Magnetic Behavior of Carboxylate and β-Diketonate Lanthanide Complexes Containing Stable Organometallic Moieties in the Core-Forming Ligand
Abstract
:1. Introduction
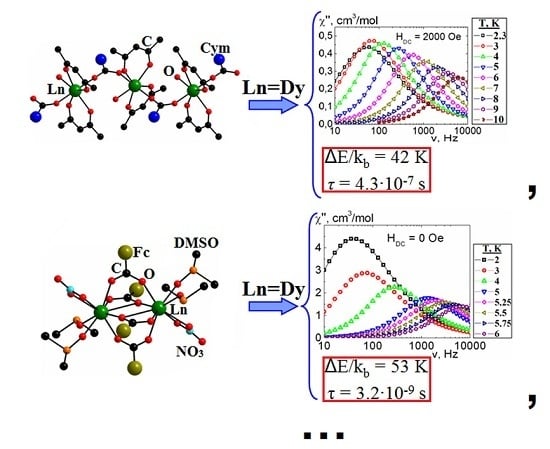
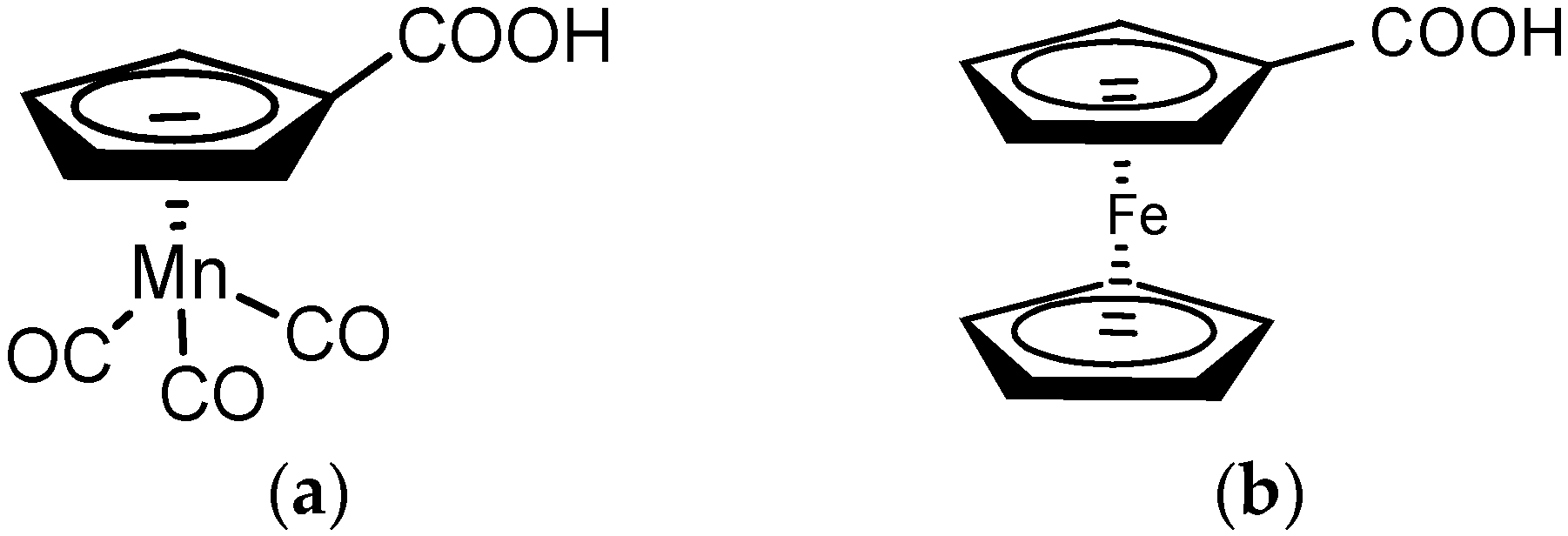
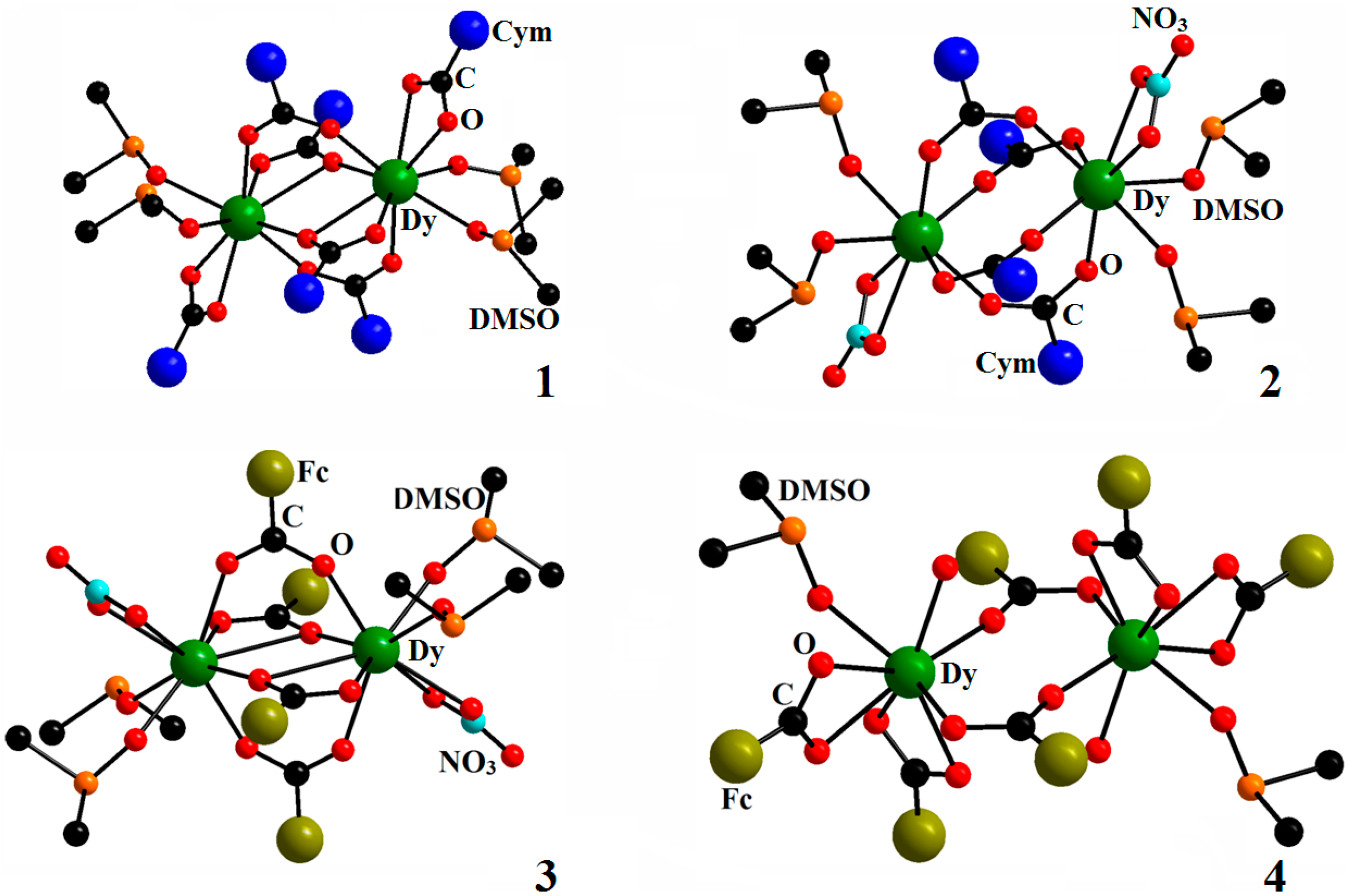
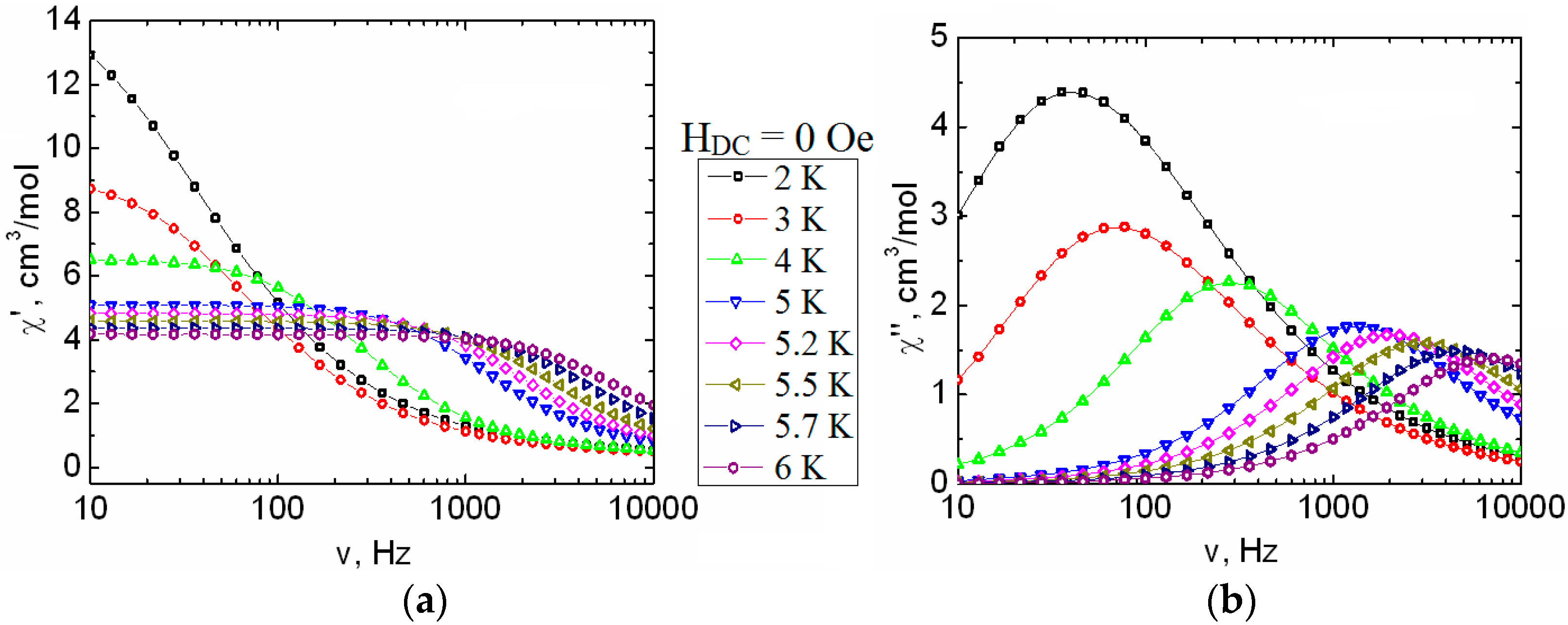
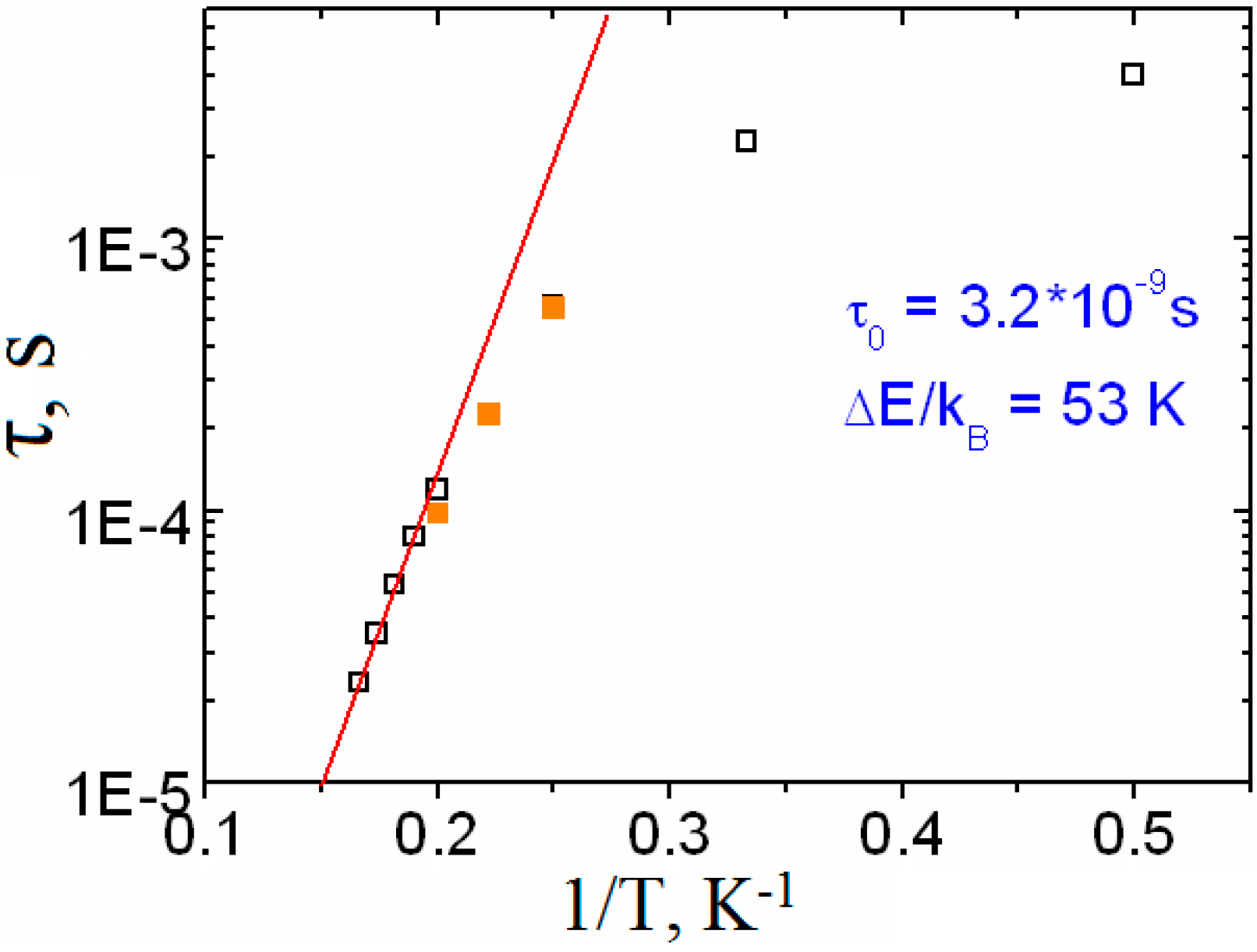
2. Results and Discussion
3. Experimental Section
3.1. Materials and Methods
3.2. Synthesis of Complexes 1–4
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Caneschi, A.; Gatteschi, R.; Sessoli, R.; Barra, A.L.; Brunel, L.C.; Guillot, M. Alternating current susceptibility, high field magnetization, and millimeter band EPR evidence for a ground S = 10 state in [Mn12O12(CH3COO)16(H2O)4]∙2CH3COOH∙4H2O. J. Am. Chem. Soc. 1991, 113, 5873–5874. [Google Scholar] [CrossRef]
- Sessoli, R.; Gatteschi, D.; Caneschi, A.; Novak, M.A. Magnetic bistability in a metal-ion cluster. Nature 1993, 365, 141–142. [Google Scholar] [CrossRef]
- Sessoli, R.; Powell, A.K. Strategies towards single molecule magnets based on lanthanide ions. Coord. Chem. Rev. 2009, 253, 2328–2341. [Google Scholar] [CrossRef]
- Bünzli, J.-C.G. Lanthanide coordination chemistry: From old concepts to coordination polymers. J. Coord. Chem. 2014, 67, 3706–3733. [Google Scholar] [CrossRef]
- Castro, G.; Regueiro-Figueroa, M.; Esteban-Gomez, D.; Perez-Lourido, P.; Platas-Iglesias, C.; Valencia, L. Magnetic anisotropies in rhombic lanthanide(III) complexes do not conform to bleaney’s theory. Inorg. Chem. 2016, 55, 3490–3497. [Google Scholar] [CrossRef] [PubMed]
- Gregson, M.; Chilton, N.F.; Ariciu, A.-M.; Tuna, F.; Crowe, I.F.; Lewis, W.; Blake, A.J.; Collison, D.; McInnes, E.J.L.; Winpenny, R.E.P.; et al. A Monometallic Lanthanide Bis(methanediide) Single molecule magnet with a large energy barrier and complex spin relaxation behaviour. Chem. Sci. 2016, 7, 155–165. [Google Scholar] [CrossRef]
- Pointillart, F.; Guizouarn, T.; Lefeuvre, S.; Golhen, S.; Cador, O.; Ouahab, L. Rational design of a lanthanide-based complex featuring Different single-molecule magnets. Chem. Eur. J. 2015, 21, 16929–16934. [Google Scholar] [CrossRef] [PubMed]
- Anastasiadis, N.C.; Kalofolias, D.A.; Philippidis, A.; Tzani, S.; Raptopoulou, C.P.; Psycharis, V.; Milios, C.J.; Escuer, A.; Perlepes, S.P. A family of dinuclear lanthanide(III) complexes from the use of a tridentate Schiff base: Structural and physical studies, and the case of a DyIII2 emissive single-molecule magnet. Dalton Trans. 2015, 44, 10200–10209. [Google Scholar] [CrossRef] [PubMed]
- Yatoo, M.A.; Cosquer, G.; Morimoto, M.; Irie, M.; Breedlove, B.K.; Yamashita, M. 1D chains of lanthanoid ions and a dithienylethene ligand showing slow relaxation of the magnetization. Magnetochemistry 2016, 2, 21–28. [Google Scholar] [CrossRef]
- Huang, C. Rare Earth Coordination Chemistry: Fundamentals and Applications; John Wiley & Sons: Singapore, 2010. [Google Scholar]
- Woodruff, D.N.; Winpenny, R.E.P.; Layfield, R.A. Lanthanide Single-Molecule Magnets. Chem. Rev. 2013, 113, 5110–5148. [Google Scholar] [CrossRef] [PubMed]
- Chilton, N.F.; Langley, S.K.; Moubaraki, B.; Soncini, A.; Batten, S.R.; Murray, K.S. Single molecule magnetism in a family of mononuclear β-diketonate lanthanide(III) complexes: Rationalization of magnetic anisotropy in complexes of low symmetry. Chem. Sci. 2013, 4, 1719–1730. [Google Scholar] [CrossRef]
- Mereacre, V.; Schlageter, M.; Powell, A.K. A temperature induced ferrocene-ferrocenium interconversion in a ferrocene functionalized μ3-O chromium carboxylate. J. Magn. Magn. Mater. 2015, 381, 478–480. [Google Scholar] [CrossRef]
- Mereacre, V.; Schlageter, M.; Eichhöfer, A.; Bauer, T.; Wolny, J.A.; Schünemann, V.; Powell, A.K. Unusual metal-ligand charge transfer in ferrocene functionalized μ3-O iron carboxylates observed with Mössbauer spectroscopy. J. Magn. Magn. Mater. 2016, 407, 87–91. [Google Scholar] [CrossRef]
- Koroteev, P.S.; Kiskin, M.A.; Dobrokhotova, Z.V.; Bogomyakov, A.M.; Efimov, N.N.; Novotortsev, V.M. Synthesis, structure, solid-state thermal decomposition and magnetic properties of binuclear Nd, Gd and Eu cymantrenecarboxylates. Polyhedron 2011, 30, 2523–2529. [Google Scholar] [CrossRef]
- Koroteev, P.S.; Dobrokhotova, Z.V.; Kiskin, M.A.; Lermontov, A.S.; Efimov, N.N.; Bogomyakov, A.S.; Tyurin, A.V.; Bykov, M.A.; Demina, L.I.; Velikodny, Y.A.; et al. Synthesis, structure, thermal behavior, thermodynamic, magnetic and luminescent properties of Pr, Sm, Eu, and Gd cymantrenecarboxylates. Polyhedron 2012, 43, 36–46. [Google Scholar] [CrossRef]
- Koroteev, P.S.; Dobrokhotova, Z.V.; Ilyukhin, A.B.; Motornova, M.S.; Novotortsev, V.M. Synthesis, structure, solid-state thermolysis, and catalytic properties of binuclear Ce, Nd, Eu, and Gd cymantrenecarboxylate complexes with DMSO. Russ. Chem. Bull. 2012, 61, 1069–1078. [Google Scholar] [CrossRef]
- Koroteev, P.S.; Efimov, N.N.; Dobrokhotova, Z.V.; Ilyukhin, A.B.; Gavrikov, A.V.; Novotortsev, V.M. Binuclear and polynuclear cymantrenecarboxylate complexes of heavy lanthanides. Russ. J. Coord. Chem. 2015, 41, 149–161. [Google Scholar] [CrossRef]
- Koroteev, P.S.; Dobrokhotova, Z.V.; Ilyukhin, A.B.; Efimov, N.N.; Kirdyankin, D.I.; Tyurin, A.V.; Velikodny, A.Y.; Kovba, M.L.; Novotortsev, V.M. Lanthanide cymantrenecarboxylate complexes with an Ln:Mn ratio of 1:2 as precursors for LnMn2O5 phases. Synthesis, structure, physicochemical properties, and thermal decomposition. Polyhedron 2013, 65, 110–121. [Google Scholar] [CrossRef]
- Koroteev, P.S.; Dobrokhotova, Z.V.; Ilyukhin, A.B.; Efimov, N.N.; Kirdyankin, D.I.; Tyurin, A.V.; Gavrikov, A.V.; Novotortsev, V.M. Polymeric lanthanide acetates with peripheral cymantrenecarboxylate groups—Synthesis, magnetism and thermolysis. Polyhedron 2015, 85, 941–952. [Google Scholar] [CrossRef]
- Koroteev, P.S.; Dobrokhotova, Z.V.; Ilyukhin, A.B.; Efimov, N.N.; Gavrikov, A.V.; Novotortsev, V.M. Polymer lanthanide cymantrenecarboxylates. Russ. J. Coord. Chem. 2015, 41, 805–816. [Google Scholar] [CrossRef]
- Gavrikov, A.V.; Koroteev, P.S.; Dobrokhotova, Z.V.; Ilyukhin, A.B.; Efimov, N.N.; Kirdyankin, D.I.; Bykov, M.A.; Ryumin, M.A.; Novotortsev, V.M. Novel heterometallic polymeric lanthanide acetylacetonates with bridging cymantrenecarboxylate groups—Synthesis, magnetism and thermolysis. Polyhedron 2015, 102, 48–59. [Google Scholar] [CrossRef]
- Koroteev, P.S.; Dobrokhotova, Z.V.; Efimov, N.N.; Ilyukhin, A.B.; Novotortsev, V.M. New binuclear ferrocenecarboxylates of rare-earth metals as precursors for ferrites: Syntheses, structures, and solid-phase thermolysis. Russ. J. Coord. Chem. 2014, 40, 495–504. [Google Scholar] [CrossRef]
- Koroteev, P.S.; Dobrokhotova, Z.V.; Ilyukhin, A.B.; Efimov, N.N.; Rouzieres, M.; Kiskin, M.; Clerac, R.; Novotortsev, V.M. Synthesis, structure, and physical properties of new rare earth ferrocenoylacetonates. Dalton Trans. 2016, 45, 6405–6417. [Google Scholar] [CrossRef] [PubMed]
- Koroteev, P.S.; Dobrokhotova, Z.V.; Ilyukhin, A.B.; Efimov, N.N.; Novotortsev, V.M. Synthesis, structure, and magnetic properties of lanthanide ferrocenoylacetonates with nitrate and 2,2′-bipyridine ligands. J. Coord. Chem. 2016, 69, 2723–2735. [Google Scholar] [CrossRef]
- Benelli, C.; Gatteschi, D. Magnetism of lanthanides in molecular materials with transition-metal ions and organic radicals. Chem. Rev. 2002, 102, 2369–2387. [Google Scholar] [CrossRef] [PubMed]
- Kahn, O. Molecular Magnetism; Wiley-Blackwell: New York, NY, USA, 1993; p. 380. [Google Scholar]
- Panagiotopoulos, A.; Zafiropoulos, T.F.; Perlepes, S.P.; Bakalbassis, E.; Masson-Ramade, I.; Kahn, O.; Terzis, A.; Raptopoulou, C.P. Molecular structure and magnetic mroperties of acetato-bridged lanthanide(III) dimers. Inorg. Chem. 1995, 34, 4918–4920. [Google Scholar] [CrossRef]
- Costes, J.P.; Clemente-Juan, J.M.; Dahan, F.; Nicodeme, F.; Verelst, M. Unprecedented Ferromagnetic Interaction in Homobinuclear Erbium and Gadolinium Complexes: Structural and Magnetic Studies. Angew. Chem. Int. Ed. 2002, 41, 323–325. [Google Scholar] [CrossRef]
- Koroteev, P.S.; Efimov, N.N.; Dobrokhotova, Z.V.; Fomina, I.G.; Ilyukhin, A.B.; Eremenko, I.L.; Novotortsev, V.M. Magnetostructural correlation for the Gd complexes with bridging oxygen. Russ. Chem. Bull. Int. Ed. 2013, 62, 1768–1771. [Google Scholar] [CrossRef]
- John, D.; Urland, W. Crystal Structure and Magnetic Behaviour of the New Gadolinium Carboxylates Gd2(ClF2CCOO)6(hypy)2, Gd2(F3CCOO)6(hypy)2, Gd2(F2HCCOO)6(hypy)2 and Gd2(Cl2HCCOO)6(H2O)2(hypy)2. Eur. J. Inorg. Chem. 2006, 3503–3509. [Google Scholar] [CrossRef]
- Roy, L.E.; Hughbanks, T. Magnetic Coupling in Dinuclear Gd Complexes. J. Am. Chem. Soc. 2006, 128, 568–575. [Google Scholar] [CrossRef] [PubMed]
- Cañadillas-Delgado, L.; Fabelo, O.; Cano, J.; Pasán, J.; Delgado, F.S.; Lloret, F.; Julve, M.; Ruiz, C. Dinuclear and two- and three-dimensional gadolinium(III) complexes with mono- and dicarboxylate ligands: Synthesis, structure and magnetic properties. CrystEngComm 2009, 11, 2131–2142. [Google Scholar] [CrossRef]
- Ito, M.; Hamada, D.; Ono, H.; Matsumoto, N.; Sunatsuki, Y.; Re, N. Binuclear tetra-acetate bridged Gd(III) complex [Gd2(μ2-O2CMe)4(H2L)2](ClO4)2·2H2O·2MeOH (H2L = bis(5-methylimidazol-4-yl-methyliden eamino propyl)methylamine): Synthesis, structure, and magnetic properties. Inorg. Chim. Acta 2016, 443, 274–278. [Google Scholar] [CrossRef]
- Tumanski, S. Handbook of Magnetic Measurements; CRC Press, Taylor & Francis Group: Boca Raton, FL, USA, 2011; p. 62. [Google Scholar]
- Bag, P.; Rastogi, C.K.; Biswas, S.; Sivakumar, S.; Mereacre, V.; Chandrasekhar, V. Homodinuclear lanthanide {Ln2} (Ln = Gd, Tb, Dy, Eu) complexes prepared from an o-vanillin based ligand: Luminescence and single-molecule magnetism behavior. Dalton Trans. 2015, 44, 4328–4340. [Google Scholar] [CrossRef] [PubMed]
- Long, J.; Habib, F.; Lin, P.; Korobkov, I.; Enright, G.; Ungur, L.; Wernsdorfer, W.; Chibotaru, L.F.; Murugesu, M. Single-molecule magnet behavior for an antiferromagnetically superexchange-coupled dinuclear dysprosium(III) complex. J. Am. Chem. Soc. 2011, 133, 5319–5328. [Google Scholar] [CrossRef] [PubMed]
- Sakamoto, S.; Fujinami, T.; Nishi, K.; Matsumoto, N.; Mochida, N.; Ishida, T.; Sunatsuki, Y.; Re, N. Carbonato-Bridged NiII2LnIII2 (LnIII = GdIII, TbIII, DyIII) Complexes Generated by Atmospheric CO2 Fixation and Their Single-Molecule-Magnet Behavior: [(μ4-CO3)2{NiII(3-MeOsaltn)(MeOH or H2O)LnIII(NO3)}2]·solvent [3-MeOsaltn = N,N′-Bis(3-methoxy-2-oxybenzylidene)-1,3-propanediaminato]. Inorg. Chem. 2013, 52, 7218–7229. [Google Scholar] [PubMed]
- Ehama, K.; Ohmichi, Y.; Sakamoto, S.; Fujinami, T.; Matsumoto, N.; Mochida, N.; Ishida, T.; Sunatsuki, Y.; Tsuchimoto, M.; Re, N. Synthesis, Structure, Luminescent, and Magnetic Properties of Carbonato-Bridged ZnII2LnIII2 Complexes [(μ4-CO3)2{ZnIILnLnIII(NO3)}2] (LnIII = GdIII, TbIII, DyIII; L1 = N,N′-Bis(3-methoxy-2-oxybenzylidene)-1,3-propanediaminato, L2 = N,N′-Bis(3-ethoxy-2-oxybenzylidene)-1,3-propanediaminato). Inorg. Chem. 2013, 52, 12828–12841. [Google Scholar] [PubMed]
- Koroteev, P.S.; Ilyukhin, A.B.; Efimov, N.N.; Minin, V.V.; Tyurin, A.V.; Dobrokhotova, Z.V.; Novotortsev, V.M. Charge transfer adducts of binuclear rare earth 3,5-dinitrobenzoates with N,N-dimethylaniline and toluene. Polyhedron 2015, 89, 238–249. [Google Scholar] [CrossRef]
- Katoh, K.; Horii, Y.; Yasuda, N.; Wernsdorfer, W.; Toriumi, K.; Breedlove, B.K.; Yamashita, M. Multiple-decker phthalocyaninato dinuclear lanthanoid(III) single-molecule magnets with dual-magnetic relaxation processes. Dalton Trans. 2012, 41, 13582–13600. [Google Scholar] [CrossRef] [PubMed]
- Katoh, K.; Asano, R.; Miura, A.; Horii, Y.; Morita, T.; Breedlove, B.K.; Yamashita, M. Effect of f–f interactions on quantum tunnelling of the magnetization: Mono- and dinuclear Dy(III) phthalocyaninato triple-decker single-molecule magnets with the same octacoordination environment. Dalton Trans. 2014, 43, 7716–7725. [Google Scholar] [CrossRef] [PubMed]
- Ungur, L.; Chibotaru, L.F. Strategies toward High-Temperature Lanthanide-Based Single-Molecule Magnets. Inorg. Chem. 2016. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Xu, G.; Gamez, P.; Zhao, L.; Lin, S.; Deng, R.; Tang, J.; Zhang, H. Two-step relaxation in a linear tetranuclear dysprosium(III) aggregate showing single-molecule magnet behavior. J. Am. Chem. Soc. 2010, 132, 8538–8539. [Google Scholar] [CrossRef] [PubMed]
- McKinney, J.D.; Anderson, P.A.; Hamor, T.A.; Jones, C.J.; Paxton, K.; Porch, A. The synthesis, solid state conductivity and X-ray crystal structure of [Fe{η5-C5H5)(E-η5-C5H4–CH=CH-9-C16H9)][1,4-{(CN)2C=}2C6H4]. J. Organomet. Chem. 1998, 558, 147–153. [Google Scholar] [CrossRef]
- Perevalova, É.G.; Grandberg, K.I.; Zharikova, N.A.; Gubin, S.P.; Nesmeyanov, A.N. Electronic influence of ferrocenyl as a substituent. Russ. Chem. Bull. 1966, 15, 796–802. [Google Scholar] [CrossRef]
- Nesmeyanov, A.N.; Anisimov, K.N.; Kolobova, N.E.; Makarov, Y.V. Metalation of cyclopentadienyltricarbonylmanganese. Russ. Chem. Bull. 1968, 17, 672. [Google Scholar] [CrossRef]
- Bruker. APEX2 and SAINT; Bruker AXS Inc.: Madison, WI, USA, 2007. [Google Scholar]
- Sheldrick, G.M. SADABS; University of Göttingen: Göttingen, Germany, 1997. [Google Scholar]
- Sheldrick, G. Crystal structure refinement with SHELXL. Acta Crystallogr. Sect. C 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
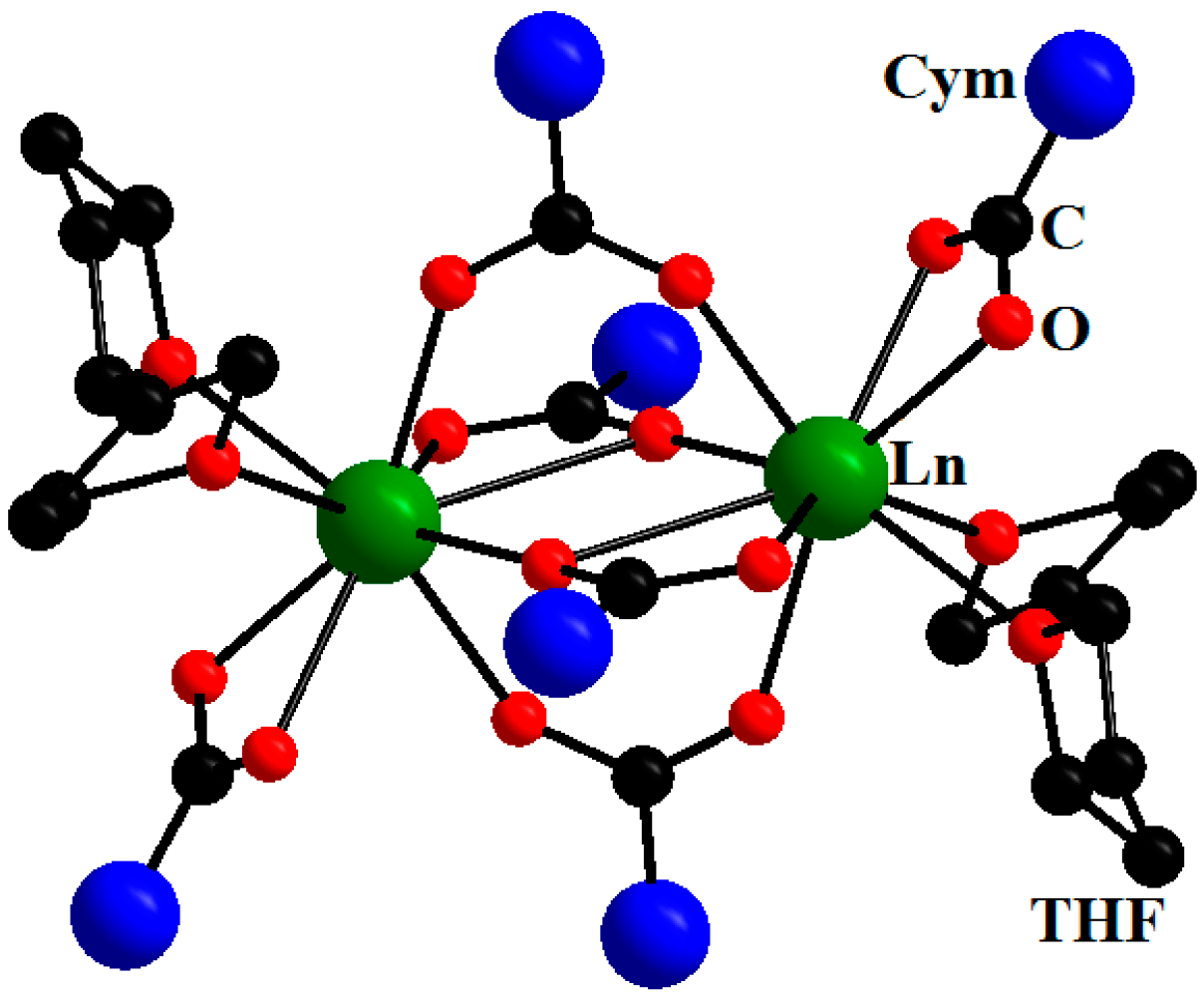
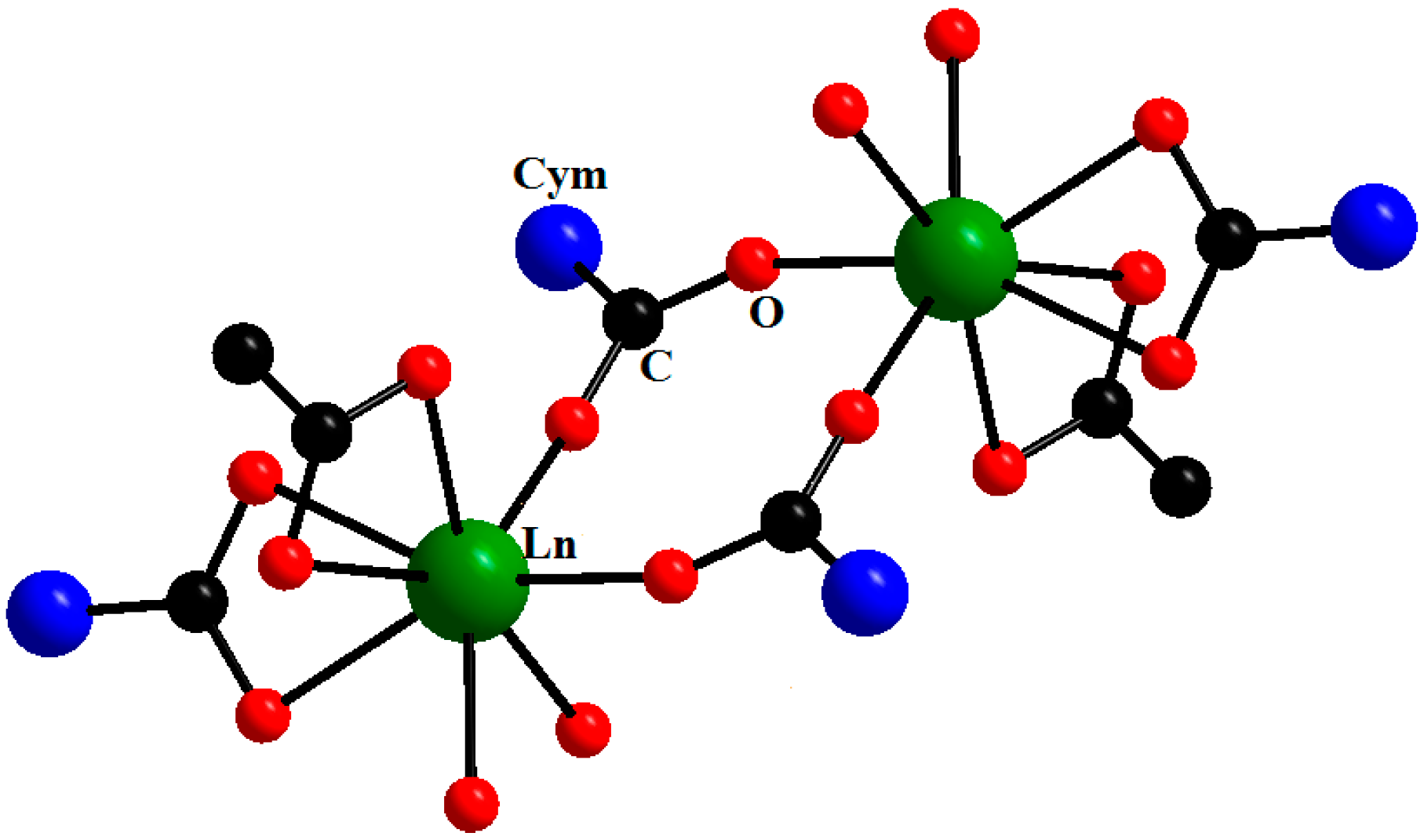
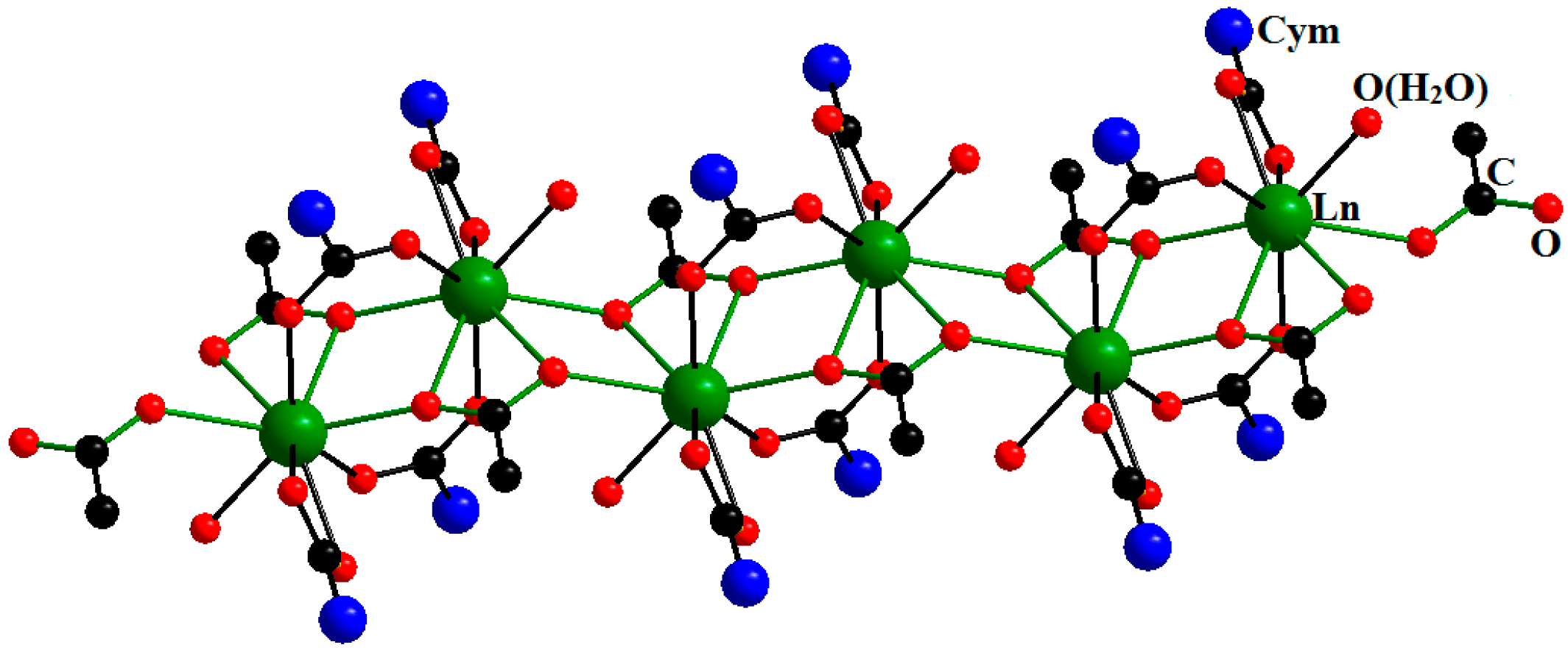
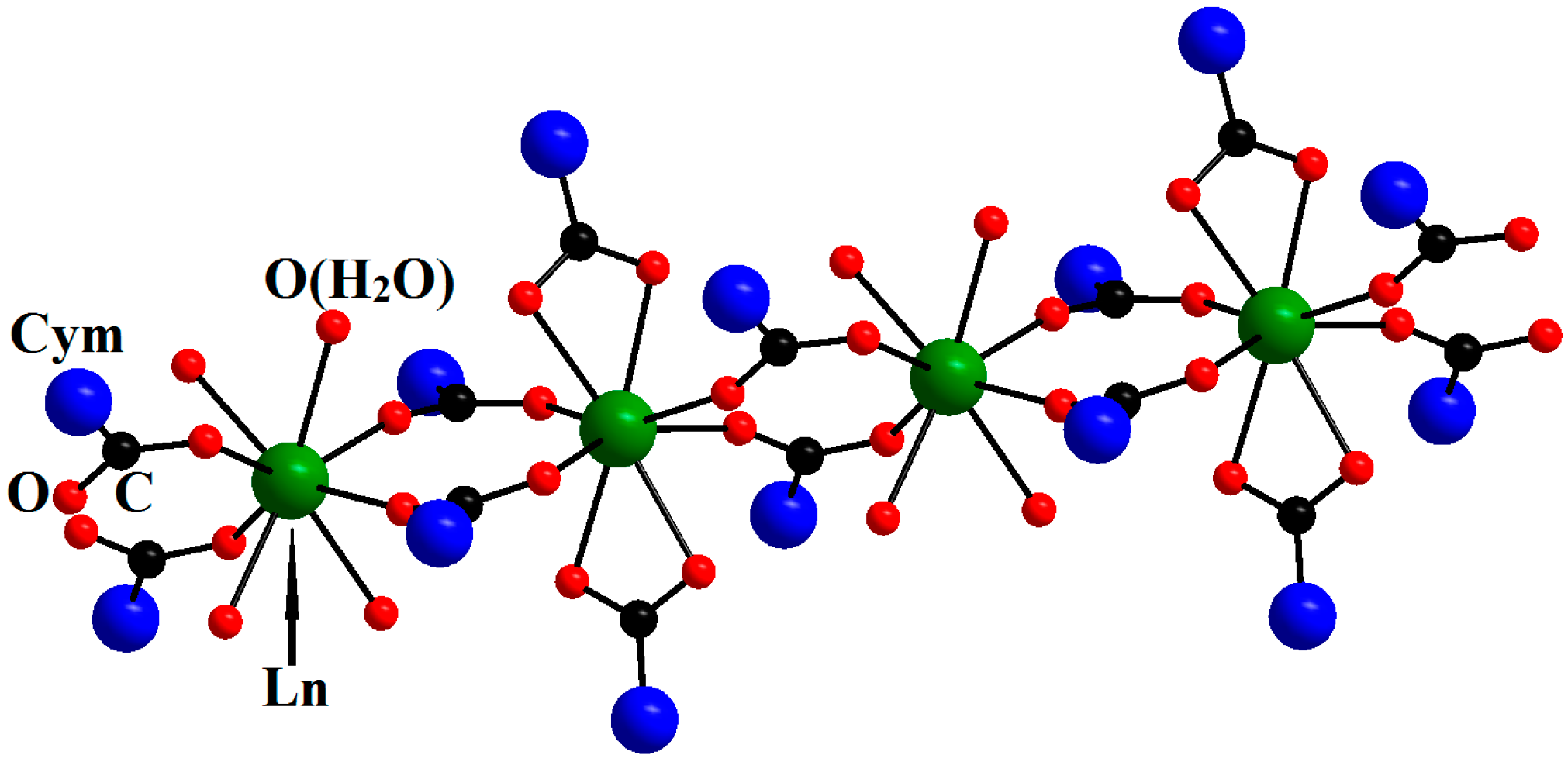
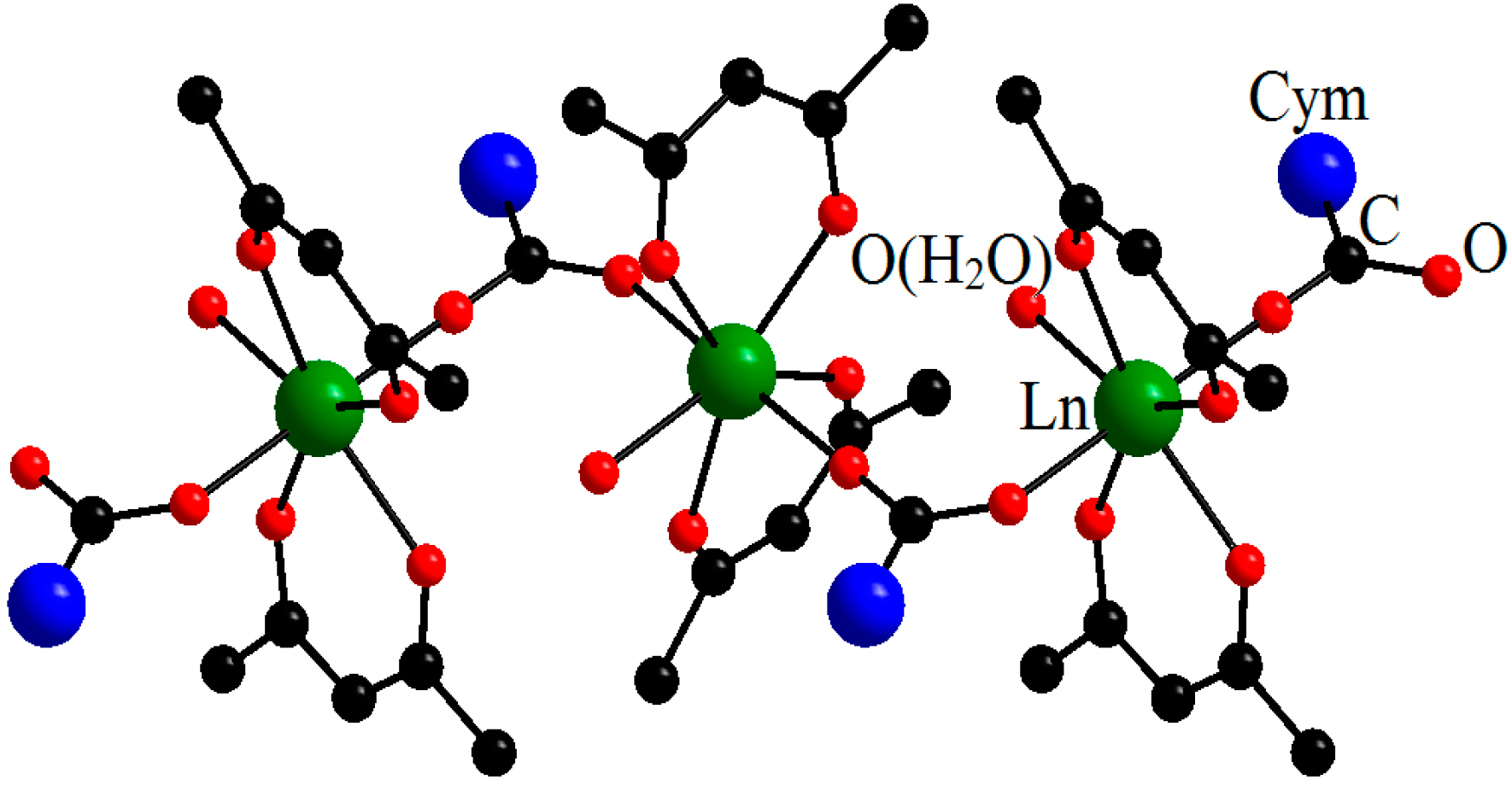

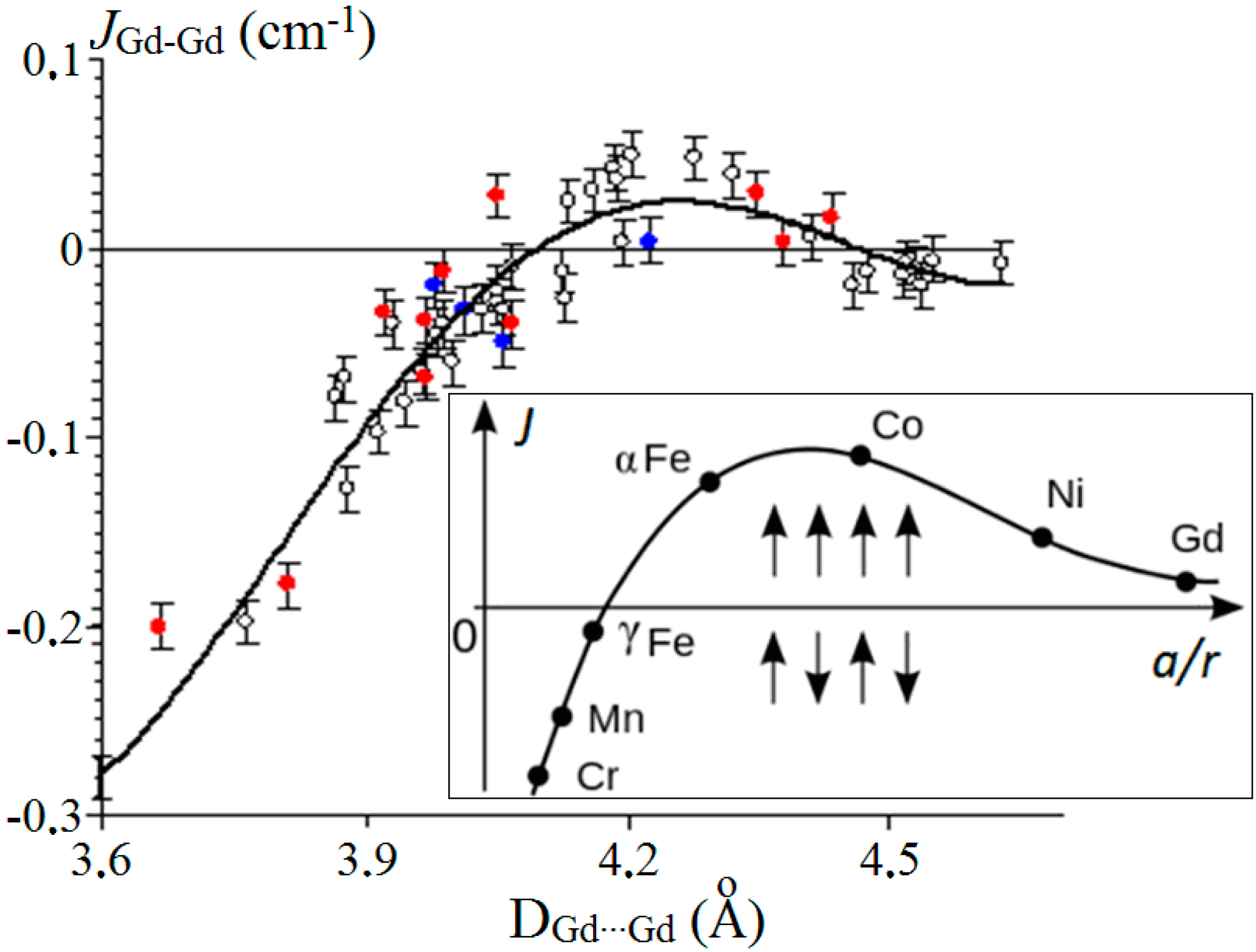

| Compound | DGd…Gd′, Å | JGd-Gd′, cm−1 | Reference |
|---|---|---|---|
| [Gd2(H2L)2(piv)4]·2CHCl3 | 3.665 | −0.19 | [36] |
| [Gd2(valdien)2(NO3)2] | 3.811 | −0.178 | [37] |
| [Gd2(O2CMe)4(H2L1)2](ClO4)2·2H2O·2MeOH | 3.920 | −0.033 | [34] |
| [Gd(O2CCym)2(OAc)(H2O)2]n∙2nH2O | 3.968 | −0.038 | [20] |
| [Gd2(O2CCym)4(NO3)2(DME)2] | 3.970 | −0,068 | [19] |
| [Gd2(Piv)6(phen)2] * | 3.978 | −0.020 | [30] |
| [(CO3)2{Ni(3-MeOsaltn)(MeOH)Gd(NO3)}2] | 3.989 | −0.012 | [38] |
| [Gd2(Piv)6(bipy)2] * | 4.014 | −0.033 | [30] |
| [(CO3)2{ZnL2Gd(NO3)}2] | 4.050 | 0.028 | [39] |
| [Gd2(O2CCym)6(DMSO)4] * | 4.058 | −0.050 | [30] |
| [Gd2(O2CFc)4(NO3)2(DMSO)4] | 4.067 | −0.040 | [23] |
| [Gd2(Piv)6(bath)2] * | 4.224 | 0.004 | [30] |
| [Gd2(O2CFc)6(DMSO)2(H2O)2]·2DMSO·2CH2Cl2 | 4.348 | 0.029 | [23] |
| [Gd2(O2CC6H3(NO2)2)6(DMSO)4]·3MePh | 4.380 | 0.003 | [40] |
| [Gd2(O2CC6H3(NO2)2)6(DMSO)4]·3(Me2N)C6H5 | 4.435 | 0.017 | [40] |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Efimov, N.N.; Koroteev, P.S.; Gavrikov, A.V.; Ilyukhin, A.B.; Dobrokhotova, Z.V.; Novotortsev, V.M. Magnetic Behavior of Carboxylate and β-Diketonate Lanthanide Complexes Containing Stable Organometallic Moieties in the Core-Forming Ligand. Magnetochemistry 2016, 2, 38. https://doi.org/10.3390/magnetochemistry2040038
Efimov NN, Koroteev PS, Gavrikov AV, Ilyukhin AB, Dobrokhotova ZV, Novotortsev VM. Magnetic Behavior of Carboxylate and β-Diketonate Lanthanide Complexes Containing Stable Organometallic Moieties in the Core-Forming Ligand. Magnetochemistry. 2016; 2(4):38. https://doi.org/10.3390/magnetochemistry2040038
Chicago/Turabian StyleEfimov, Nikolay N., Pavel S. Koroteev, Andrey V. Gavrikov, Andrey B. Ilyukhin, Zhanna V. Dobrokhotova, and Vladimir M. Novotortsev. 2016. "Magnetic Behavior of Carboxylate and β-Diketonate Lanthanide Complexes Containing Stable Organometallic Moieties in the Core-Forming Ligand" Magnetochemistry 2, no. 4: 38. https://doi.org/10.3390/magnetochemistry2040038