Neutral Low-Dimensional Assemblies of a Mn(III) Schiff Base Complex and Octacyanotungstate(V): Synthesis, Characterization, and Magnetic Properties
Abstract
:1. Introduction
2. Results and Discussion
2.1. Preparation and Characterization
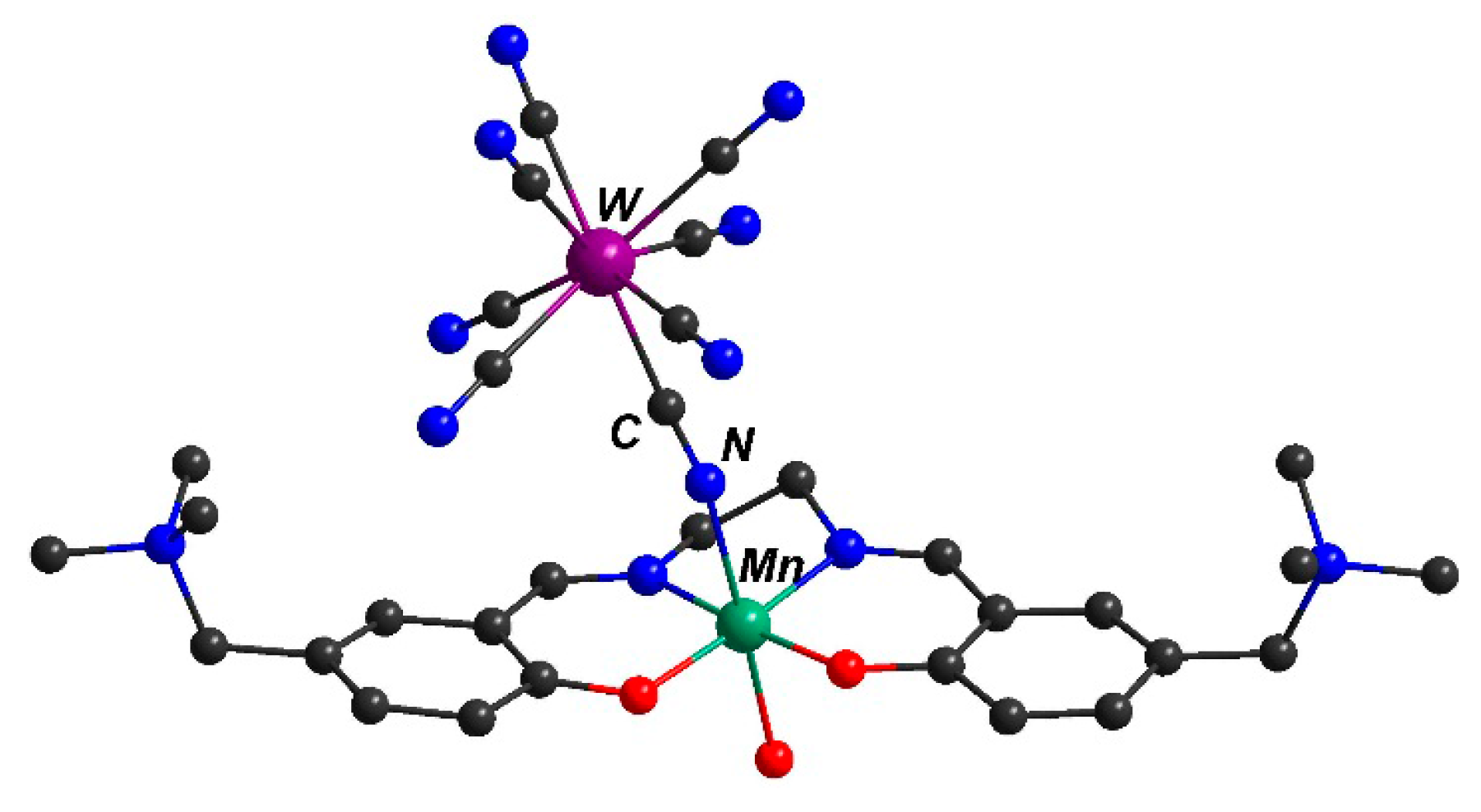
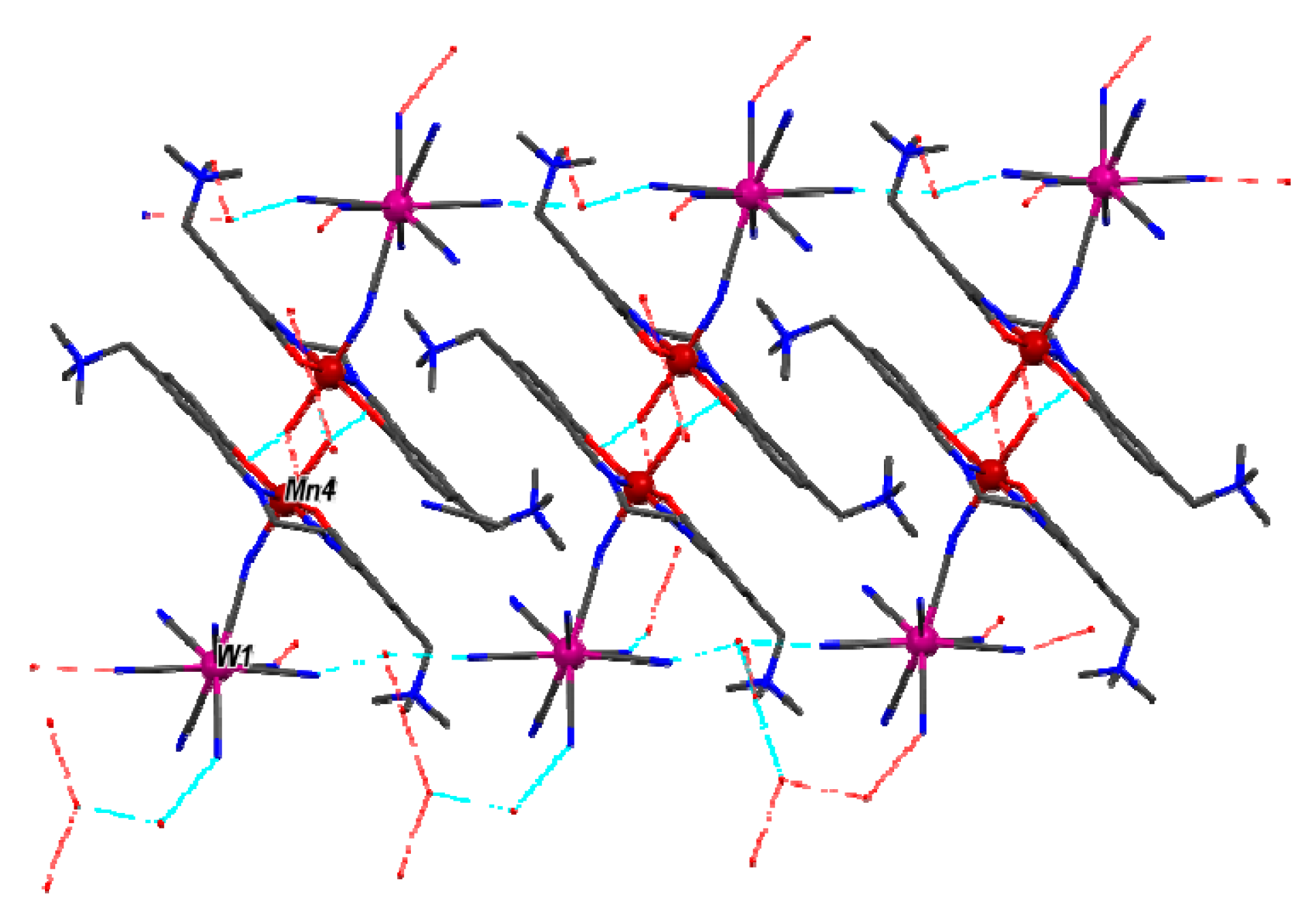
2.2. Description of the Molecular Structure
2.3. Magnetic Properties
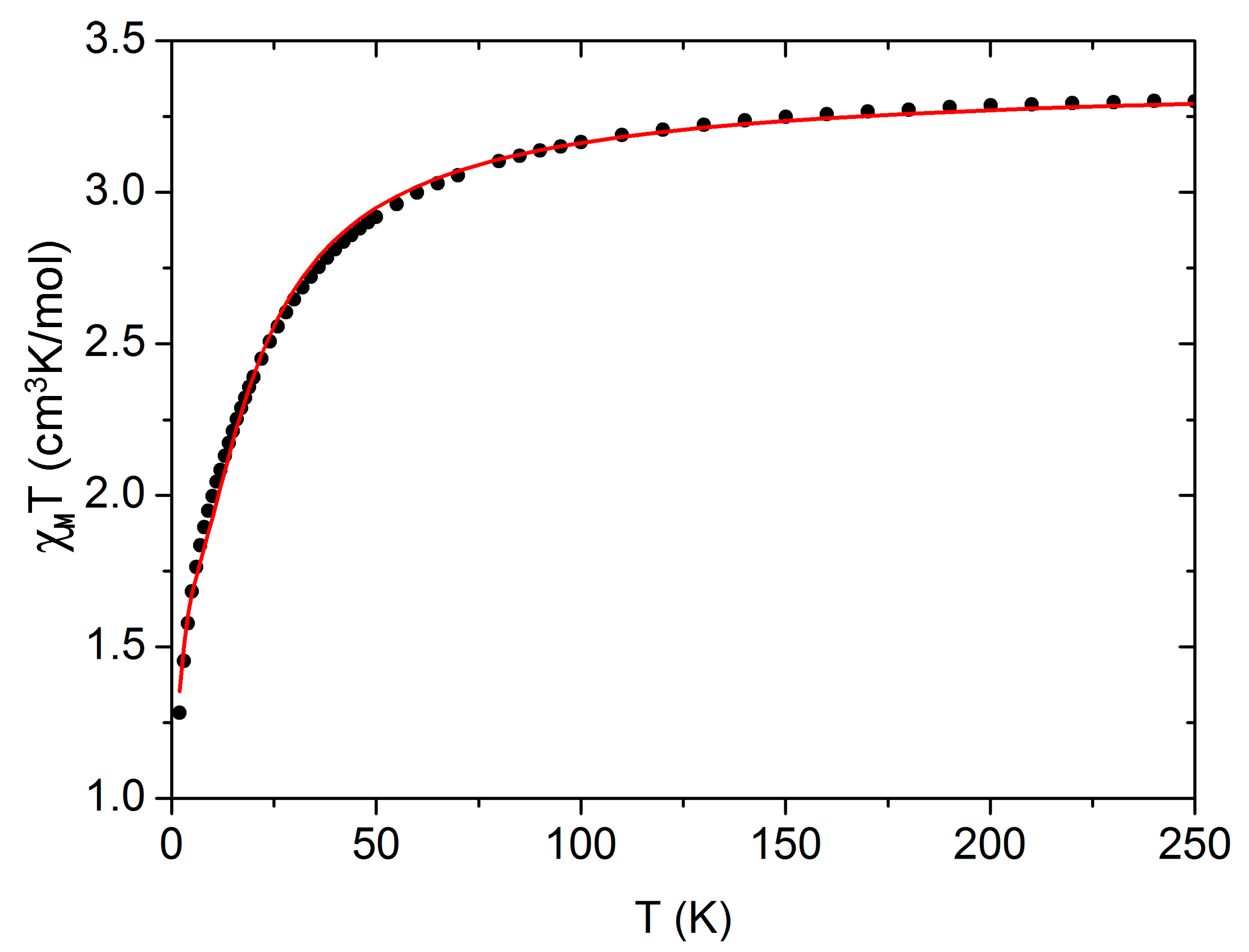
2.3.1. Magnetic Behavior of 1
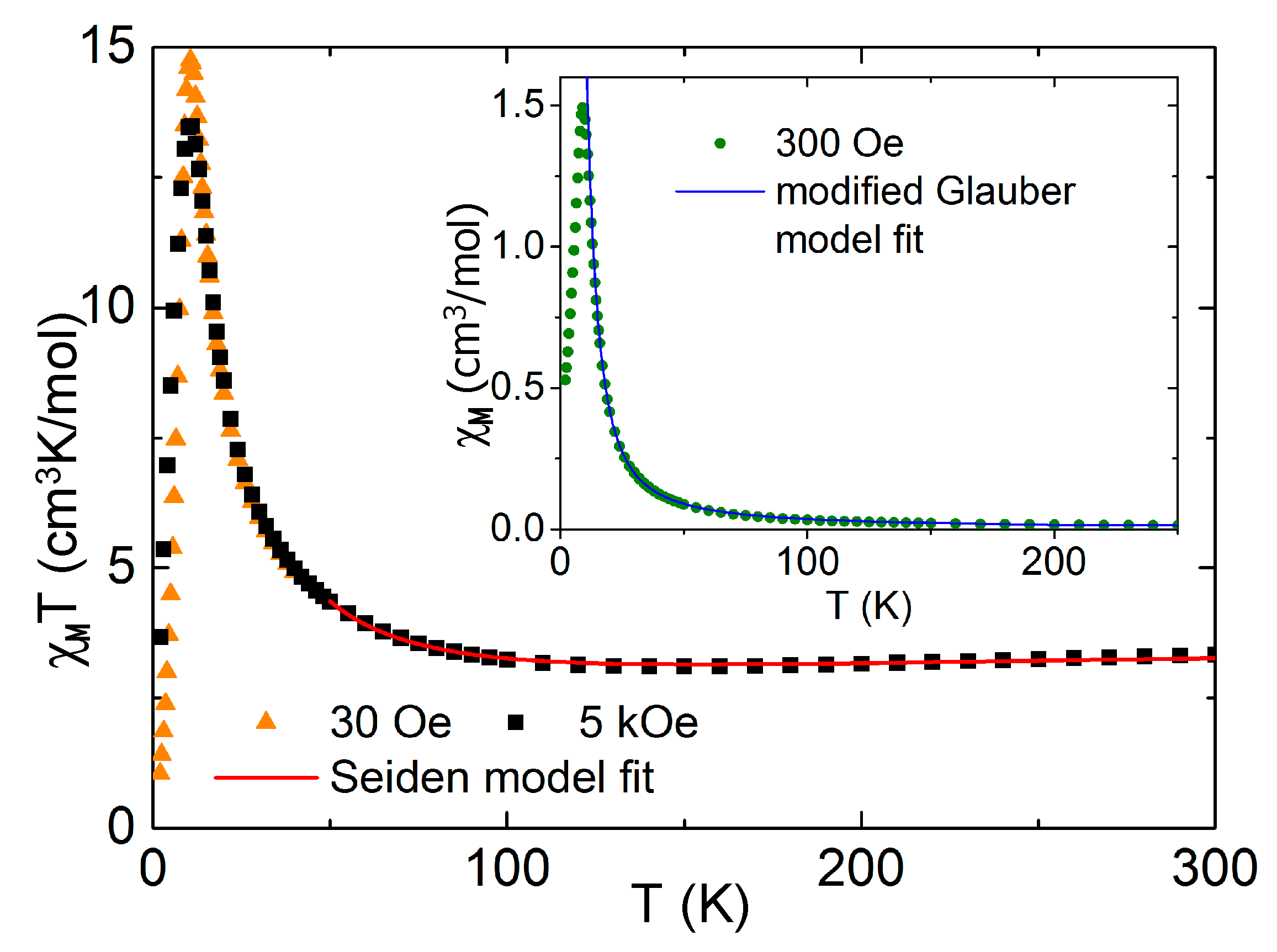
2.3.2. Magnetic Behavior of 2
3. Experimental Section
3.1. Single-Crystal X-Ray Diffraction
3.2. Magnetic Measurements
3.3. Synthetic Details
3.3.1. Synthesis of Complex 1, [Mn(5TMAMsalen)(H2O)][W(CN)8](H2O)4.75(CH3CN)
3.3.2. Synthesis of Complex 2, [Mn(5TMAMsalen)W(CN)8](H2O)8(CH3CN)
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Gatteschi, D.; Sessoli, R.; Villain, J. Molecular Nanomagnets; Oxford University Press: Oxford, UK, 2006; pp. 1–408. [Google Scholar]
- Coulon, C.; Miyasaka, H.; Clérac, R. Single-Chain Magnets: Theoretical Approach and Experimental Systemsin. In Structure and Bonding; Winpenny, R., Ed.; Springer: Berlin/Heidelberg, Germany, 2006; pp. 163–206. [Google Scholar]
- Bogani, L.; Wernsdorfer, W. Molecular spintronics using single-molecule magnets. Nat. Mater. 2008, 7, 179–186. [Google Scholar] [CrossRef]
- Juan, B.; Fernando, L.; Fernández, J.F. (Eds.) Molecular Magnets: Physics and Applications. In NanoScience and Technology; Springer: Berlin/Heidelberg, Germany, 2014; pp. 1–395. [Google Scholar]
- Lescouëzec, R.; Toma, L.; Vaissermann, J.; Verdaguer, M.; Delgado, F.S.; Ruiz-Pérez, C.; Lloret, F.; Julve, M. Design of single chain magnets through cyanide-bearing six-coordinate complexes. Coord. Chem. Rev. 2005, 249, 2691–2729. [Google Scholar] [CrossRef]
- Miyasaka, H.; Clerac, R. Synthetic Strategy for Rational Design of Single-Chain Magnets. Bull. Chem. Soc. Jpn. 2005, 78, 1725–1748. [Google Scholar] [CrossRef]
- Miyasaka, H.; Julve, M.; Yamashita, M.; Clérac, R. Slow Dynamics of the Magnetization in One-Dimensional Coordination Polymers: Single-Chain Magnets. Inorg. Chem. 2009, 48, 3420–3437. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.-L.; Wang, Z.-M.; Gao, S. Strategies towards single-chain magnets. Coord. Chem. Rev. 2010, 254, 1081–1100. [Google Scholar] [CrossRef]
- Wang, S.; Ding, X.-H.; Li, Y.-H.; Huang, W. Dicyanometalate chemistry: A type of versatile building block for the construction of cyanide-bridged molecular architectures. Coord. Chem. Rev. 2012, 256, 439–464. [Google Scholar] [CrossRef]
- Kang, L.-C.; Zuo, J.-L. Multifunctional Molecular Materials; Ouahab, L., Ed.; Pan Stanford Publishing: Singapore, 2013; pp. 105–131. [Google Scholar]
- Zhang, W.-X.; Breedlove, B.; Ishikawa, R.; Yamashita, M. Single-chain magnets: Beyond the Glauber model. RSC Adv. 2013, 3, 3772–3798. [Google Scholar] [CrossRef]
- Gatteschi, D.; Vindigni, A. Single-Chain Magnets. In Molecular Magnets; Springer: Berlin/Heidelberg, Germany, 2014; pp. 191–220. [Google Scholar]
- Ishikawa, R.; Katoh, K.; Breedlove, B.K.; Yamashita, M. MnIII(tetrabiphenylporphyrin)–TCNE Single-Chain Magnet via Suppression of the Interchain Interactions. Inorg. Chem. 2012, 51, 9123–9131. [Google Scholar] [CrossRef] [PubMed]
- Bhargavi, G.; Rajasekharan, M.V.; Costes, J.-P.; Tuchagues, J.-P. A new end-on azido bridged MnIII single-chain magnet and its dimeric single molecule magnet polymorph. Synthesis, structure and magnetic properties of [Mn(5-Clsalpn)N3]n and phenoxo bridged [Mn(5-Clsalpn)N3]2. Dalton Trans. 2013, 42, 8113–8123. [Google Scholar] [CrossRef] [PubMed]
- Senapati, T.; Pichon, C.; Ababei, R.; Mathoniere, C.; Clérac, R. Cyanido-Bridged Fe(III)–Mn(III) Heterobimetallic Materials Built From Mn(III) Schiff Base Complexes and Di- or Tri-Cyanido Fe(III) Precursors. Inorg. Chem. 2012, 51, 3796–3812. [Google Scholar] [CrossRef] [PubMed]
- Boeckmann, J.; Wriedt, M.; Näther, C. Metamagnetism and Single-Chain Magnetic Behavior in a Homospin One-Dimensional Iron(II) Coordination Polymer. Chem. Eur. J. 2012, 18, 5284–5289. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.-X.; Shiga, T.; Miyasaka, H.; Yamashita, M. New Approach for Designing Single-Chain Magnets: Organization of Chains via Hydrogen Bonding between Nucleobases. J. Am. Chem. Soc. 2012, 134, 6908–6911. [Google Scholar] [CrossRef] [PubMed]
- Sahoo, S.; Sutter, J.-P.; Ramasesha, S. Study of Low Temperature Magnetic Properties of a Single Chain Magnet with Alternate Isotropic and Non-collinear Anisotropic Units. J. Stat. Phys. 2012, 147, 181–193. [Google Scholar] [CrossRef]
- Liu, R.N.; Hu, P.; Li, L.C.; Liao, D.Z.; Sutter, J.-P. One-dimensional lanthanide complexes bridged by nitronyl nitroxide radical ligands with non-chelating nitrogen donors: Structure and magnetic characterization. Sci. China Chem. 2012, 55, 997–1003. [Google Scholar] [CrossRef]
- Li, Z.-X.; Jie, W.; Zha, G.; Wang, T.; Xu, Y. Nitrate-Templated 1D EE-Azide-Cobalt Chain Exhibits Canted Antiferromagnetism and Slow Magnetic Relaxation. Eur. J. Inorg. Chem. 2012, 22, 3537–3540. [Google Scholar] [CrossRef]
- Liu, R.; Xiong, C.; Zhao, S.; Wu, J.; Li, Q.; Fang, D. Ruthenium (II) complexes binding to human serum albumin and inducing apoptosis of tumor cells. Inorg. Chem. Commun. 2012, 22, 104–107. [Google Scholar] [CrossRef]
- Tomkowicz, Z.; Rams, M.; Balanda, M.; Foro, S.; Nojiri, H.; Krupskaya, Y.; Kataev, V.; Buchner, B.; Nayak, S.K.; Yakhmi, J.V.; et al. Slow Magnetic Relaxations in Manganese(III) Tetra(meta-fluorophenyl)porphyrin-tetracyanoethenide. Comparison with the Relative Single Chain Magnet ortho Compound. Inorg. Chem. 2012, 51, 9983–9994. [Google Scholar] [CrossRef] [PubMed]
- Hoshino, N.; Iijima, F.; Newton, G.N.; Yoshida, N.; Shiga, T.; Nojiri, H.; Nakao, A.; Kumai, R.; Murakami, Y.; Oshio, H. Three-way switching in a cyanide-bridged [CoFe] chain. Nat. Chem. 2012, 4, 921–926. [Google Scholar] [CrossRef] [PubMed]
- Visinescu, D.; Jeon, I.-R.; Madalan, A.M.; Alexandru, M.-G.; Jurca, B.; Mathoniere, C.; Clérac, R.; Andruh, M. Self-assembly of [CuIITbIII]3+ and [W(CN)8]3−tectons: A case study of a mixture containing two complexes showing slow-relaxation of the magnetization. Dalton Trans. 2012, 41, 13578–13581. [Google Scholar] [CrossRef] [PubMed]
- Yao, M.-X.; Zheng, Q.; Qian, K.; Song, Y.; Gao, S.; Zuo, J.-L. Controlled Synthesis of Heterotrimetallic Single-Chain Magnets from Anisotropic High-Spin 3d–4f Nodes and Paramagnetic Spacers. Chem. Eur. J. 2013, 19, 294–303. [Google Scholar] [CrossRef] [PubMed]
- Mougel, V.; Chatelain, L.; Hermle, J.; Caciuffo, R.; Colineau, E.; Tuna, F.; Magnani, N.; Degeyer, A.; Pécaut, J.; Mazzanti, M. A Uranium-Based UO2+–Mn2+ Single-Chain Magnet Assembled trough Cation–Cation Interaction. Angew. Chem. Int. 2014, 53, 819–823. [Google Scholar] [CrossRef] [PubMed]
- Vaz, M.G.F.; Cassaro, R.A.A.; Akpinar, H.; Schlueter, J.A.; Lahti, P.M.; Novak, M.A. A Cobalt Pyrenylnitronylnitroxide Single-Chain Magnet with High Coercivity and Record Blocking Temperature. Chem. Eur. J. 2014, 20, 5460–5467. [Google Scholar] [CrossRef] [PubMed]
- Glauber, R.J. Time-Dependent Statistics of the Ising Model. J. Math. Phys. 1963, 4, 294–307. [Google Scholar] [CrossRef]
- Suzuki, M.; Kubo, R. Dynamics of the Ising Model near the Critical Point. J. Phys. Soc. Jpn. 1968, 24, 51–60. [Google Scholar] [CrossRef]
- Coulon, C.; Clérac, R.; Lecren, L.; Wernsdorfer, W.; Miyasaka, H. Glauber dynamics in a single-chain magnet: From theory to real systems. Phys. Rev. B 2004, 69, 132408. [Google Scholar] [CrossRef]
- Miyasaka, H.; Saitoh, A.; Abec, S. Magnetic assemblies based on Mn(III) salen analogues. Coord. Chem. Rev. 2007, 251, 2622–2664. [Google Scholar] [CrossRef]
- Wang, X.-Y.; Avendaño, C.; Dunbar, K.R. Molecular magnetic materials based on 4d and 5d transition metals. Chem. Soc. Rev. 2011, 40, 3213–3238. [Google Scholar] [CrossRef] [PubMed]
- Dreiser, J.; Pedersen, K.S.; Schnegg, A.; Holldack, K.; Nehrkorn, J.; Sigrist, M.; Tregenna-Piggott, P.; Mutka, H.; Weihe, H.; Mironov, V.S.; et al. Three-Axis Anisotropic Exchange Coupling in the Single-Molecule Magnets NEt4[MnIII2(5-Brsalen)2(MeOH)2MIII(CN)6](M=Ru, Os). Chem. Eur. J. 2013, 19, 3693–3701. [Google Scholar] [CrossRef] [PubMed]
- Qian, K.; Huang, X.-C.; Zhou, C.; You, X.-Z.; Wang, X.-Y.; Dunbar, K.R. A Single-Molecule Magnet Based on Heptacyanomolybdate with the Highest Energy Barrier for a Cyanide Compound. J. Am. Chem. Soc. 2013, 135, 13302–13305. [Google Scholar] [CrossRef] [PubMed]
- Miyasaka, H.; Saitoh, A.; Yamashita, M.; Clérac, R. A MnIII2NiII single-chain magnet separated by a thick isolating network of BPh4− anions. Dalton Trans. 2008, 2422–2427. [Google Scholar] [CrossRef]
- Choi, H.J.; Sokol, J.J.; Long, J.R. Raising the Spin-Reversal Barrier in Cyano-Bridged Single-Molecule Magnets: Linear MnIII2MIII(CN)6(M=Cr, Fe) Species Incorporating [(5-Brsalen)Mn]+ Units. Inorg. Chem. 2004, 43, 1606–1608. [Google Scholar] [CrossRef] [PubMed]
- Miyasaka, H.H.; Takahashi, M.T.; Sugiura, K.; Clérac, R.; Nojiri, H. Cyano-Bridged MnIII3MIII (MIII = Fe, Cr) Complexes: Synthesis, Structure, and Magnetic Properties. Inorg. Chem. 2005, 44, 5969–5971. [Google Scholar] [CrossRef] [PubMed]
- Tregenna-Piggott, P.L.W.; Sheptyakov, D.; Keller, L.; Klokishner, S.I.; Ostrovsky, S.M.; Palii, A.V.; Reu, O.S.; Bendix, J.; Brock-Nannestad, T.; Pedersen, K.; et al. Single-Ion Anisotropy and Exchange Interactions in the Cyano-Bridged Trimers MnIII2MIII(CN)6(MIII = Co, Cr, Fe) Species Incorporating [Mn(5-Brsalen)]+ Units: An Inelastic Neutron Scattering and Magnetic Susceptibility Study. Inorg. Chem. 2009, 48, 128–134. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.W.; Liu, J.; Harris, T.D.; Hill, S.; Long, J.R. Slow Magnetic Relaxation Induced by a Large Transverse Zero-Field Splitting in a MnIIReIV(CN)2 Single-Chain Magnet. J. Am. Chem. Soc. 2012, 134, 7521–7529. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.-H.; He, W.-R.; Ding, X.-H.; Wang, S.; Cui, L.-F.; Huang, W. Cyanide-bridged assemblies constructed from capped tetracyanometalate building blocks [MA(ligand)(CN)4]1−/2−(MA = Fe or Cr). Coord. Chem. Rev. 2012, 256, 2795–2815. [Google Scholar] [CrossRef]
- Toma, L.M.; Pasan, J.; Catalina, R.-P.; Julve, M.; Lloret, F. [FeIII(dmbpy)(CN)4]−: A new building block for designing single-chain magnets. Dalton Trans. 2012, 41, 13716–13726. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.; Kanegawa, S.; Sato, O. Slow magnetic relaxation in a 4,2-ribbon like FeIII2CoII heterobimetallic chain. Dalton Trans. 2012, 41, 13575–13577. [Google Scholar] [CrossRef] [PubMed]
- Dong, D.-P.; Liu, T.; Zheng, H.; Zhao, L.; Zhuang, P.-F.; He, C.; Duan, C.-Y. Synthesis, structures and single chain magnet behavior of a cyano-bridged {Fe2Cu} chain. Inorg. Chem. Commun. 2012, 24, 153–156. [Google Scholar] [CrossRef]
- Chorazy, S.; Nakabayashi, K.; Imoto, K.; Mlynarski, J.; Sieklucka, B.; Ohkoshi, S. Conjunction of Chirality and Slow Magnetic Relaxation in the Supramolecular Network Constructed of Crossed Cyano-Bridged CoII–WVMolecular Chains. J. Am. Chem. Soc. 2012, 134, 16151–16154. [Google Scholar] [CrossRef] [PubMed]
- Toma, L.M.; Ruiz-Perez, C.; Pasan, J.; Wernsdorfer, W.; Lloret, F.; Julve, M. Molecular Engineering to Control the Magnetic Interaction between Single-Chain Magnets Assembled in a Two-Dimensional Network. J. Am. Chem. Soc. 2012, 134, 15265–15268. [Google Scholar] [CrossRef] [PubMed]
- Bhowmick, I.; Hillard, E.A.; Dechambenoit, P.; Coulon, C.; Harris, T.D.; Clérac, R. A canted antiferromagnetic ordered phase of cyanido-bridged MnIII2ReIV single-chain magnets. Chem. Commun. 2012, 48, 9717–9719. [Google Scholar] [CrossRef] [PubMed]
- Ferbinteanu, M.; Miyasaka, H.; Wernsdorfer, W.; Nakata, K.; Sugiura, K.; Yamashita, M.; Coulon, C.; Clérac, R. Single-Chain Magnet (NEt4)[Mn2(5-MeOsalen)2Fe(CN)6] Made of MnIII-FeIII-MnIII Trinuclear Single-Molecule Magnet with an ST)9/2 Spin Ground State. J. Am. Chem. Soc. 2005, 127, 3090–3099. [Google Scholar] [CrossRef] [PubMed]
- Miyasaka, H.; Madanbashi, T.; Saitoh, A.; Motokawa, N.; Ishikawa, R.; Yamashita, M.; Bahr, S.; Wernsdorfer, W.; Clérac, R. Cyano-Bridged MnIIIMIII Single-Chain Magnets with MIII=CoIII, FeIII, MnIII, and CrIII. Chem. Eur. J. 2012, 18, 3942–3954. [Google Scholar] [CrossRef] [PubMed]
- Bleuzen, A.; Marvaud, V.; Mathoniere, C.; Sieklucka, B.; Verdaguer, M. Photomagnetism in Clusters and Extended Molecule-Based Magnets. Inorg. Chem. 2009, 48, 3453–3466. [Google Scholar] [CrossRef] [PubMed]
- Sieklucka, B.; Podgajny, R.; Przychodzen, P.; Korzeniak, T. Engineering of octacyanometalate-based coordination networks towards functionality. Coord. Chem. Rev. 2005, 249, 2203–2220. [Google Scholar] [CrossRef]
- Przychodzen, P.; Korzeniak, T.; Podgajny, R.; Sieklucka, B. Supramolecular coordination networks based on octacyanometalates: From structure to function. Coord. Chem. Rev. 2006, 250, 2234–2260. [Google Scholar] [CrossRef]
- Sieklucka, B.; Podgajny, R.; Pinkowicz, D.; Nowicka, B.; Korzeniak, T.; Bałanda, M.; Wasiutyński, T.; Pełka, R.; Makarewicz, M.; Czapla, M.; Rams, M.; et al. Towards high Tc octacyanometalate-based networks. CrystEngComm 2009, 11, 2032–2039. [Google Scholar] [CrossRef]
- You, Y.S.; Kim, D.; Do, Y.; Oh, S.J.; Hong, C.S. One-Dimensional Octacyanomolybdate-Based Cu(II)–Mo(V) Bimetallic Assembly with a Novel Rope-Ladder Chain Structure. Inorg. Chem. 2004, 43, 6899–6901. [Google Scholar] [CrossRef] [PubMed]
- Yoon, J.H.; Lee, J.W.; Ryu, D.W.; Choi, S.Y.; Yoon, S.W.; Suh, B.J.; Koh, E.K.; Kim, H.C.; Hong, C.S. Cyanide-Bridged WVMnIII Single-Chain Magnet with Isolated MnIII Moieties Exhibiting Two Types of Relaxation Dynamics. Inorg. Chem. 2011, 50, 11306–11308. [Google Scholar] [CrossRef] [PubMed]
- Yoo, H.S.; Ko, H.H.; Ryu, D.W.; Lee, J.W.; Yoon, J.H.; Lee, W.R.; Kim, H.C.; Koh, E.K.; Hong, C.S. Octacyanometalate-Based Ferrimagnetic MVMnIII (M = Mo, W) bimetallic chain racemates with slow magnetic relaxations. Inorg. Chem. 2009, 48, 5617–5619. [Google Scholar] [CrossRef] [PubMed]
- Yoon, J.H.; Lim, K.S.; Ryu, D.W.; Lee, W.R.; Yoon, S.W.; Suh, B.J.; Hong, C.S. Synthesis, Crystal Structures, and Magnetic Properties of Cyanide-Bridged WVMnIII Anionic Coordination Polymers Containing Divalent Cationic Moieties: Slow Magnetic Relaxations and Spin Crossover Phenomenon. Inorg. Chem. 2014, 53, 10437–10442. [Google Scholar] [CrossRef] [PubMed]
- Kou, H.-Z.; Ni, Z.-H.; Zhou, B.C.; Wang, R.-J.; Kou, H.-Z.; Ni, Z.-H.; Zhou, B.C.; Wang, R.-J. A cyano-bridged molecule-based magnet containing manganese(III) Schiff base and octacyanotungstate(V) building blocks. Inorg. Chem. Commun. 2004, 7, 1150–1153. [Google Scholar] [CrossRef]
- Lim, J.H.; Kang, J.S.; Kim, H.C.; Koh, E.K.; Hong, C.S. Synthesis, Crystal Structures, and Magnetic Properties of Cyano-Bridged Honeycomblike Layers MV–CuII (M = Mo, W) Chelated by a Macrocyclic Ligand. Inorg. Chem. 2006, 45, 7821–2787. [Google Scholar] [CrossRef] [PubMed]
- Nowicka, B.; Rams, M.; Stadnicka, K.; Sieklucka, B. Reversible Guest-Induced Magnetic and Structural Single-Crystal-to-Single-Crystal Transformation in Microporous Coordination Network {[Ni(cyclam)]3 [W(CN)8]2}n. Inorg. Chem. 2007, 46, 8123–8125. [Google Scholar] [CrossRef] [PubMed]
- Nowicka, B.; Bałanda, M.; Gaweł, B.; Ćwiak, G.; Budziak, A.; Łasocha, W.; Sieklucka, B. Microporous {[Ni(cyclam)]3[W(CN)8]2}n affording reversible structural and magnetic conversions. Dalton Trans. 2011, 40, 3067–3073. [Google Scholar] [CrossRef] [PubMed]
- Larionova, J.; Clerac, R.; Donnadieu, B.; Willemin, S.; Guerin, C. Synthesis and Structure of a Two-Dimensional Cyano-Bridged Coordination Polymer [Cu(cyclam)]2[Mo(CN)8]·10.5H2O (Cyclam = 1,4,8,11-Tetraazacyclodecane). Cryst. Growth Des. 2003, 3, 267–272. [Google Scholar] [CrossRef]
- Umeta, Y.; Tokoro, H.; Ozaki, N.; Ohkoshi, S. Room-temperature thermally induced relaxation effect in a two-dimensional cyano-bridged Cu-Mo bimetal assembly and thermodynamic analysis of the relaxation process. AIP Adv. 2013, 3. [Google Scholar] [CrossRef]
- Venkatakrishnan, T.S.; Sahoo, S.; Bréfuel, N.; Duhayon, C.; Paulsen, C.; Barra, A.-L.; Ramasesha, S.; Sutter, J.-P. Enhanced ion anisotropy by nonconventional coordination geometry: single-chain magnet behavior for a [{FeIIL}2{NbIV(CN)8}] helical chain compound designed with heptacoordinate FeII. J. Am. Chem. Soc. 2010, 132, 6047–6056. [Google Scholar] [CrossRef] [PubMed]
- Sieklucka, B.; Podgajny, R.; Korzeniak, T.; Nowicka, B.; Pinkowicz, D.; Kozieł, M. A decade of octacyanides in polynuclear molecular materials. Eur. J. Inorg. Chem. 2011, 3, 305–326. [Google Scholar] [CrossRef]
- Ohkoshi, S.; Hashimoto, K. Photo-magnetic and magneto-optical effects of functionalized metal polycyanides. J. Photochem. Photobiol. C Photochem. Rev. 2001, 2, 71–88. [Google Scholar] [CrossRef]
- Pinkowicz, D.; Pełka, R.; Drath, O.; Nitek, W.; Bałanda, M.; Majcher, A.M.; Poneti, G.; Sieklucka, B. Nature of Magnetic Interactions in 3D {[MII(pyrazole)4]2[NbIV(CN)8]·4H2O}n (M = Mn, Fe, Co, Ni) Molecular Magnets. Inorg. Chem. 2010, 49, 7565–7576. [Google Scholar] [CrossRef] [PubMed]
- Qian, J.; Hu, J.; Zhang, J.; Yoshikawa, H.; Awaga, K.; Zhang, C. Solvent-Induced Assembly of Octacyanometalates-Based Coordination Polymers with Unique afm1 Topology and Magnetic Properties. Cryst. Growth Des. 2013, 13, 5211–5219. [Google Scholar] [CrossRef]
- Pinkowicz, D.; Podgajny, R.; Nitek, W.; Rams, M.; Majcher, A.M.; Nuida, T.; Ohkoshi, S.; Sieklucka, B. Multifunctional Magnetic Molecular {[MnII(urea)2(H2O)]2[NbIV(CN)8]}n System: Magnetization-Induced SHG in the Chiral Polymorph. Chem. Mater. 2011, 23, 21–31. [Google Scholar] [CrossRef]
- Przychodzeń, P.; Rams, M.; Guyard-Duhayon, C.; Sieklucka, B. Antiferromagnetic coupling through cyano-bridge and H-bonds in [MnIII(3-OMesalophen)(H2O)2]2[MnIII(3-OMesalophen)(H2O)] [WV(CN)8]·2H2O. Inorg. Chem. Commun. 2005, 8, 350–354. [Google Scholar] [CrossRef]
- Przychodzeń, P.; Lewiński, K.; Bałanda, M.; Pełka, R.; Rams, M.; Wasiutyński, T.; Guyard-Duhayon, C.; Sieklucka, B. Crystal structures and magnetic properties of two low-dimensional materials constructed from [MnIII(salen)H2O]+ and [M(CN)8]3−/4− (M = Mo or W) precursors. Inorg. Chem. 2004, 43, 2967–2974. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Wang, Q.-L.; Su, C.-Y.; Hu, L.-N.; Li, L.-C.; Liao, D.-Z. A W(V)–Mn(III) bimetallic assembly built by manganese(III) Schiff-base and octacyanotungstate(V) building blocks: Structure and magnetic property. Inorg. Chem. Commun. 2014, 40, 26–30. [Google Scholar] [CrossRef]
- Wang, T.-W.; Wang, J.; Ohkoshi, S.; Song, Y.; You, X.-Z. Manganese(II)-octacyanometallate(V) bimetallic ferrimagnets with Tc from 41 to 53 K obtained in acidic media. Inorg. Chem. 2010, 49, 7756–7763. [Google Scholar] [CrossRef] [PubMed]
- Ohkoshi, S.; Ikeda, S.; Hozumi, T.; Kashiwagi, T.; Hashimoto, K. Photoinduced magnetization with a high curie temperature and a large coercive field in a cyano-bridged cobalt–tungstate bimetallic assembly. J. Am. Chem. Soc. 2006, 128, 5320–5321. [Google Scholar] [CrossRef] [PubMed]
- Mathoniére, C.; Podgajny, R.; Guionneau, P.; Labrugere, C.; Sieklucka, B. Photomagnetism in Cyano-Bridged Hexanuclear Clusters [MnII(bpy)2]4[MIV(CN)8]2·xH2O (M = Mo, x = 14, and M = W, x = 9). Chem. Mater. 2005, 17, 442–449. [Google Scholar] [CrossRef]
- Song, Y.; Zhang, P.; Ren, X.-M.; Shen, X.-F.; Li, Y.-Z.; You, X.-Z. Octacyanometalate-based single-molecule magnets: CoII9MV6 (M = W, Mo). J. Am. Chem. Soc. 2005, 127, 3708–3709. [Google Scholar] [CrossRef] [PubMed]
- Nowicka, B.; Korzeniak, T.; Stefańczyk, O.; Pinkowicz, D.; Chorąży, S.; Podgajny, R.; Sieklucka, B. The impact of ligands upon topology and functionality of octacyanidometallate-based assemblies. Coord. Chem. Rev. 2012, 256, 1946–1971. [Google Scholar] [CrossRef]
- Ko, H.H.; Lim, J.H.; Yoo, H.S.; Kang, J.S.; Kim, H.C.; Koh, E.K.; Hong, C.S. Two WV–MnIII bimetallic assemblies built by octacyanotungstate(V) and MnIII Schiff bases: molecular structures and a spin-flop transition. Dalton Trans. 2007, 20, 2070–2076. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.W.; Ryu, D.W.; Lee, J.W.; Yoon, J.H.; Kim, H.C.; Lee, H.; Cho, B.K.; Hong, C.S. One-dimensional cyanide-bridged MnIIIWV bimetallic complexes: Metamagnetism, spontaneous resolution, and slow magnetic relaxation. Inorg. Chem. 2009, 48, 9066–9068. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.W.; Kwak, H.Y.; Yoon, J.H.; Kim, H.C.; Koh, E.K.; Hong, C.S. Intermolecular contact-tuned magnetic nature in one-dimensional 3d–5d bimetallic systems: From a metamagnet to a single-chain magnet. Inorg. Chem. 2008, 47, 10214–10216. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.W.; Lim, K.S.; Yoon, J.H.; Ryu, D.W.; Koo, B.H.; Koh, E.K.; Hong, C.S. Cyanide-bridged single molecule magnet based on a manganese(III) complex with TTF-fused Schiff base ligand. Sci. China Chem. 2012, 55, 1012–1017. [Google Scholar] [CrossRef]
- Lim, K.S.; Hong, C.S. [W(CN)6(L)]1−/2− (L = bidentate ligand) as a useful building unit to construct molecule-based magnetic systems. Dalton Trans. 2013, 42, 14941–14950. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, R.; Nakano, M.; Breedlove, B.K.; Yamashita, M. Syntheses, structures, and magnetic properties of discrete cyano-bridged heterodinuclear complexes composed of MnIII(salen)-type complex and MIII(CN)6 anion (MIII = Fe, Mn, Cr). Polyhedron 2013, 64, 346–351. [Google Scholar] [CrossRef]
- Kiernan, P.M.; Griffith, W.P. Studies on transition-metal cyano-complexes. Part I. Octacyanoniobates(III), -niobates(IV), -molybdates(V), and -tungstates(V). J. Chem. Soc. Dalton Trans. 1975, 23, 2489–2494. [Google Scholar] [CrossRef]
- Peresypkina, E.V.; Vostrikova, K.E. 2[Mn(acacen)]+ + 1[Fe(CN)5NO]2− polynuclear heterobimetallic coordination compounds of different dimensionality in the solid state. Dalton Trans. 2012, 41, 4100–4106. [Google Scholar] [CrossRef] [PubMed]
- Rams, M.; Peresypkina, E.V.; Mironov, V.S.; Wernsdorferand, W.; Vostrikova, K.E. Magnetic Relaxation of 1D Coordination Polymers (X)2[Mn(acacen)Fe(CN)6], X = Ph4P+, Et4N+. Inorg. Chem. 2014, 53, 10291–10210. [Google Scholar] [CrossRef] [PubMed]
- Mironov, V.S.; Chibotaru, L.F.; Ceulemans, A. Exchange interaction in the YbCrBr93− mixed dimer: The origin of a strong Yb3+−Cr3+ exchange anisotropy. Phys. Rev. B 2003, 67, 014424–014428. [Google Scholar] [CrossRef]
- Zorina, E.N.; Zauzolkova, N.V.; Sidorov, A.A.; Aleksandrov, G.G.; Lermontov, A.S.; Kiskin, M.A.; Bogomyakov, A.S.; Mironov, V.S.; Novotortsev, V.M.; Eremenko, I.L. Novel polynuclear architectures incorporating Co2+ and K+ ions bound by dimethylmalonate anions: Synthesis, structure, and magnetic properties. Inorg. Chim. Acta 2013, 396, 108–118. [Google Scholar] [CrossRef]
- Samsonenko, D.G.; Paulsen, C.; Lhotel, E.; Mironov, V.S.; Vostrikova, K.E. [MnIII(Schiff base)]3[ReIV(CN)7], highly anisotropic 3D coordination framework: Synthesis, crystal structure, magnetic investigations, and theoretical analysis. Inorg. Chem. 2014, 53, 10217–10231. [Google Scholar] [CrossRef] [PubMed]
- Mironov, V.S. Origin of Dissimilar Single-Molecule Magnet Behavior of Three MnII2MoIII Complexes Based on [MoIII(CN)7]4− Heptacyanomolybdate: Interplay of MoIII–CN–MnII Anisotropic Exchange Interactions. Inorg. Chem. 2015, 54, 11339–11355. [Google Scholar] [CrossRef] [PubMed]
- Schaffer, C.E. A perturbation representation of weak covalent bonding. The symmetry basis for the angular overlap model of the ligand field. Struct. Bond. 1968, 5, 68–95. [Google Scholar]
- Tokyo Institute of Technology, Dept. Organic & Organic Materials Engineering. Atomic Parameters for Extended Huckel Calculation. Available online: http://www.op.titech.ac.jp/lab/mori/EHTB/EHTB~1.html (accessed on 31 January 2017).
- Lee, S. Electron localization and the structure of transition metal chains. J. Am. Chem. Soc. 1989, 111, 7754–7761. [Google Scholar] [CrossRef]
- Seiden, J. Propriétés statiques d'une chaîne isotrope alternée de spins quantiques 1/2 et de spins classiques. J. Phys. Lett. 1983, 44, 947–952. [Google Scholar] [CrossRef]
- Carlin, R.L.; Van Duyneveldt, A.J. Magnetic Properties of Transition Metal Compounds; Springer: Berlin, Germany, 1977. [Google Scholar]
- Wöhlert, S.; Fic, T.; Tomkowicz, Z.; Ebbinghaus, S.G.; Rams, M.; Haase, W.; Näther, C. Structural and Magnetic Studies of a New Co(II) Thiocyanato Coordination Polymer Showing Slow Magnetic Relaxations and a Metamagnetic Transition. Inorg. Chem. 2013, 52, 12947–12957. [Google Scholar] [CrossRef] [PubMed]
- Prosvirin, A.V.; Zhao, H.; Dunbar, K.R. A Mn(III) chain derived from Mn12–acetate that exhibits both glauber dynamics and antiferromangetic ordering regimes. Inorg. Chim. Acta 2012, 389, 118–121. [Google Scholar] [CrossRef]
- Mydosh, J.A. Spin Glasses: An Experimental Introduction; Taylor and Francis: London, UK, 1993; p. 67. [Google Scholar]
- Cole, K.S.; Cole, R.H. Dispersion and Absorption in Dielectrics I. Alternating Current Characteristics. J. Chem. Phys. 1941, 9, 341. [Google Scholar] [CrossRef]
- Sakamoto, F.; Sumiya, T.; Fujita, M.; Tada, T.; Tan, X.S.; Suzuki, E.; Okura, I.; Fujii, Y. T-site selective photocleavage of DNA by cationic Schiff base complex of manganese(III). Chem. Lett. 1998, 11, 1127–1128. [Google Scholar] [CrossRef]
- Baadsgaard, H.; Treadwell, W.D. Zur Kenntnis der komplexen Wolframcyanide K4[W(CN)8] 2H2O und K3[W(CN)8] H2O. Helv. Chim. Acta 1955, 38, 1669–1679. [Google Scholar] [CrossRef]
- Goodenow, E.L.; Garner, C.S. The Exchange Reaction between Octacyanotungstate(IV) and Octacyanotungstate(V) Ions. J. Am. Chem. Soc. 1955, 77, 5272–5274. [Google Scholar] [CrossRef]
- NETZSCH Proteus Thermal Analysis v.4.8.1.; NETZSCH55 Geratebau: Bayern, Germany, 2005.
- CrysAlisPro; v. 1.171.33.46 (rel. 27-08-2CrysAlis171.NET); Oxford Diffraction Ltd.: Oxford, UK, 2009.
- De Meulenaar, J.; Tompa, H. The absorption correction in crystal structure analysis. Acta Crystallogr. 1965, 19, 1014–1018. [Google Scholar] [CrossRef]
- Cascarano, G.; Altomare, A.; Giacovazzo, C.; Guagliardi, A.; Moliterni, A.G.G.; Siliqi, D.; Burla, M.C.; Polidori, G.; Camalli, M. SIRWARE. Acta Crystallogr. Sect. A 1996, 52, C79. [Google Scholar] [CrossRef]
- University of Oxford. Crystallography Program “Crystalsv1461”. Available online: http://www.xtl.ox.ac.uk/crystals.1.html (accessed on 31 January 2017).
- Altomare, A.; Burla, M.C.; Camalli, M.; Cascarano, G.L.; Giacovazzo, C.; Guagliardi, A.; Grazia, A.; Moliterni, G.; Polidori, G.; Spagna, R. SIR97: A new tool for crystal structure determination and refinement. J. App. Cryst. 1999, 32, 115–119. [Google Scholar] [CrossRef]
- Betteridge, P.W.; Carruthers, J.R.; Cooper, R.I.; Prout, K.; Watkin, D.J. CRYSTALS version 12: Software for guided crystal structure analysis. J. Appl. Cryst. 2003, 36, 1487. [Google Scholar] [CrossRef]




| 1 | (Bu4N)3[W(CN)8] [83] | 2 |
|---|---|---|
| 2165.7 sh | - | 2167.6 sh |
| 2161.8 | - | 2161.8 |
| 2138.7 | 2141 | 2146.4 |
| 2127.1 | 2130 | 2129.0 sh |
| 2121.3 | 2123 | - |
| 2100.0 | - | 2111.7 |
| 2084.0 | - | 2088.0 |
| 2033.0 | - | 2038.4 |
| [Mn(5TMAMsalmen)]3+ Moiety | |
|---|---|
| Mn–Ophenolate | 1.873(4) |
| 1.895(3) | |
| Mn–Nimine | 1.985(4) |
| 1.989(4) | |
| Mn–Owater | 2.247(4) |
| Mn–Ncyanide | 2.244(4) |
| Mn–N≡C | 160.8(3) |
| Nimine–C–C–Nimine | 43.3(6) |
| [W(CN)8]3− Moiety | |
| M–Ccyanide | 2.154(6) |
| 2.152(5) | |
| 2.159(5) | |
| 2.157(6) | |
| 2.168(6) | |
| 2.152(5) | |
| 2.153(6) | |
| 2.151(7) | |
| Formula | C34H43MnN13O5.75W |
| formula weight | 964.58 |
| crystal system | triclinic |
| space group | PĪ |
| Lattice Parameters: | |
| a (Å) | 11.1881(4) |
| b (Å) | 12.7756(5) |
| c (Å) | 18.5278(8) |
| α (°) | 89.805(3)° |
| β (°) | 74.570(4)° |
| γ (°) | 72.773(3)° |
| V (Å3) | 2430.09(17) |
| Z | 2 |
| Dcalc (g cm3) | 1.318 |
| F (000) | 966 |
| λ (Mo Kα) Å | 0.71069 |
| μ (Mo Ka) (mm−1) | 2.67 |
| No. of Measured Reflections: | |
| total | 20,799 |
| unique | 11,156 |
| reflections with I > 2.0σ(I) | 9319 |
| No. of variables | 506 |
| Rint | 0.037 |
| R1 (I > 2σ(I)) a | 0.053 |
| wR2 (All reflections) b | 0.055 |
| Goodness-of-fit (S) c | 0.9 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Majcher, A.M.; Pilet, G.; Mironov, V.S.; Vostrikova, K.E. Neutral Low-Dimensional Assemblies of a Mn(III) Schiff Base Complex and Octacyanotungstate(V): Synthesis, Characterization, and Magnetic Properties. Magnetochemistry 2017, 3, 16. https://doi.org/10.3390/magnetochemistry3020016
Majcher AM, Pilet G, Mironov VS, Vostrikova KE. Neutral Low-Dimensional Assemblies of a Mn(III) Schiff Base Complex and Octacyanotungstate(V): Synthesis, Characterization, and Magnetic Properties. Magnetochemistry. 2017; 3(2):16. https://doi.org/10.3390/magnetochemistry3020016
Chicago/Turabian StyleMajcher, Anna M., Guillaume Pilet, Vladimir S. Mironov, and Kira E. Vostrikova. 2017. "Neutral Low-Dimensional Assemblies of a Mn(III) Schiff Base Complex and Octacyanotungstate(V): Synthesis, Characterization, and Magnetic Properties" Magnetochemistry 3, no. 2: 16. https://doi.org/10.3390/magnetochemistry3020016






