Edible Composite Coating of Chitosan and Curdlan Maintains Fruit Quality of Postharvest Cherry Tomatoes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of the Coating Solution and Cherry Tomato Treatment
2.3. Determination of Indexes Related to Cherry Tomato Preservation
2.3.1. Determination of Appearance of Rottenness
2.3.2. Determination of Weight Loss, Firmness, Soluble Solids and Titratable Acidity
2.3.3. Determination of Respiration and Ethylene
2.3.4. Determination of Enzymatic Activities
2.3.5. Determination of MDA Content and Membrane Permeability
2.3.6. Determination of Vitamin C and Lycopene
2.3.7. Determination of Antioxidant Activities
2.4. Statistical Analysis
3. Results
3.1. Rottenness
3.2. Weight Loss, Firmness, Soluble Solids and Titratable Acidity
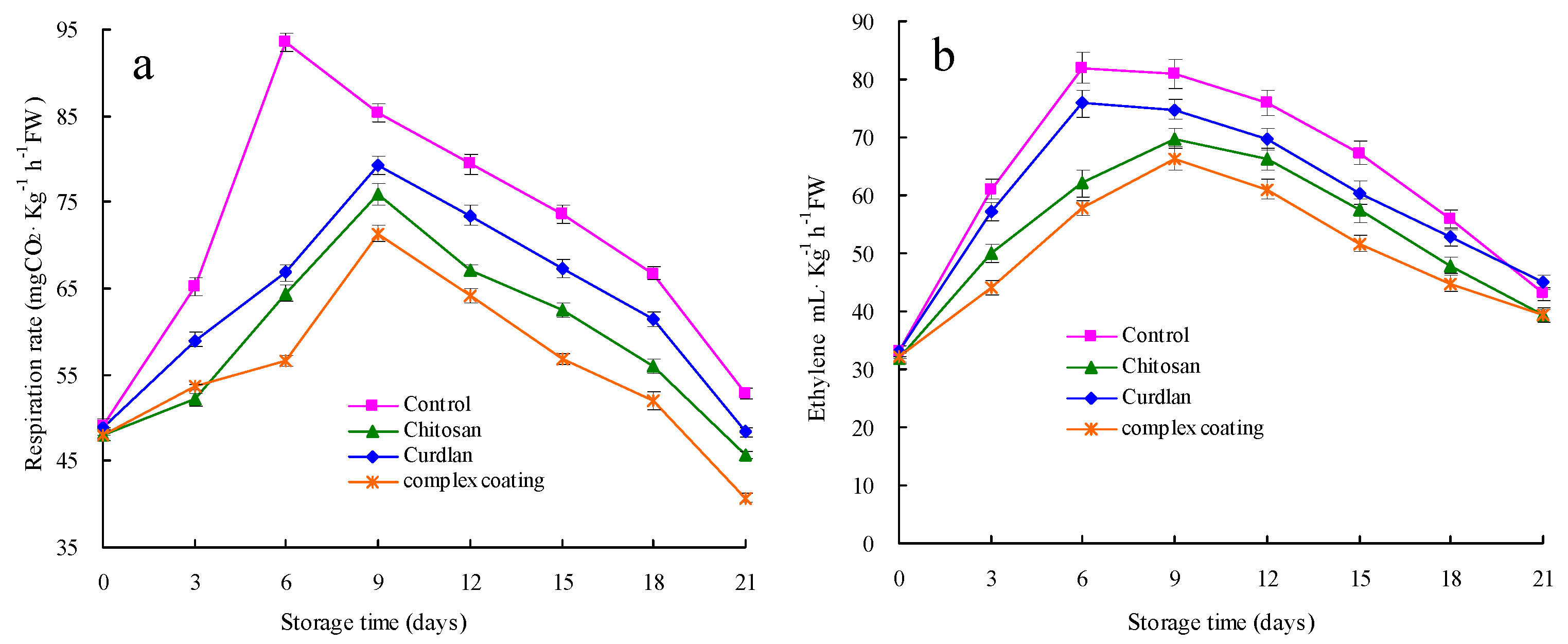
3.3. Respiration Rate and Ethylene
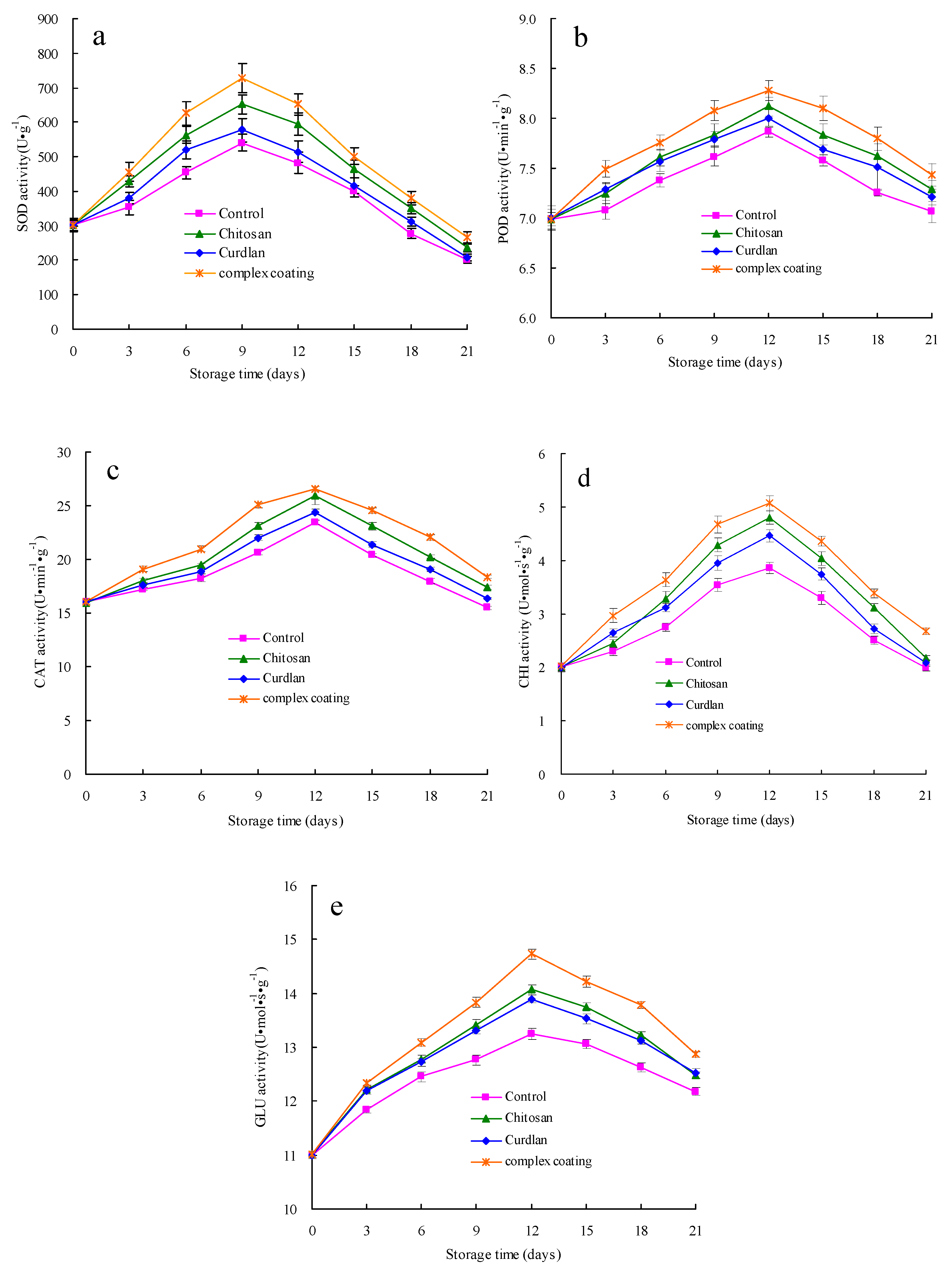
3.4. SOD, POD, CAT, GLU and CHI Activities
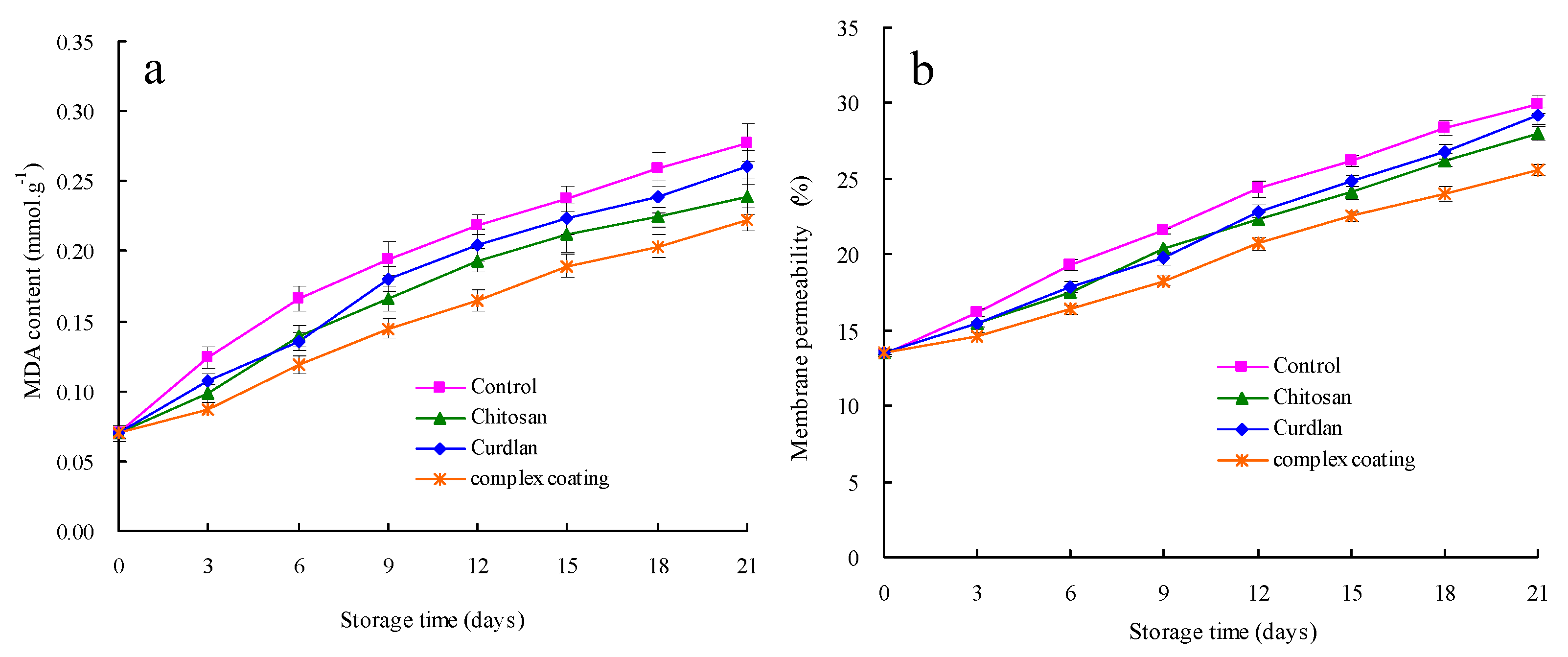
3.5. MDA Content and Membrane Permeability
3.6. Vitamin C and Lycopene
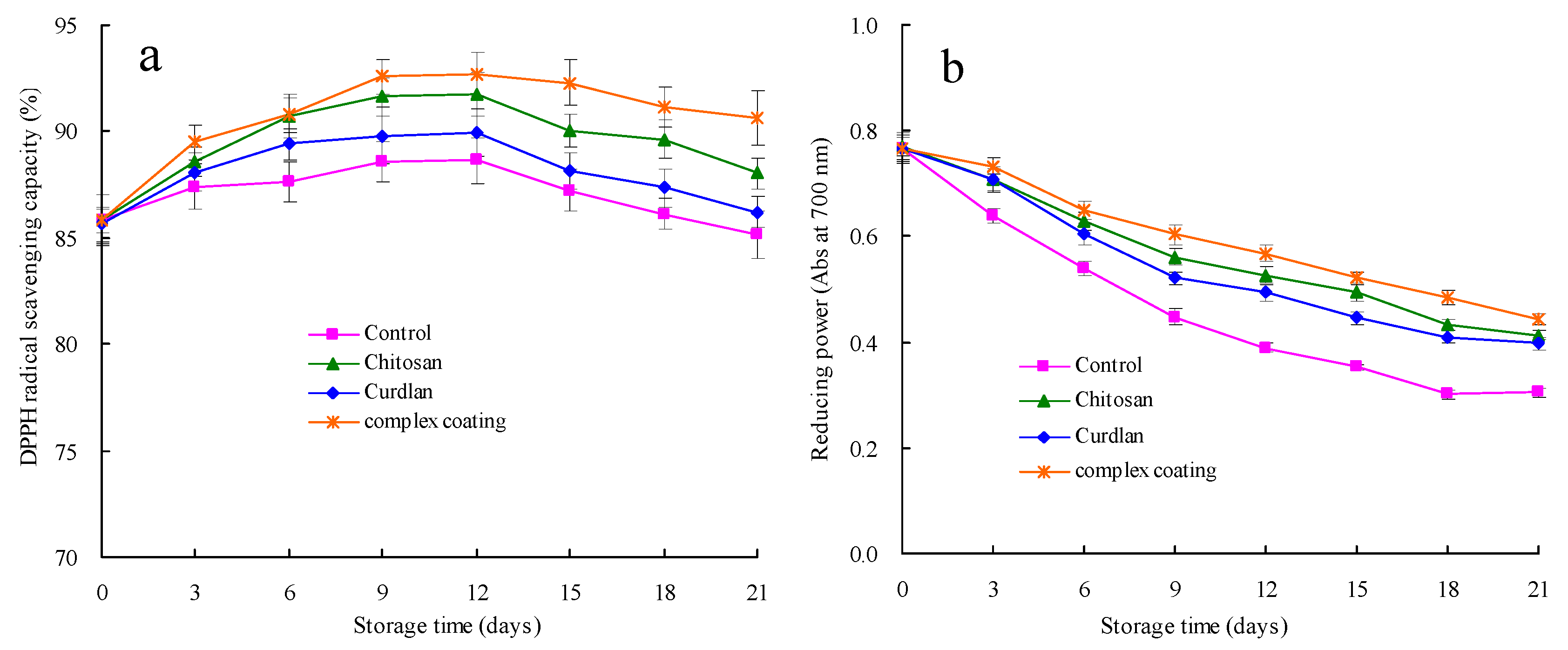
3.7. Antioxidant Capacities in Terms of DPPH Radical Scavenging Capacity and Reducing Power
4. Discussion
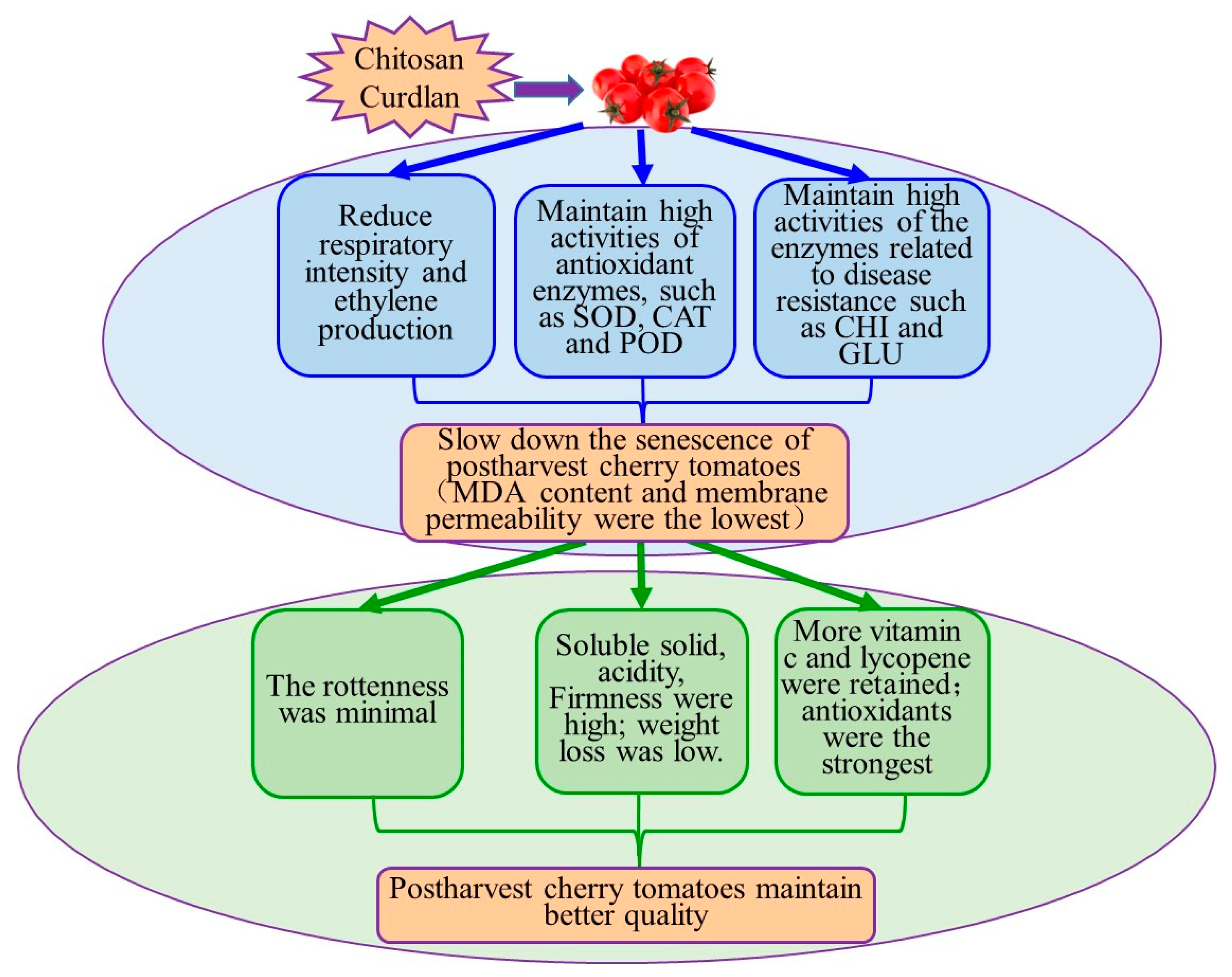
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zheng, J.R.; Li, Y.H.; Nie, J.; Tan, D.L.; Xie, Y.M.; Zhang, C.Y. Overview and research progress of protected cherry tomato industry. Guangdong Agric. Sci. 2020, 47, 212–220. [Google Scholar]
- Spring Woods. Why Does FAO Give Priority to Promoting These “Four Fruits”? Sohu.com. 2017. Available online: https://www.sohu.com/a/190638721_99965757 (accessed on 8 September 2017).
- Nehela, Y.; Taha, N.A.; Elzaawely, A.A.; Xuan, T.D.; Amin, M.A.; Ahmed, M.E.; El-Nagar, A. Benzoic Acid and Its Hydroxylated Derivatives Suppress Early Blight of Tomato (Alternaria solani) via the Induction of Salicylic Acid Biosynthesis and Enzymatic and Nonenzymatic Antioxidant Defense Machinery. J. Fungi 2021, 7, 663. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhou, Y.; Wang, Z.; Cai, R.; Yue, T.; Cui, L. Preparation and Characterization of Chitosan–Nano-ZnO Composite Films for Preservation of Cherry Tomatoes. Foods 2021, 10, 3135. [Google Scholar] [CrossRef]
- Razali, Z.; Somasundram, C.; Nurulain, S.Z.; Kunasekaran, W.; Alias, M.R. Postharvest Quality of Cherry Tomatoes Coated with Mucilage from Dragon Fruit and Irradiated with UV-C. Polymers 2021, 13, 2919. [Google Scholar] [CrossRef] [PubMed]
- D’Aquino, S.; Mistriotis, A.; Briassoulis, D.; Lorenzo, M.L.D.; Malinconico, M.; Palma, A. Influence of modified atmosphere packaging on postharvest quality of cherry tomatoes held at 20 °C. Postharvest Biol. Technol. 2016, 115, 103–112. [Google Scholar] [CrossRef]
- Steelheart, C.; Alegre, M.L.; Bahima, J.V.; Senn, M.E.; Simontacchi, M.; Bartoli, C.G.; Grozeff, G.E.G. Nitric oxide improves the effect of 1-methylcyclopropene extending the tomato (Lycopersicum esculentum L.) fruit postharvest life. Sci. Hortic. 2019, 255, 193–201. [Google Scholar] [CrossRef]
- Wang, D.; Randhawa, M.S.; Azam, M.; Liu, H.; Ejaz, S.; Ilahy, R.; Qadri, R.; Khan, M.I.; Umer, M.A.; Khan, M.A.; et al. Exogenous melatonin treatment reduces postharvest senescence and maintains the quality of papaya fruit during cold storage. Front. Plant Sci. 2022, 13, 1039373. [Google Scholar] [CrossRef]
- Zhang, X.Y.; Zhou, Y.; Dhanasekaran, S.; Wang, J.Y.; Zhou, H.Y.; Gu, X.Y.; Li, B.; Zhao, L.N.; Zhang, H.Y. Insights into the defense mechanisms involved in the induction of resistance against black spot of cherry tomatoes by Pichia caribbica. LWT-Food Sci. Technol. 2022, 169, 113973. [Google Scholar] [CrossRef]
- Bajaj, K.; Adhikary, T.; Gill, P.P.S.; Kumar, A. Edible coatings enriched with plant-based extracts preserve postharvest quality of fruits: A review. Prog. Org. Coat. 2023, 182, 107669. [Google Scholar] [CrossRef]
- Chavan, P.; Lata, K.; Kaur, T.; Jambrak, A.R.; Sharma, S.; Roy, S.; Sinhmar, A.; Thory, R.; Singh, G.P.; Aayush, K.; et al. Recent advances in the preservation of postharvest fruits using edible films and coatings: A comprehensive review. Food Chem. 2023, 418, 135916. [Google Scholar] [CrossRef]
- Panahirad, S.; Dadpour, M.; Peighambardoust, S.H.; Soltanzadeh, M.; Gullón, B.; Alirezalu, K.; Lorenzo, J.M. Applications of carboxymethyl cellulose- and pectin-based active edible coatings in preservation of fruits and vegetables: A review. Trends Food Sci. Technol. 2021, 110, 663–673. [Google Scholar] [CrossRef]
- Khan, M.R.; Di Giuseppe, F.A.; Torrieri, E.; Sadiq, M.B. Recent advances in biopolymeric antioxidant films and coatings for preservation of nutritional quality of minimally processed fruits and vegetables. Food Packag. Shelf 2021, 30, 100752. [Google Scholar] [CrossRef]
- Basumatary, I.B.; Mukherjee, A.; Katiyar, V.; Kumar, S. Biopolymer-based nanocomposite films and coatings: Recent advances in shelf-life improvement of fruits and vegetables. Crit. Rev. Food Sci. 2022, 62, 1912–1935. [Google Scholar] [CrossRef]
- Ruiz-Herrera, J.; Ortiz-Castellanos, L. Cell wall glucans of fungi. A review. Cell Surf. 2019, 5, 100022. [Google Scholar] [CrossRef] [PubMed]
- Godana, E.A.; Yang, Q.; Zhao, L.; Zhang, X.; Liu, J.; Zhang, H. Pichia anomala induced with chitosan triggers defense response of table grapes against post-harvest blue mold disease. Front. Microbiol. 2021, 12, 704519. [Google Scholar] [CrossRef]
- Godana, E.A.; Yang, Q.Y.; Wang, K.L.; Zhang, H.Y.; Zhang, X.Y.; Zhao, L.N.; Abdelhai, M.H. Penicillium expansum in postharvest grapes and its possible inhibition mechanism. LWT-Food Sci. Technol. 2020, 124, 109188. [Google Scholar] [CrossRef]
- Prakash, S.; Rajeswari, K.; Divya, P.; Ferlin, M.; Rajeshwari, C.T.; Vanavil, B. Optimization and production of curdlan gum using Bacillus cereus PR3 isolated from rhizosphere of leguminous plant. Prep. Biochem. Biotech. 2018, 48, 408–418. [Google Scholar] [CrossRef]
- Cai, Z.; Zhang, H. Recent progress on curdlan provided by functionalization strategies. Food Hydrocolloid. 2016, 68, 128–135. [Google Scholar] [CrossRef]
- GB 28304-2012; Food Additive-Cordran. Food Safety National Standard. Chinese Ministry of Health: Beijing, China, 2012. Available online: https://sppt.cfsa.net.cn:8086/db (accessed on 25 June 2012).
- Álvarez, A.; Manjarres, J.J.; Ramírez, C.; Bolívar, G. Use of an exopolysaccharide-based edible coating and lactic acid bacteria with antifungal activity to preserve the postharvest quality of cherry tomato. LWT-Food Sci. Technol. 2021, 151, 112225. [Google Scholar] [CrossRef]
- Cao, J.K.; Jiang, W.B.; Zhao, Y.M. Experimental Guidance of Postharvest Physiology and Biochemistry of Fruits and Vegetables; China Light Industry Press: Beijing, China, 2020; Available online: http://www.chlip.com.cn/book/show.php/id-6417.html (accessed on 1 September 2017).
- Zhang, J.H.; Zeng, L.; Sun, H.L.; Zhang, J.H.; Chen, S.Y. Using chitosan combined treatment with citric acid as edible coatings to delay postharvest ripening process and maintain tomato (Solanum lycopersicon Mill) Quality. J. Food Nutr. Res. 2017, 56, 144–150. [Google Scholar]
- Mazumder, M.N.N.; Misran, A.; Ding, P.; Wahab, P.E.M.; Mohamad, A. effect of harvesting stages and calcium chloride application on postharvest quality of tomato fruits. Coatings 2021, 11, 1445. [Google Scholar] [CrossRef]
- Zeng, C.Z.; Tan, P.P.; Liu, Z.X. Effect of exogenous ARA treatment for improving postharvest quality in cherry tomato (Solanum lycopersicum L.) fruits. Sci. Hortic. 2020, 261, 108959. [Google Scholar] [CrossRef]
- Li, S.G.; Xu, Y.H.; Bi, Y.; Zhang, B.; Shen, S.L.; Jiang, T.J.; Zheng, X.L. Melatonin treatment inhibits gray mold and induces disease resistance in cherry tomato fruit during postharvest. Postharvest Biol. Technol. 2019, 157, 110962. [Google Scholar] [CrossRef]
- Chen, J.Y.; He, L.H.; Jiang, Y.M.; Wang, Y.; Joyce, D.C.; Ji, Z.L.; Lu, W.J. Role of phenylalanine ammonia-lyase in heat pretreatment-induced chilling tolerance in banana fruit. Physiol. Plant. 2008, 132, 318–328. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.Y.; Li, N. Effects of carbon monoxide on quality, nutrients and antioxidant activity of post-harvest jujube. J. Sci. Food Agric. 2014, 94, 1013–1019. [Google Scholar] [CrossRef]
- Jayaprakasha, G.K.; Singh, R.P.; Sakariah, K.K. Antioxidant activity of grape seed (Vitis vinifera) extracts on peroxidation models in vitro. Food Chem. 2001, 73, 285–290. [Google Scholar] [CrossRef]
- Jahani, R.; Behnamian, M.; Dezhsetan, S.; Karimirad, R.; Chamani, E. Chitosan nano-biopolymer/Citrus paradisi peel oil delivery system enhanced shelf-life and postharvest quality of cherry tomato. Int. J. Biol. Macromol. 2023, 225, 1212–1223. [Google Scholar] [CrossRef]
- Yuan, X.; Li, C.; Xie, J.; Li, K.; Chen, S.; Yuan, L.; Hu, C.; Wang, X.; Zhao, X. Combination of Selenium and Methyl Jasmonate Controls Postharvest Tomato Gray Mold by Damaging the Membrane System. Horticulturae 2022, 8, 782. [Google Scholar] [CrossRef]
- Li, W.H.; Liu, Z.Y.; Li, X.J.; Li, X.H. Quality maintenance of 1-Methylcyclopropene combined with titanium dioxide photocatalytic reaction on postharvest cherry tomatoes. J. Food Process. Pres. 2022, 46, e16500. [Google Scholar]
- Rahman, M.A.; Islam, M.A.; Sultan, M.T.; Kamal, A.H.M.; Khan, R.A.; Razzak, M.; Chowdhury, M.T.I.; Khan, M.S.I.; Mollah, M.Z.I. Assessment of chitosan as preservative on shelf life and major nutrient contents on fruits and vegetables. Am. J. Agric. Sci. 2019, 6, 1–10. [Google Scholar]
- Belay, Z.A.; Oluwafemi, J.C. Role of integrated omics in unravelling fruit stress and defence responses during postharvest: A review. Food Chem.-Mol. Sci. 2022, 5, 100118. [Google Scholar] [CrossRef] [PubMed]
- Khetabi, A.E.; Lahlali, R.; Ezrari, S.; Radouane, N.; Lyousfi, N.; Banani, H.; Askarne, L.; Tahiri, A.; Ghadraoui, L.E.; Belmalha, S.; et al. Role of plant extracts and essential oils in fighting against postharvest fruit pathogens and extending fruit shelf life: A review. Trends Food Sci. Tech. 2022, 120, 402–417. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, Y.; Yan, K.; Zhang, H.; Song, Y.; Chang, Y.; Liu, K.; Zhang, S.; Cui, M. Edible Composite Coating of Chitosan and Curdlan Maintains Fruit Quality of Postharvest Cherry Tomatoes. Horticulturae 2023, 9, 1033. https://doi.org/10.3390/horticulturae9091033
Yu Y, Yan K, Zhang H, Song Y, Chang Y, Liu K, Zhang S, Cui M. Edible Composite Coating of Chitosan and Curdlan Maintains Fruit Quality of Postharvest Cherry Tomatoes. Horticulturae. 2023; 9(9):1033. https://doi.org/10.3390/horticulturae9091033
Chicago/Turabian StyleYu, Youwei, Kejing Yan, Huanhuan Zhang, Yanyin Song, Yuan Chang, Kunyu Liu, Shaoying Zhang, and Meilin Cui. 2023. "Edible Composite Coating of Chitosan and Curdlan Maintains Fruit Quality of Postharvest Cherry Tomatoes" Horticulturae 9, no. 9: 1033. https://doi.org/10.3390/horticulturae9091033



