. (In Russian) [Google Scholar]
- Bityutskii, N.P.; Yakkonen, K.L.; Semenov, K.N. Zinc deficiency in cucumber plants can be alleviated by fullerenol. J. Plant Nutr. 2023, 46, 1504–1518. [Google Scholar] [CrossRef]
- Bityutskii, N.P.; Yakkonen, K.L.; Lukina, K.A.; Semenov, K.N. Fullerenol increases effectiveness of foliar iron fertilization in iron deficient cucumber. PLoS ONE 2020, 15, e0232765. [Google Scholar] [CrossRef]
- Kabata-Pendias, A.; Pendias, H. Trace elements in soils and plants. In Biogeochemistry of Trace Elements; 3rd ed.; CRC Press: Boca Raton, FL, USA; London, UK; New York, NY, USA; Washington, DC, USA, 2001; 403p. [Google Scholar]
- Marschner, H. Mineral Nutrition of Higher Plants, 3rd ed.; Academic Press: London, UK, 2012; 651p, ISBN 978-0-12-384905-2. [Google Scholar] [CrossRef]
- Tsonev, T.; Lidon, F.J.C. Zinc in plants-an overview. Emir. J. Food Agric. (EJFA) 2012, 24, 322–333. [Google Scholar]
- Hafeez, B.M.; Khanif, Y.M.; Saleem, M. Role of zinc in plant nutrition-a review. Am. J. Exp. Agric. 2013, 3, 374–391. [Google Scholar] [CrossRef]
- Shams Tabrez, K.; Malik, A. (Eds.) Microbial Biofertilizers and Micronutrient Availability: The Role of Zinc in Agriculture and Human Health; Springer Nature: Cham, Switzerland, 2021. [Google Scholar] [CrossRef]
- Sharma, A.; Kumar, V.; Shahzad, B.; Ramakrishnan, M.; Singh Sidhu, G.P.; Bali, A.S.; Handa, N.; Kapoor, D.; Yadav, P.; Khanna, K.; et al. Photosynthetic Response of Plants Under Different Abiotic Stresses: A Review. J. Plant Growth Regul. 2019, 39, 509–531. [Google Scholar] [CrossRef]
- Buturi, C.V.; Mauro, R.P.; Fogliano, V.; Leonardi, C.; Giuffrida, F. Mineral Biofortification of Vegetables as a Tool to Improve Human Diet. Foods 2021, 10, 223. [Google Scholar] [CrossRef]
- Chaudhry, F.M.; Sharif, M.; Latif, A.; Qureshi, R.H. Zinc-copper antagonism in the nutrition of rice (Oryza sativa L.). Plant Soil 1973, 38, 573–580. [Google Scholar] [CrossRef]
- Poudel, P.; Di Gioia, F.; Lambert, J.D.; and Connolly, E.L. Zinc biofortification through seed nutri-priming using alternative zinc sources and concentration levels in pea and sunflower microgreens. Front. Plant Sci. 2023, 14, 1177844. [Google Scholar] [CrossRef]
- Hygienic Requirements for the Safety and Nutritional Value of Food: Sanitary and Epidemiological Rules and Regulations SanPiN 2.3.2.1078-01. approved by the Chief State Sanitary Doctor of the Russian Federation 06.11.2001, Moskow, Russian Federation, 44p. (In Russian). Available online: https://www.russiangost.com/p-149772-sanpin-2321078-01.aspx (accessed on 28 May 2023).
- Commission Regulation (EU) No 1258/2011. Amending Regulation (EC) No 1881/2006 as regards maximum levels for nitrates in foodstuffs. Off. J. Eur. Union 2011, 320, 15–17. Available online: http://eurlex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2011:320:0015:0017:EN:PDF (accessed on 28 May 2023).
- Gorenjak, A.H.; Cencič, A. Nitrate in vegetables and their impact on human health (a review). Acta Aliment. 2013, 42, 158–172. [Google Scholar] [CrossRef]
- Xu, J.; Yang, J.; Duan, X.; Jiang, Y.; Zhang, P. Increased expression of native cytosolic Cu/Zn superoxide dismutase and ascorbate peroxidase improves tolerance to oxidative and chilling stresses in cassava (Manihot esculenta Crantz). BMC Plant Biol. 2014, 14, 208. [Google Scholar] [CrossRef] [PubMed]
- Ali, S.; Mehmood, A.; Khan, N. Uptake, translocation, and consequences of nanomaterials on plant growth and stress adaptation. J. Nanomater. 2021, 6677616. [Google Scholar] [CrossRef]
- МR 2.3.1.1915-04; Recommended Levels of Food Intake and Biologically Active Substances: Guidelines. Federal Center for State Sanitary and Epidemiological Surveillance of the Ministry of Health of Russia: Moskow, Russia, 2004; 46p.
- Dietary Reference Intakes for Vitamin A, Vitamin K, Arsenic, Boron, Chromium, Copper, Iodine, Manganese, Mohbdenium, Nikel, Silicon, Vanadium and Zinc Food and Nutrition Board (FNB), Institute of Medicine (IOM); Copyright by National Academy of Sciences; The National Academies Press: Washington, DC, USA, 2002; 773p. [CrossRef]
- Dietary Reference Intakes for Vitamin C, Vitamin E, Selenium and Carotenoids; National Academy of Sciences: Washington, DC, USA, 2000; 529p. [CrossRef]





| Experiment Series | Growing Equipment [62] | Method of Plant Cultivation | Method of Treatment with Test Substance | Condition | |||||
|---|---|---|---|---|---|---|---|---|---|
| Air Temperature: Day/Night | Air Humidity | Light Source/Duration of the Light Period | Light Intensity | pH of Root Inhabited Zone, Relative Units | EC, mS cm−1 | ||||
| 1 | Plant growing light equipment | Geoponic | Introduction into soil | +22–+24 °C/+18–+20 °C | 75–80% (soil humidity: 60–70%) | High-pressure sodium lamps (DnaZ-400, “Reflax” LLC, Moscow, Russia)/14 h per day | 70–75 W/m2 in the PAR | Soil: 5.8 | Soil: no data |
| 2 | Rhizotron | Panoponic [63] | Introduction into nutrient solution | +22–+24 °C/ +18–+20 °C | 60–70% | 75–80 W/m2 in the PAR region | Nutrient solution 6.0 | Nutrient solution 1.0 | |
| 3 | Plant growing light equipment | Foliar treatment | + 23–+25 °C / +21–+23 °C | 60–70%. | High-pressure sodium lamps (DnaZ-400, “Reflax” LLC, Moscow, Russia)/16 h per day | 95–105 W/m2 in the PAR region | Nutrient solution 5.6–5.8 | Nutrient solution 1.5 | |
| 4 | Plant growing light equipment | Foliar treatment | +22–+24 °C/ +18–+20 °C | 75–80%. | High-pressure sodium lamps (DNaZ-400, “Reflax” LLC, Moscow, Russia)/14 h per day | 70–75 W/m2 in the PAR area | Nutrient solution 6.0–6.2 | Nutrient solution 1.6 | |
| Substance Name | Amount of Substance, mmol/L |
|---|---|
| Calcium nitrate | 4.2 |
| Potassium nitrate | 3.6 |
| Potassium phosphate monosubstituted | 1.8 |
| Magnesium sulfate heptahydrate | 1.0 |
| Ammonium nitrate | 1.8 |
| Urea | 0.3 |
| Iron citrate ammonium | 0.0178 |
| Boric acid | 0.0467 |
| Manganese sulfate pentahydrate | 0.00789 |
| Zinc sulfate heptahydrate | 0.00070 |
| Copper sulfate pentahydrate | 0.00068 |
| Soils Indicators | Concentrations of Applied Fullerenol, mg/kg of Soil | |||
|---|---|---|---|---|
| 0 (Control) | 1 | 10 | 100 | |
| pH KCl, units pH | 5.10 a | 5.10 a | 5.10 a | 5.10 a |
| Ammonium nitrogen, mg/kg | 6.84 ± 0.34 a | 5.09 ± 0.25 b | 6.52 ± 0.33 a | 5.01 ± 0.25 b |
| Nitrate nitrogen, mg/kg | 0.53 ± 0.03 c | 0.50 ± 0.03 c | 0.72 ± 0.04 b | 0.84 ± 0.04 a |
| Phosphorus: mobile in terms of P2O5, mg/kg | 130.00 ± 6.50 b | 121.00 ± 6.05 b | 125.00 ± 6.25 b | 174.00 ± 8.70 a |
| Potassium: mobile in terms of K2O, mg/kg | 48.00 ± 2.40 a | 42.00 ± 2.10 b | 48.00 ± 2.40 a | 52.00 ± 2.60 a |
| Calcium: exchangeable, mmol/100 g | 5.00 ± 0.25 a | 4.38 ± 0.22 b | 4.63 ± 0.23 ab | 4.88 ± 0.24 a |
| Magnesium: exchangeable, mmol/100 g | <0.10 ± 0.005 c | <0.10 ± 0.005 c | 1.25 ± 0.063 b | 1.50 ± 0.075 a |
| Iron: mobile, mg/kg | 12.00 ± 0.60 b | 12.60 ± 0.63 b | 13.80 ± 0.69 a | 11.40 ± 0.57 b |
| Copper: mobile, mg/kg | 0.18 ± 0.009 c | 0.22 ± 0.011 b | 0.25 ± 0.013 a | 0.23 ± 0.012 ab |
| Zinc: mobile, mg/kg | 2.40 ± 0.12 c | 2.17 ± 0.11 c | 3.20 ± 0.16 b | 4.49 ± 0.22 a |
| Manganese: mobile, mg/kg | 7.31 ± 0.37 a | 7.39 ± 0.37 a | 7.77 ± 0.39 a | 6.43 ± 0.32 b |
| Concentrations of C60 Fullerenol Introduced into the Soil, mg/kg of Soil | Chlorophyll a, µg 100 g−1 FM | Chlorophyll b, µg 100 g−1 FM | Total Chlorophyll, µg 100 g−1 FM | Carotenoid, µg 100 g−1 FM |
|---|---|---|---|---|
| Control | 70.82 ± 1.43 b | 25.24 ± 1.16 c | 96.06 ± 4.44 c | 21.68 ± 1.00 b |
| 1 | 80.83 ± 3.73 a | 30.35 ± 1.40 a | 111.18 ± 5.14 a | 24.74 ± 1.14 a |
| 10 | 70.88 ± 1.42 b | 27.38 ± 1.27 bc | 98.26 ± 4.54 bc | 22.42 ± 1.04 b |
| 100 | 78.14 ± 1.76 a | 28.41 ± 1.31 ab | 106.55 ± 4.92 ab | 24.79 ± 1.15 a |
| Concentrations of C60 Fullerenol Introduced into the Soil, mg/kg of Soil | Number of Leaves, pcs. | Leaves’ Area, cm2 | Stem Cross-Sectional Area cm2 | Leaves | Stems | Roots | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Raw Mass, g/Plant | Dry Mass, g/Plant | % Dry Matter | Raw Mass, g/Plant | Dry Mass, g/Plant | % Dry Matter | Raw Mass, g/Plant | Dry Mass, g/Plant | % Dry Matter | ||||
| Control | 4.4 ± 0.3 a | 240.1 ± 9.3 bc | 0.218 ± 0.014 b | 5.04 ± 0.25 b | 1.23 ± 0.07 a | 24.4 ± 0.2 c | 3.92 ± 0.25 a | 0.43 ± 0.05 ab | 11.0 ± 0.2 b | 2.28 ± 0.34 b | 1.96 ± 0.03 b | 8.6 ± 0.2 a |
| 1 | 4.9 ± 0.3 a | 259.4 ± 10.3 a | 0.242 ± 0.014 a | 5.45 ± 0.17 a | 1.33 ± 0.11 a | 24.4 ± 0.2 c | 4.12 ± 0.28 a | 0.40 ± 0.05 b | 9.7 ± 0.2 c | 2.67 ± 0.48 a | 2.06 ± 0.03 a | 7.7 ± 0.2 b |
| 10 | 4.5 ± 0.3 a | 245.2 ± 11.7 b | 0.238 ± 0.014 a | 5.14 ± 0.32 b | 1.31 ± 0.09 a | 25.5 ± 0.3b | 4.00 ± 0.22 a | 0.47 ± 0.05 a | 11.7 ± 0.3 a | 2.48 ± 0.40 ab | 1.94 ± 0.05 b | 7.8 ± 0.2 b |
| 100 | 4.4 ± 0.3 a | 230.2 ± 11.1 c | 0.231 ± 0.014 ab | 5.10 ± 0.35 b | 1.36 ± 0.08 a | 26.7 ± 0.3 a | 3.93 ± 0.26 a | 0.46 ± 0.03 a | 11.7 ± 0.2 a | 2.33 ± 0.32 b | 1.85 ± 0.04 c | 7.9 ± 0.2 b |
| Concentrations of C60 Fullerenol Introduced into the Soil, mg/kg of Soil | POX, U s−1 g−1 | CAT, µM H2O2 mg−1 Protein min−1 | LPO, µM g−1 |
|---|---|---|---|
| Leaves | |||
| Control | 19.46 ± 0.90 a | 269.73 ± 12.46 c | 0.0157 ± 0.0007 a |
| 1 | 18.18 ± 0.84 a | 330.55 ± 15.27 b | 0.0092 ± 0.0004 c |
| 10 | 19.45 ± 0.91 a | 373.56 ± 17.26 a | 0.0101 ± 0.0005 bc |
| 100 | 15.94 ± 0.74 b | 293.56 ± 13.57 c | 0.0108 ± 0.0007 b |
| Roots | |||
| Control | 49.32 ± 2.28 b | 6.61 ± 0.31 d | 0.0150 ± 0.0007 a |
| 1 | 37.94 ± 1.75 c | 44.83 ± 2.07 b | 0.0132 ± 0.0006 b |
| 10 | 26.18 ± 1.21 d | 16.96 ± 0.78 c | 0.0117 ± 0.0005 c |
| 100 | 55.99 ± 2.59 a | 49.35 ± 2.28 a | 0.0121 ± 0.0006b c |
| Experience Variant | Raw Mass, g | Selenium Content mg/kg a.d.m. |
|---|---|---|
| Aboveground part | ||
| Control (NS) | 35.1 ± 2.0 c | 49.0 ± 2.8 c |
| NS + 0.0001% Na2SeO4 | 4.3 ± 0.3 d | 51.0 ± 2.9 c |
| NS + 0.0001% Na2SeO4+0,0001% C60-L-Arg | 85.8 ± 4.9 b | 92.5 ± 5.2 a |
| NS + 0.0001% Na2SeO4+0,0001% C60-L-Gly | 119.4 ± 6.8 a | 64.4 ± 3.6 b |
| Roots | ||
| Control (NS) | 10.4 ± 0.6 c | 58.6 ± 3.3 c |
| NS + 0,0001% Na2SeO4 | 1.8 ± 0.1 d | 69.3 ± 3.9 b |
| NS + 0.0001% Na2SeO4+0.0001% C60-L-Arg | 18.7 ± 1.1 b | 101.5 ± 5.7 a |
| NS + 0.0001% Na2SeO4+0.0001% C60-L-Gly | 34.4 ± 1.9 a | 72.2 ± 4.1 b |
| Indicators | Foliar Treatment with Mono- and Mixed Solutions | |||
|---|---|---|---|---|
| Control (Water) | 0.0001% Na2SeO4 | Na2SeO4 + 0.0001% C60-L-Arg | 0,0001% Na2SeO4 + 0.0001% C60-L-Gly | |
| % Dry matter | 5.6 ± 0.3 a | 6.2 ± 0.4 a | 6.0 ± 0.3 a | 5.8 ± 0.3 a |
| Total saccharide % a.d.m | 35.6 ± 2.0 c | 42.8 ± 2.4 ab | 39.8 ± 2.3 b | 45.0 ± 2.6 a |
| Monosaccharide, % a.d.m | 34.3 ± 1.9 b | 41.7 ± 2.4 a | 35.9 ± 2.0 b | 43.6 ± 2.5 a |
| Disaccharide, % a.d.m. | 1.3 ± 0.1 bc | 1.1 ± 0.1 c | 3.9 ± 0.2 a | 1.4 ± 0.1 b |
| Vitamin C, mg/100 g r.m. | 18.9 ± 1.1 c | 24.6 ± 1.4 a | 22.0 ± 1.2 b | 25.3 ± 1.4 a |
| Nitrates, mg/kg r.m. | <29.7 ± 1.5 a | <29.7 ± 1.5 a | <29.7 ± 1.5 a | <29.7 ± 1.5 a |
| Carotene, mg/kg r.m. | 50.3 ± 2.8 c | 85.3 ± 4.8 a | 59.7 ± 3.4 b | 65.1 ± 3.7 b |
| Indicator | Foliar Treatment with Mono- and Mixed Solutions | |||||
|---|---|---|---|---|---|---|
| Control | ZnSO4 | Fullerenol C60 | Fullerenol C60 + ZnSO4 | Fullerenol C70 | Fullerenol C70 +ZnSO4 | |
| Raw ash, % a.d.m. | 12.36 ± 0.46 c | 12.80 ± 0.36 bc | 12.84 ± 0.34 abc | 13.40 ± 0.38 ab | 13.49 ± 0.34 a | 13.45 ± 0.46 ab |
| N, % a.d.m. | 4.60 ± 0.54 a | 4.98 ± 0.41 a | 4.97 ± 0.43 a | 4.70 ± 0.36 a | 4.80 ± 0.45 a | 5.05 ± 0.48 a |
| P, % a.d.m. | 1.08 ± 0.23 a | 1.07 ± 0.20 a | 1.02 ± 0.18 a | 1.07 ± 0.16 a | 1.05 ± 0.16 a | 1.10 ± 0.20 a |
| K, % a.d.m. | 5.03 ± 0.35 b | 5.67 ± 0.41 ab | 5.20 ± 0.38 ab | 5.37 ± 0.40 ab | 5.74 ± 0.35 a | 5.54 ± 0.43 ab |
| Ca, % a.d.m. | 1.10 ± 0.09 ab | 1.09 ± 0.07 abc | 1.18 ± 0.06 a | 1.06 ± 0.06 bcd | 1.00 ± 0.05 cd | 0.97 ± 0.07 d |
| Mg, % a.d.m. | 0.29 ± 0.02 b | 0.30 ± 0.05 ab | 0.30 ± 0.03 ab | 0.31 ± 0.03 ab | 0.34 ± 0.03 a | 0.31 ± 0.05 ab |
| Fe, mg/kg a.d.m. | 51.70 ± 3.62 c | 54.50 ± 5.43 c | 52.20 ± 4.30 c | 97.10 ± 5.66 a | 74.20 ± 4.75 b | 57.60 ± 5.88 a |
| Mn, mg/kg a.d.m. | 40.0 ± 3.28 c | 48.00 ± 3.73 a | 45.30 ± 3.17 abc | 41.40 ± 3.39 bc | 42.90 ± 3.17 abc | 45.90 ± 3.62 ab |
| Cu, mg/kg a.d.m. | 21.9 ± 0.45 b | 10.40 ± 0.52 d | 10.90 ± 0.38 d | 9.32 ± 0.34e | 12.40 ± 0.48c | 25.70 ± 0.68 a |
| Zn, mg/kg a.d.m. | 28.90 ± 0.50 f | 37.30 ± 0.45c | 34.10 ± 0.41 d | 45.90 ± 0.43 a | 30.80 ± 0.34e | 40.90 ± 0.46 |
| Dry matter, % | 3.20 ± 0.2 a | 2.80 ± 0.3 a | 2.80 ± 0.2 a | 3.00 ± 0.3 a | 3.20 ± 0.2 a | 3.00 ± 0.2 a |
| Vitamin C, mg/100 g r.m. | 9.40 ± 0.7 a | 8.10 ± 0.7 b | 8.80 ± 0.5 ab | 8.60 ± 0.7 ab | 8.70 ± 0.6 ab | 8.50 ± 0.7 ab |
| Nitrates, mg/kg r.m. | 159.0 ± 10.6 c | 151.80 ± 10.9 c | 141.70 ± 10.0 c | 219.50 ± 11.8 a | 117.90 ± 10.4 d | 195.60 ± 11.9 b |
| Total saccharide, % a.d.m. | 29.10 ± 3.2 ab | 28.30 ± 2.9 ab | 28.70 ± 2.6 ab | 31.10 ± 2.9 a | 25.60 ± 2.5 b | 26.70 ± 2.8 ab |
| Monosaccharide, % a.d.m. | 28.60 ± 3.4 ab | 27.50 ± 2.9 abc | 27.80 ± 2.5 abc | 30.60 ± 2.9 a | 23.30 ± 2.7 c | 25.60 ± 2.7 bc |
| Disaccharide, % a.d.m. | 0.50 ± 0.03 a | 0.80 ± 0.05 d | 0.90 ± 0.04 c | 0.50 ± 0.03 e | 2.40 ± 0.02 a | 1.10 ± 0.03 b |
| Experience Variant | Stem Cross-Sectional Area cm2 | Leaves’ Area, cm2 | Leaves | Stems | ||||
|---|---|---|---|---|---|---|---|---|
| Raw Mass, g/Plant | Dry Mass, g/Plant | % Dry Matter | Raw Mass, g/Plant | Dry Mass, g/Plant | % Dry Matter | |||
| Control (water) | 1183.0 ± 158.4 d | 2412.0 ± 656.3 c | 338.0 ± 91.7 a | 33.6 ± 8.9 a | 10.0 ± 0.8 ab | 320.0 ± 58.5 a | 18.1 ± 4.3 a | 5.7 ± 0.7 a |
| ZnSO4 | 1398.0 ± 158.4 abc | 3149.0 ± 339.5 a | 361.0 ± 37.3 a | 34.5 ± 2.0 a | 9.6 ± 0.2b | 297.0 ± 20.1 a | 13.5 ± 2.4 a | 4.6 ± 0.5b |
| Fullerenol C60 | 1224.0 ± 237.6 cd | 2418.0 ± 80.3 c | 348.0 ± 101.8 a | 36.9 ± 5.0 a | 10.6 ± 0.3 a | 299.5 ± 27.0 a | 15.9 ± 2.8 a | 5.3 ± 0.2 a |
| Fullerenol C60+ ZnSO4 | 1575.0 ± 181.1 a | 3053.0 ± 56.6 a | 360.0 ± 37.3 a | 37.3 ± 1.9 a | 10.4 ± 0.2 a | 327.0 ± 21.6 a | 18.1 ± 2.3 a | 5.5 ± 0.2 a |
| Fullerenol C70 | 1360.0 ± 69.0 bcd | 2618.0 ± 339.5 bc | 343.5 ± 48.7 a | 36.5 ± 5.5 a | 10.6 ± 0.3 a | 318.5 ± 39.0 a | 17.9 ± 2.6 a | 5.6 ± 0.2 a |
| Fullerenol C70 + ZnSO4 | 1441.0 ± 66.8 ab | 2843.0 ± 509.2 ab | 354.0 ± 65.6 a | 37.9 ± 5.9 a | 10.7 ± 0.4 a | 320.0 ± 62.4 a | 18.0 ± 4.1 a | 5.6 ± 0.4 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Panova, G.G.; Semenov, K.N.; Zhuravleva, A.S.; Khomyakov, Y.V.; Volkova, E.N.; Mirskaya, G.V.; Artemyeva, A.M.; Iamalova, N.R.; Dubovitskaya, V.I.; Udalova, O.R. Obtaining Vegetable Production Enriched with Minor Micronutrients Using Fullerene Derivatives. Horticulturae 2023, 9, 828. https://doi.org/10.3390/horticulturae9070828
Panova GG, Semenov KN, Zhuravleva AS, Khomyakov YV, Volkova EN, Mirskaya GV, Artemyeva AM, Iamalova NR, Dubovitskaya VI, Udalova OR. Obtaining Vegetable Production Enriched with Minor Micronutrients Using Fullerene Derivatives. Horticulturae. 2023; 9(7):828. https://doi.org/10.3390/horticulturae9070828
Chicago/Turabian StylePanova, Gayane G., Konstantin N. Semenov, Anna S. Zhuravleva, Yuriy V. Khomyakov, Elena N. Volkova, Galina V. Mirskaya, Anna M. Artemyeva, Nailia R. Iamalova, Victoriya I. Dubovitskaya, and Olga R. Udalova. 2023. "Obtaining Vegetable Production Enriched with Minor Micronutrients Using Fullerene Derivatives" Horticulturae 9, no. 7: 828. https://doi.org/10.3390/horticulturae9070828