Separation of the α- and β-Anomers of Carbohydrates by Diffusion-Ordered NMR Spectroscopy
Abstract
:1. Introduction
2. Results and Discussion
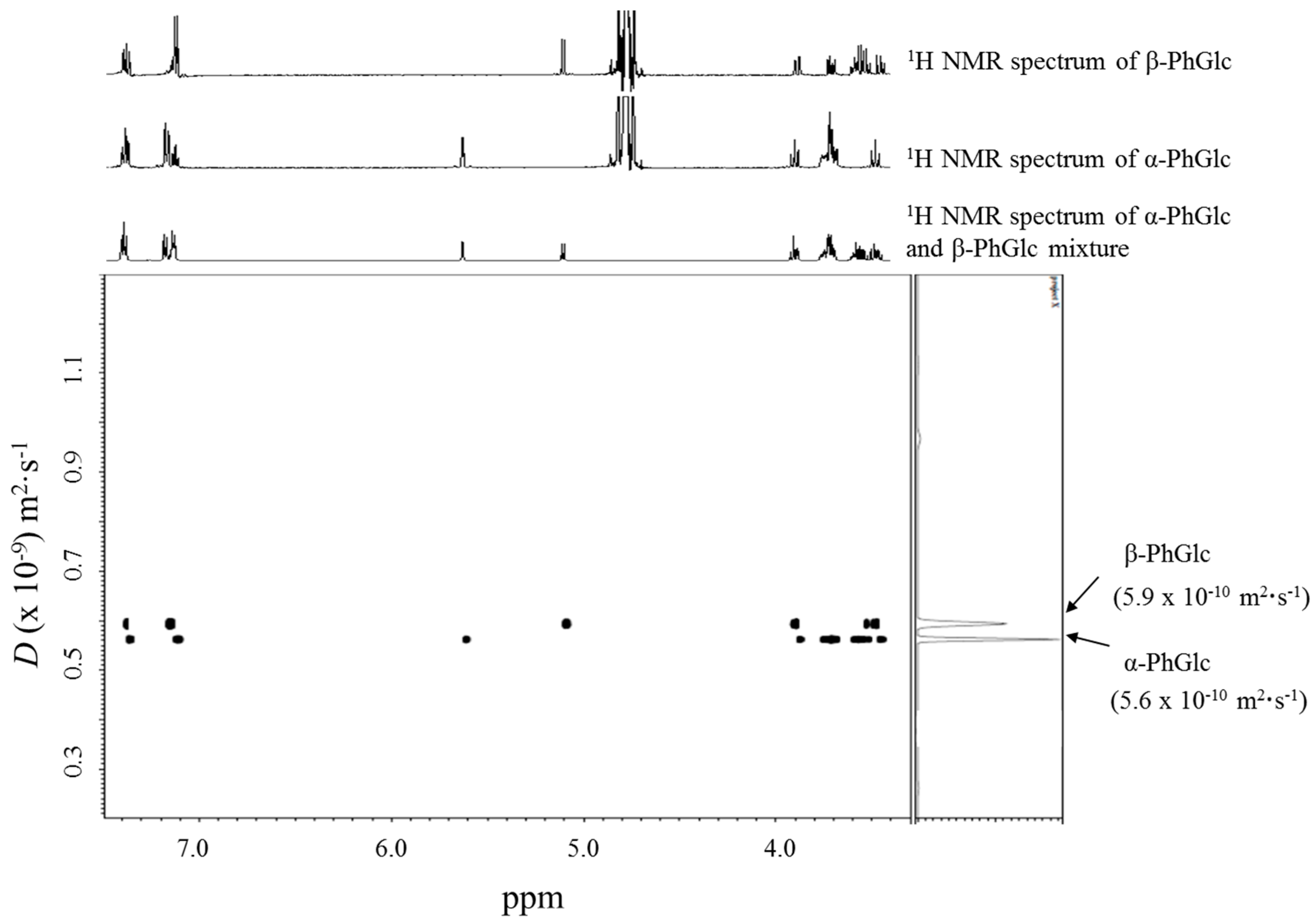
2.1. DOSY Separation of the α- and β-Anomeric Isomers of Glycopyranosides
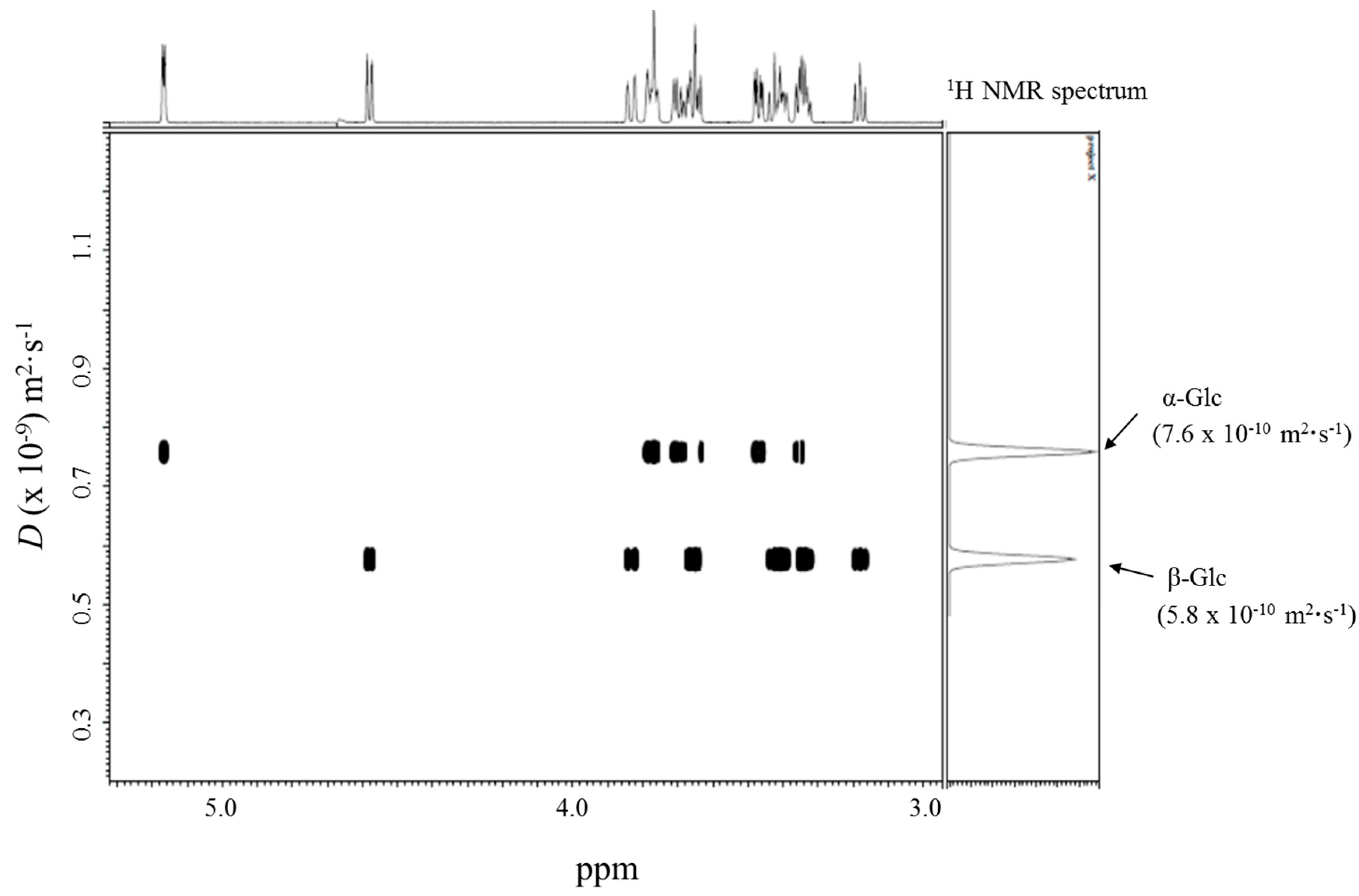
2.2. DOSY Separation of the α- and β-Anomeric Isomers of Glycopyranoses
2.3. DOSY Separation of a Mixture of Two Kinds of Glycopyranosides Having Similar Aglycon Structures
3. Materials and Methods
4. Conclusions
Supplementary Materials
Author Contributions
Conflicts of Interest
References
- Johnson, C.S. Diffusion ordered nuclear magnetic resonance spectroscopy: Principles and applications. Prog. Nucl. Magn. Reson. Spectrosc. 1999, 34, 203–256. [Google Scholar] [CrossRef]
- Antalek, B. Using Pulsed Gradient Spin Echo NMR for Chemical Mixture Analysis: How to Obtain Optimum Results. Concepts Magn. Reson. 2002, 14, 225–258. [Google Scholar] [CrossRef]
- Kavakka, J.S.; Kilpeläinen, I.; Heikkinen, S. General Chromatographic NMR Method in Liquid State for Synthetic Chemistry: Polyvinylpyrrolidone Assisted DOSY Experiments. Org. Lett. 2009, 6, 1349–1352. [Google Scholar] [CrossRef] [PubMed]
- Kavakka, J.S.; Parviainen, V.; Wahala, K.; Kilpeläinen, I.; Heikkinen, S. Enhanced chromatographic NMR with polyethyleneglycol. A novel resolving agent for diffusion ordered spectroscopy. Magn. Reson. Chem. 2010, 48, 777–781. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Wu, R.; Bai, Z.; Yang, Y.; Li, S.; Dou, X. Evaluation of the separation performance of polyvinylpyrrolidone as a virtual stationary phase for chromatographic NMR. Magn. Reson. Chem. 2014, 52, 486–490. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Tan, T.; Kenne, L.; Sandström, C. The use of diffusion-ordered spectroscopy and complexation agents to analyze mixtures of catechins. New J. Chem. 2009, 33, 1057–1063. [Google Scholar] [CrossRef]
- Oda, Y.; Kobayashi, N.; Yamanoi, T.; Katsuraya, K.; Takahashi, K.; Hattori, K. β-Cyclodextrin Conjugates with Glucose Moieties Designed as Drug Carriers: Their Syntheses, Evaluations Using Concanavalin A and Doxorubicin, and Structural Analyses by NMR Spectroscopy. Med. Chem. 2008, 4, 244–255. [Google Scholar] [CrossRef] [PubMed]
- Oda, Y.; Matsuda, S.; Yamanoi, T.; Murota, A.; Katsuraya, K. Identification of the inclusion complexation between phenyl β-d-(13C6)-glucopyranoside and α-cyclodextrin using 2D 1H or 13C DOSY spectrum. Supramol. Chem. 2009, 21, 638–642. [Google Scholar] [CrossRef]
- Haiber, S.; Nilsson, M.; Morris, G.A. Matrix-assisted diffusion-ordered spectroscopy: Application of surfactant solutions to the resolution of isomer spectra. Magn. Reson. Chem. 2012, 50, 458–465. [Google Scholar]
- Reile, I.; Aspers, R.L.E.G.; Feiters, M.C.; Rutjes, F.P.J.T.; Tessari, M. Resolving DOSY spectra of isomers by methanol-d4 solvent effects. Magn. Reson. Chem. 2017, 55, 759–762. [Google Scholar] [CrossRef] [PubMed]
- Gramosa, N.V.; Ricardo, N.M.S.P.; Adams, R.W.; Morris, G.A.; Nilsson, M. Matrix-assisted diffusion-ordered spectroscopy: Choosing a matrix. Magn. Reson. Chem. 2016, 54, 815–820. [Google Scholar] [CrossRef] [PubMed]
- Adams, R.W.; Aguilar, J.A.; Cassani, J.; Morris, G.A.; Nilsson, M. Resolving natural product epimer spectra by matrix-assisted DOSY. Org. Biomol. Chem. 2011, 20, 7062–7064. [Google Scholar] [CrossRef] [PubMed]
- Codling, D.J.; Zheng, G.; Stait-Gardner, T.; Yang, S.; Nilsson, M.; Price, W.S. Diffusion Studies of Dihydroxybenzene Isomers in Water−Alcohol Systems. J. Phys. Chem. B 2013, 117, 2734–2741. [Google Scholar] [CrossRef] [PubMed]
- Chaudhari, S.R.; Suryaprakash, N. Diffusion ordered spectroscopy for resolution of double bonded cis, trans-isomers. J. Mol. Struct. 2012, 1017, 106–108. [Google Scholar] [CrossRef]
- Viel, S.; Capitani, D.; Mannina, L.; Segre, A. Diffusion-Ordered NMR Spectroscopy: A Versatile Tool for the Molecular Weight Determination of Uncharged Polysaccharides. Biomacromolecules 2003, 4, 1843–1847. [Google Scholar] [CrossRef] [PubMed]
- Groves, P.; Rasmussen, M.O.; Molero, M.D.; Samain, E.; Cañada, F.J.; Driguez, H.; Jiménez-Barbero, J. Diffusion ordered spectroscopy as a complement to size exclusion chromatography in oligosaccharide analysis. Glycobiology 2004, 14, 451–456. [Google Scholar] [CrossRef] [PubMed]
- Cao, R.; Komura, F.; Nonaka, A.; Kato, T.; Fukumashi, J.; Matsui, T. Quantitative analysis of D-(+)-glucose in fruit juices using diffusion ordered-1H nuclear magnetic resonance spectroscopy. Anal. Sci. 2014, 30, 383–388. [Google Scholar] [CrossRef] [PubMed]
- Politi, M.; Groves, P.; Chávez, M.I.; Cañada, F.J.; Jiménez-Barberoa, J. Useful applications of DOSY experiments for the study of mushroom polysaccharides. Carbohydr. Res. 2006, 341, 84–89. [Google Scholar] [CrossRef] [PubMed]
- Kählig, H.; Dietrich, K.; Dorner, S. Analysis of Carbohydrate Mixtures by Diffusion Difference NMR Spectroscopy. Monatsh. Chem. 2002, 133, 589–598. [Google Scholar] [CrossRef]
- Díaz, M.D.; Berger, S. Studies of the complexation of sugars by diffusion-ordered NMR spectroscopy. Carbohydr. Res. 2000, 329, 1–5. [Google Scholar] [CrossRef]
- Nagy, L.; Gyetvai, G.; Nagy, G. Determination of the Diffusion Coefficient of Monosaccharides with Scanning Electrochemical Microscopy (SECM). Electroanalysis 2009, 21, 542–549. [Google Scholar] [CrossRef]
- Mori, N.; Sugai, E.; Fuse, Y.; Funazukuri, T. Infinite Dilution Binary Diffusion Coefficients for Six Sugars at 0.1 MPa and Temperatures from (273.2 to 353.2). J. Chem. Eng. Data 2007, 52, 40–43. [Google Scholar]
- Ihnat, M.; Goring, D.A.I. Shape of the cellodextrins in aqueous solution at 25 °C. Can. J. Chem. 1967, 45, 2353–2361. [Google Scholar] [CrossRef]
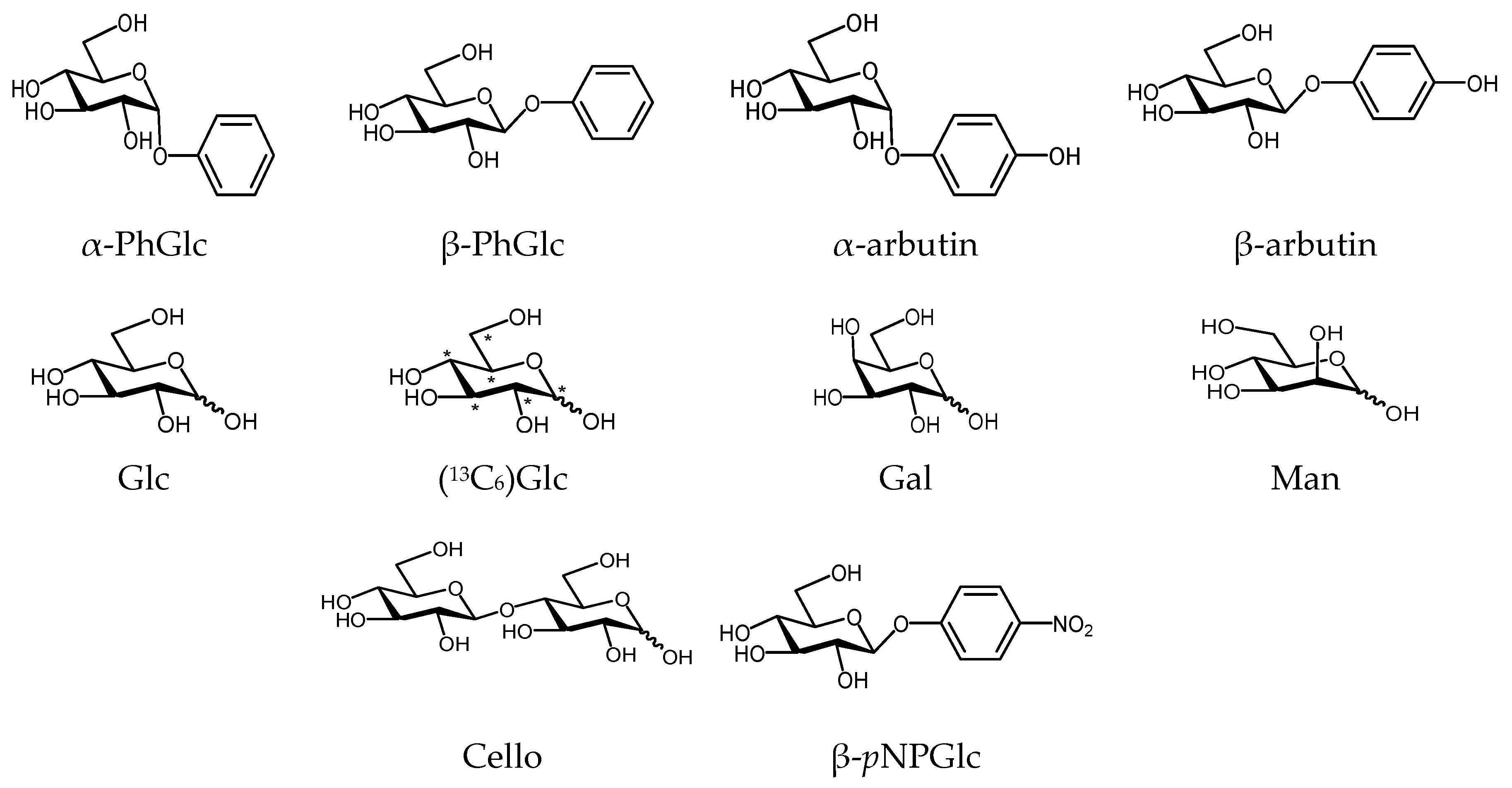
| Entry | Carbohydrate (Conditions, 30 °C, D2O) | Diffusion Coefficient D (× 10−10) m2·s−1 | Reported Diffusion Coefficient D (× 10−10) m2·s−1 (Conditions) [Lit.] |
|---|---|---|---|
| 1 | α-PhGlc (10 mM) | 5.6 | - |
| β-PhGlc (10 mM) | 5.9 | - | |
| 2 | α-arbutin (10 mM) | 5.9 | - |
| β-arbutin (10 mM) | 5.8 | - | |
| 3 | Glc (20 mM) | - | 6.3 (100 mM, H2O) [21] |
| α-anomer | 7.6 | - | |
| β-anomer | 5.8 | - | |
| 4 | (13C6)Glc (20 mM) | - | |
| α-anomer | 9 | ||
| β-anomer | 5.4 | ||
| 5 | Gal (20 mM) | - | 8.7 (100 mM, H2O) [21] |
| α-anomer | 10.9 | - | |
| β-anomer | 5.8 | - | |
| 6 | Man (20 mM) | - | 7.0 (25 °C, H2O) [22] |
| α-anomer | 7.4 | - | |
| β-anomer | 6.85 | - | |
| 7 | Cello (20 mM) | - | 5.2 (25 °C, H2O) [23] |
| α-anomer | 5. 5 | - | |
| β-anomer | 5.95 | - | |
| 8 | α-PhGlc (10 mM) | 5.77 | - |
| α-arbutin (10 mM) | 5.49 | - | |
| 9 | β-arbutin (10 mM) | 7.2 | - |
| β-pNPGlc (10 mM) | 5.6 | - |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yamanoi, T.; Oda, Y.; Katsuraya, K. Separation of the α- and β-Anomers of Carbohydrates by Diffusion-Ordered NMR Spectroscopy. Magnetochemistry 2017, 3, 38. https://doi.org/10.3390/magnetochemistry3040038
Yamanoi T, Oda Y, Katsuraya K. Separation of the α- and β-Anomers of Carbohydrates by Diffusion-Ordered NMR Spectroscopy. Magnetochemistry. 2017; 3(4):38. https://doi.org/10.3390/magnetochemistry3040038
Chicago/Turabian StyleYamanoi, Takashi, Yoshiki Oda, and Kaname Katsuraya. 2017. "Separation of the α- and β-Anomers of Carbohydrates by Diffusion-Ordered NMR Spectroscopy" Magnetochemistry 3, no. 4: 38. https://doi.org/10.3390/magnetochemistry3040038





