Hybrid Molecular Compound Exhibiting Slow Magnetic Relaxation and Electrical Conductivity
Abstract
:1. Introduction
2. Results
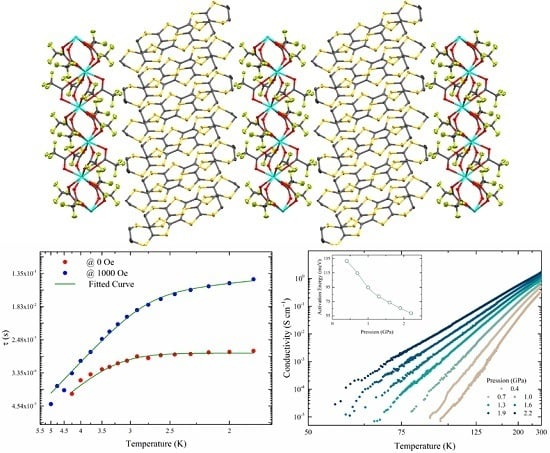
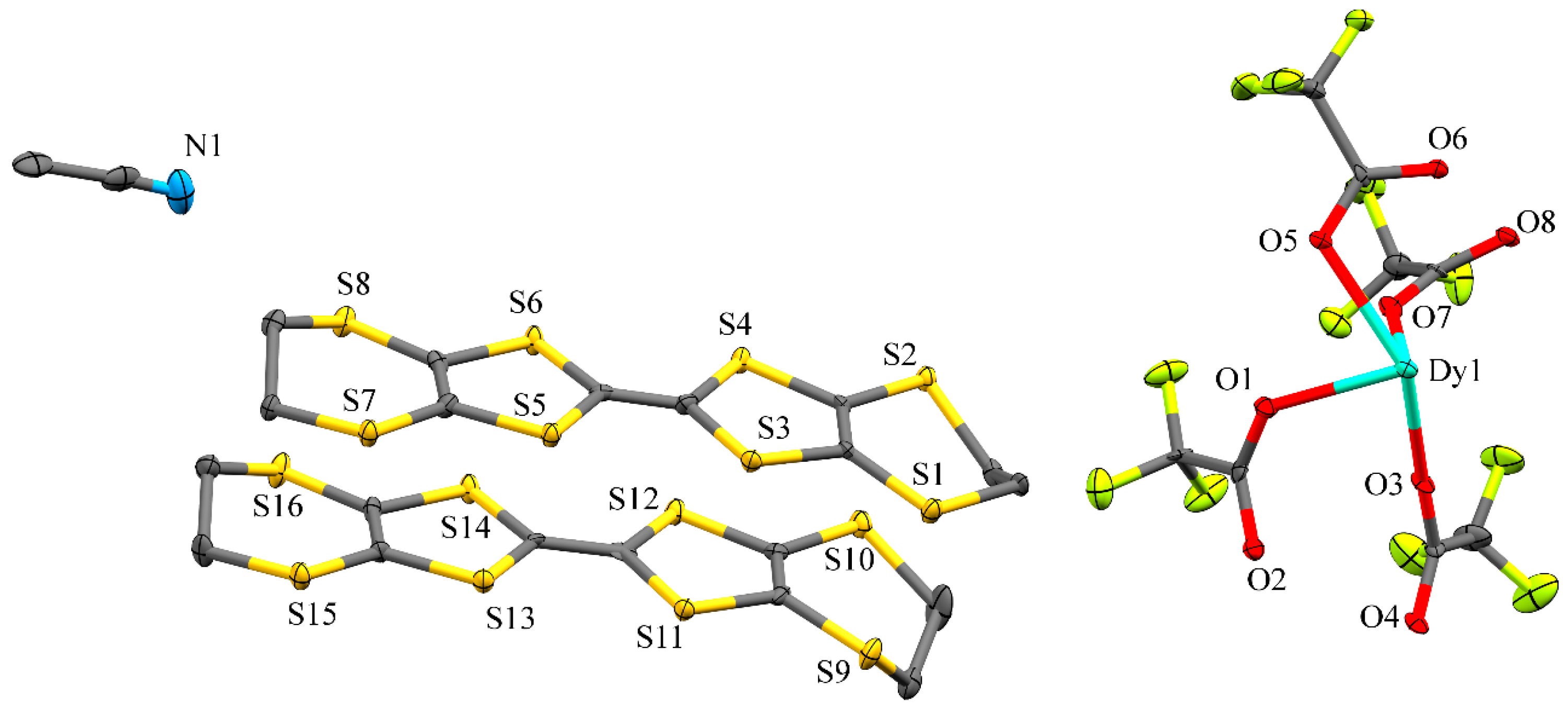
2.1. Crystal Structure
2.2. Optical Properties
2.3. Electrical Conductivity
2.4. Magnetic Properties
3. Materials and Methods
3.1. Synthesis
3.2. Physical Measurements
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Ouahab, L. Organic/inorganic supramolecular assemblies and synergy between physical properties. Chem. Mater. 1997, 9, 1909–1926. [Google Scholar] [CrossRef]
- Enoki, T.; Miyazaki, A. Magnetic TTF-based charge-transfer complexes. Chem. Rev. 2004, 104, 5449–5478. [Google Scholar] [CrossRef] [PubMed]
- Williams, J.M.; Ferraro, J.R.; Thorn, R.J.; Carlson, K.D.; Geiger, U.; Wang, H.H.; Kini, A.M.; Whangbo, M.H. Organic Superconductors: Synthesis, Structure, Properties and Theory; Grimes, R.N., Ed.; Prentice Hall: Englewood Cliffs, NJ, USA, 1992. [Google Scholar]
- Martin, L.; Turner, S.S.; Day, P.; Mabbs, F.E.; McInnes, E.J.L. New molecular superconductor containing paramagnetic chromium (iii) ions. Chem. Commun. 1997, 15, 1367–1368. [Google Scholar] [CrossRef]
- Fujiwara, H.; Fujiwara, E.; Nakazawa, Y.; Narymbetov, B.Z.; Kato, K.; Kobayashi, H.; Kobayashi, A.; Tokumoto, M.; Cassoux, P. A novel antiferromagnetic organic superconductor k-(BETS)2FeBr4 [Where BETS = Bis(ethylenedithio) tetraselenafulvalene]. J. Am. Chem. Soc. 2001, 123, 306–314. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, K.; Kitagawa, Y.; Onishi, T.; Isobe, H.; Kawakami, T.; Nagao, H.; Takamizawa, S. Spin-mediated superconductivity in cuprates, organic conductors and π–d conjugated systems. Coord. Chem. Rev. 2002, 226, 235–249. [Google Scholar] [CrossRef]
- Day, P.; Kurmoo, M.; Mallah, T.; Marsden, I.R.; Friend, R.H.; Pratt, F.L.; Hayes, W.; Chasseau, D.; Gaultier, J.; Bravic, G.; et al. Structure and properties of tris[bis(ethylenedithio) tetrathiaful-valenium]tetrachlorocopper hydrate (BEDT-TTF)3CuCl4·H2O: First evidence for coexistence of localized and conduction electrons in a metallic charge-transfer salt. J. Am. Chem. Soc. 1992, 114, 10722–10729. [Google Scholar] [CrossRef]
- Ojima, E.; Fujiwara, H.; Kato, K.; Kobayashi, H.; Tanaka, H.; Kobayashi, A.; Tokumoto, M.; Cassoux, P. Antiferromagnetic Organic Metal Exhibiting Superconducting Transition, K-(BETS)2FeBr4 [BETS Bis(ethylenedithio) tetraselenafulvalene]. J. Am. Chem. Soc. 1999, 121, 5581–5582. [Google Scholar] [CrossRef]
- Bogani, L.; Wernsdorfer, W. Molecular spintronics using single-molecule magnets. Nat. Mater. 2008, 7, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Kurmoo, M.; Graham, A.W.; Day, P.; Coles, S.J.; Hursthouse, M.B.; Caulfield, J.L.; Singleton, J.; Pratt, F.L.; Hayes, W.; Ducasse, L.; et al. Superconducting and Semiconducting Magnetic Charge Transfer Salt: (BEDT-TTF)4AFe(C2O4)3C6H5CN (A = H2O, K, NH4). J. Am. Chem. Soc. 1995, 117, 12209–12227. [Google Scholar] [CrossRef]
- Hiraga, H.; Miyasaka, H.; Nakata, K.; Kajiwara, K.; Takaishi, S.; Oshima, Y.; Nojiri, H.; Yamashita, M. Hybrid molecular materials exhibiting single-molecule magnet behaviour and molecular conductivity. Inorg. Chem. 2007, 46, 9661–9671. [Google Scholar] [CrossRef] [PubMed]
- Hiraga, H.; Miyasaka, H.; Takaishi, S.; Kajiwara, T.; Yamashita, M. Hybridized complexes of ½ MnIII 2 single-molecule magnets and Ni dithiolate complexes. Inorg. Chim. Acta 2008, 361, 3863–3872. [Google Scholar] [CrossRef]
- Kubo, K.; Shiga, T.; Yamamoto, T.; Tajima, A.; Moriwaki, T.; Ikemoto, Y.; Yamashita, M.; Sessini, E.; Mercuri, M.-L.; Deplano, P.; et al. Electronic state of a conducting single molecule magnet based on Mn-salen type and Ni-Dithiolene complexes. Inorg. Chem. 2011, 50, 9337–9344. [Google Scholar] [CrossRef] [PubMed]
- Ueki, S.; Nogami, T.; Ishida, T.; Tamura, M. ET and TTF salts with lanthanide complex ions showing frequency-dependent ac magnetic susceptibility. Mol. Cryst. Liq. Cryst. 2006, 455, 129–134. [Google Scholar] [CrossRef]
- Johnson, D.A.; Waugh, A.B.; Hambley, T.W.; Taylor, J.C. Synthesis and Crystal Structure of 1,1,1,5,5,5-Hexafluoro-2-aminopentan-4-one (HFAP). J. Fluor. Chem. 1985, 27, 371–378. [Google Scholar] [CrossRef]
- Ruiz-Martínez, A.; Casanova, D.; Alvarez, S. Polyhedral structures with an odd number of vertices: Nine-coordinate metal compounds. Chem. Eur. J. 2008, 14, 1291–1303. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, S.; Alemany, P.; Casanova, D.; Cirera, J.; Llunell, M.; Avnir, D. Shape maps and polyhedral interconversion paths in transition metal chemistry. Coord. Chem. Rev. 2005, 249, 1693–1708. [Google Scholar]
- Mori, T. Structural genealogy of BEDT-TTF-based organic conductors I. parallel molecules: β and β Phases. Bull. Chem. Soc. Jpn. 1998, 71, 2509–2526. [Google Scholar] [CrossRef]
- Mori, T.; Mori, H.; Tanaka, S. Structural genealogy of BEDT-TTF-based organic conductors II. inclined molecules: θ, α, and κ Phases. Bull. Chem. Soc. Jpn. 1999, 72, 179–197. [Google Scholar] [CrossRef]
- Shibaeva, R.P.; Yagubskii, E.B. Molecular conductors and superconductors based on Trihalides of BEDT-TTF and some of its analogues. Chem. Rev. 2004, 104, 5347–5378. [Google Scholar] [CrossRef] [PubMed]
- Guionneau, P.; Kepert, C.J.; Bravic, G.; Chasseau, D.; Truter, M.R.; Kurmoo, M.; Day, P. Determining the charge distribution in BEDT-TTF salts. Synth. Metal. 1997, 86, 1973–1974. [Google Scholar] [CrossRef]
- Rosokha, S.V.; Kochi, J.K. Molecule and electronic structure of the long-bonded π-dimers of tetrathiafulvalene cation-radical in intermolecular electron transfer and in (solid-state) conductivity. J. Am. Chem. Soc. 2007, 129, 828–838. [Google Scholar] [CrossRef] [PubMed]
- Cosquer, G.; Pointillart, F.; Le Guennic, B.; Le Gal, Y.; Golhen, S.; Cador, O.; Ouahab, L. 3d4f heterobimetallic dinuclear and tetranuclear complexes. Inorg. Chem. 2012, 51, 8488–8501. [Google Scholar] [CrossRef] [PubMed]
- Pokhodnya, K.I.; Cassoux, P.; Feltre, L.; Meneghtti, M. Optical excitations in a quarter-filled Ni(dmit)2 based compound described by a dimerized octamer model. Synth. Met. 1999, 103, 2187. [Google Scholar] [CrossRef]
- Romaniello, P.; Lelj, F.; Arca, M.; Devillanova, F.A. Structural and new spectroscopic properties of neutral [M(dmit)2](dmit = C3S52−, 1,3-dithiole-2-thione-4,5-dithiolate) and [M(H2timdt)2](H2timdt = H2C3N2S31−, monoanion of imidazolidine-2,4,5-trithione) complexes within the density functional approach. Theor. Chem. Acc. 2007, 117, 621–635. [Google Scholar] [CrossRef]
- Visentini, G.; Masino, M. Experimental determination of BEDT-TTF electron-molecular vibration constants through optical microreflectance. Phys. Rev. B 1998, 58, 9460–9467. [Google Scholar] [CrossRef]
- Tajima, H.; Yakushi, K.; Kuroda, H. Polarized reflectance spectrum of β-(BEDT-TTF)2I3 single crystal. Solid State Commun. 1985, 56, 159–163. [Google Scholar] [CrossRef]
- Yamamoto, T.; Uruichi, M.; Yamamoto, K.; Yakushi, K.; Kawamoto, A.; Taniguchi, H. Examination of the Charge-Sensitive Vibrational Modes in Bis(ethylenedithio)tetrathiafulvalene. J. Phys. Chem. B 2005, 109, 15226–15235. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, T.; Nakazawa, Y.; Tamura, M.; Fukunaga, T.; Kato, R.; Yakushi, K. Vibrational Spectra of [Pd(dmit)2] Dimer (dmit = 1,3-dithiole-2-thione-4,5-dithiolate): Methodology for examining charge, inter-molecular interactions, and orbital. J. Phys. Soc. Jpn. 2011, 80. [Google Scholar] [CrossRef]
- Jacobsen, C.S.; Tanner, D.B. Electronic structure of some p-(C10H8S8)2X compounds as studied by infrared spectroscopy. Phys. Rev. B. 1987, 35, 9605–9612. [Google Scholar] [CrossRef] [Green Version]
- Świetlik, R.; Połomska, M.; Ouahab, L.; Guillevic, J. Infrared and Raman studies of the k-phase charge-transfer salts formed by BEDT-TTF and magnetic anions M(CN)63− (where M = CoIII, FeIII, CrIII). J. Mater. Chem. 2001, 11, 1313–1318. [Google Scholar] [CrossRef]
- Sugimoto, T.; Fujiwara, H.; Noguchi, S.; Murata, K. New aspects of π–d interactions in magnetic molecular conductors. Sci. Technol. Adv. Mater. 2009, 10, 024302. [Google Scholar] [CrossRef] [PubMed]
- Alemany, P.; Pouget, J.-P.; Canadell, E. Structural and electronic control of the metal to insulator transition and local orderings in the θ-(BEDT-TTF)2X organic conductors. J. Phys. Condens. Matter 2015, 27, 465702. [Google Scholar] [CrossRef] [PubMed]
- Kahn, O. Molecular Magnetism; VCH Publishers: Weinheim, Germany, 1993. [Google Scholar]
- Kinoshita, M.; Novoa, J.J.; Inoue, K.; Rawson, J.M.; Arčon, D. π-Electron Magnetism. From Molecules to Magnetic Materials; Veciana, J., Ed.; Springer: Berlin/Heidelberg, Germany, 2001. [Google Scholar]
- Tian, H.; Wei, S.; Zheng, N.; Bo, N.; Peng, C. Magnetic blocking from exchange interactions: Slow relaxation of the magnetization and hysteresis loop observed in a dysprosium–nitronyl nitroxide chain compound with an antiferromagnetic ground state. Chem. Eur. J. 2013, 19, 994–1001. [Google Scholar]
- Yatoo, M.A.; Cosquer, G.; Morimoto, M.; Irie, M.; Breedlove, B.K.; Yamashita, M. 1D chains of lanthanoid ions and a dithienylethene ligand showing slow relaxation of the magnetization. Magnetochemistry 2016, 2, 21. [Google Scholar] [CrossRef]
- Zeng, D.; Ren, M.; Bao, S.-S.; Zheng, L.M. Tuning the coordination geometries and magnetic dynamics of [Ln(hfac)4]− through alkali metal counterions. Inorg. Chem. 2014, 53, 795–801. [Google Scholar] [CrossRef] [PubMed]
- Crystal Clear-SM, 1.4.0 SP1; Rigaku Corporation: Tokyo, Japan, 17 April 2008.
- Altomare, A.; Burla, M.C.; Camalli, M.; Cascarano, G.L.; Giacovazzo, C.; Guagliardi, A.; Moliterni, A.G.G.; Polidori, G.; Spagna, R. SIR97: A new tool for crystal structure determination and refinement. J. Appl. Crystallogr. 1999, 32, 115–119. [Google Scholar] [CrossRef]
- Farrugia, L.J. WinGX and ORTEP for Windows: An update. J. Appl. Crystallogr. 2012, 45, 849–854. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Cryst. C 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]







| Field | 0 Oe | 1000 Oe (Low f ) |
|---|---|---|
| Calculation Equation | ||
| A | - | 3.3 × 10−12 |
| τ0 (s) | 8.8 × 10−8 | 5.0 × 10−8 |
| Δ (cm−1) | 21.3 | 22.1 |
| QTM (s) | 1.1 × 10−3 | - |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shen, Y.; Cosquer, G.; Breedlove, B.K.; Yamashita, M. Hybrid Molecular Compound Exhibiting Slow Magnetic Relaxation and Electrical Conductivity. Magnetochemistry 2016, 2, 44. https://doi.org/10.3390/magnetochemistry2040044
Shen Y, Cosquer G, Breedlove BK, Yamashita M. Hybrid Molecular Compound Exhibiting Slow Magnetic Relaxation and Electrical Conductivity. Magnetochemistry. 2016; 2(4):44. https://doi.org/10.3390/magnetochemistry2040044
Chicago/Turabian StyleShen, Yongbing, Goulven Cosquer, Brian K. Breedlove, and Masahiro Yamashita. 2016. "Hybrid Molecular Compound Exhibiting Slow Magnetic Relaxation and Electrical Conductivity" Magnetochemistry 2, no. 4: 44. https://doi.org/10.3390/magnetochemistry2040044