How to Make a Better Magnet? Insertion of Additional Bridging Ligands into a Magnetic Coordination Polymer
Abstract
:1. Introduction
2. Results and Discussion
2.1. Synthesis and X-ray Crystal Structure Description
2.2. IR Spectroscopy
2.3. Identity and Purity Confirmation by Powder X-ray Diffraction
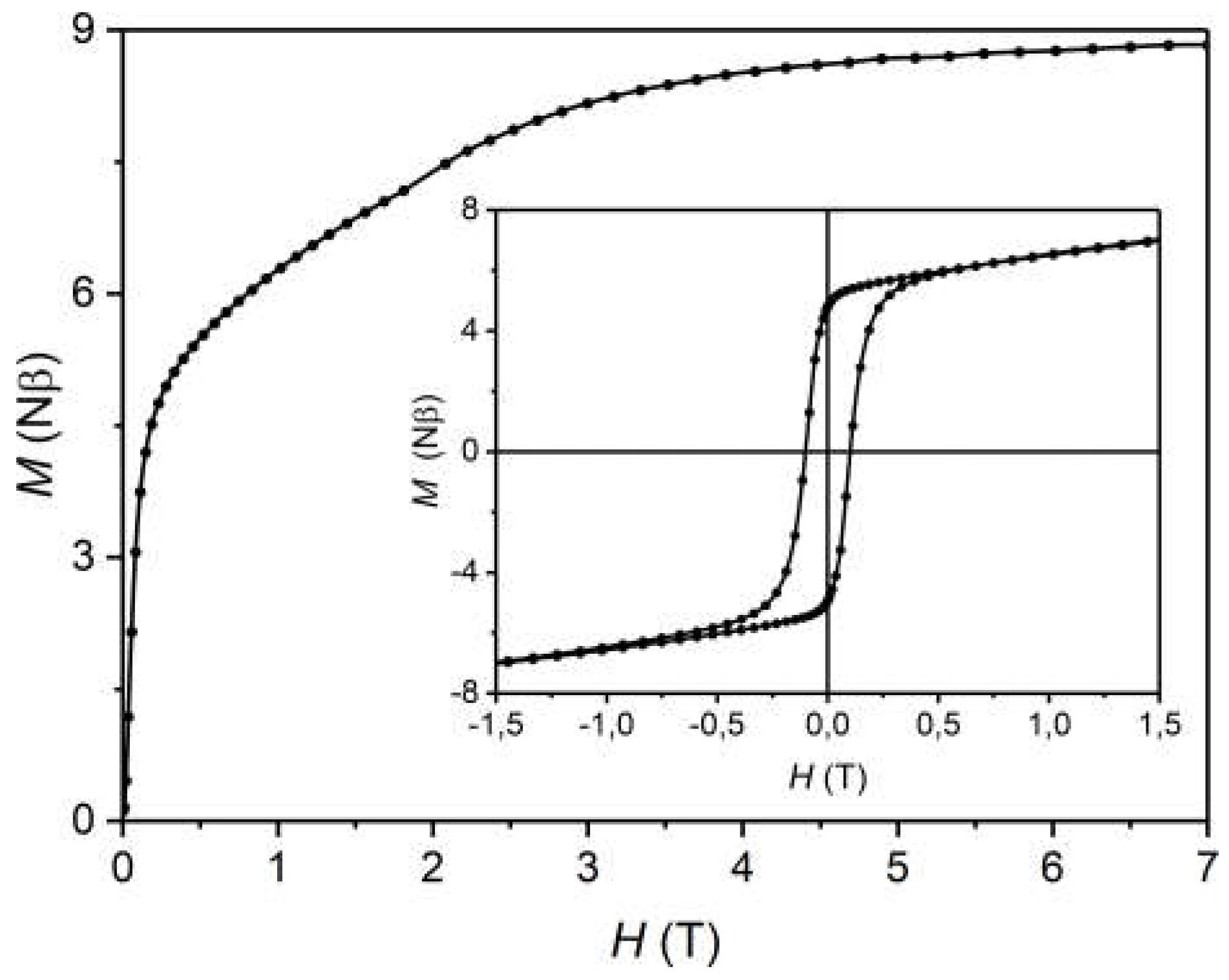
2.4. Magnetic Properties
3. Materials and Methods
3.1. Materials
3.2. Synthesis of {(NH4)[(H2O)FeII-(µ-HCOO)-FeII(H2O)][NbIV(CN)8]·3H2O}n (Fe2NbHCOO)
3.3. Single-Crystal X-ray Diffraction
3.4. Magnetic Measurements
3.5. Other Physical Measurements and Calculations
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Pinkowicz, D.; Czarnecki, B.; Reczyński, M.; Arczyński, M. Multifunctionality in Molecular Magnetism. Sci. Prog. 2015, 98, 346–378. [Google Scholar] [CrossRef] [PubMed]
- Miller, J.S.; Ohkoshi, S. High-Tc Ordered Molecular Magnets. In Molecular Magentic Materials. Concepts and Applications, 1st ed.; Sieklucka, B., Pinkowicz, D., Eds.; Wiley: Weinheim, Germany, 2017; pp. 161–186. ISBN 978-3-527-33953-2. [Google Scholar]
- Tokoro, H.; Ohkoshi, S. Novel magnetic functionalities of Prussian blue analogs. Dalton Trans. 2011, 40, 6825–6833. [Google Scholar] [CrossRef] [PubMed]
- Nowicka, B.; Korzeniak, T.; Stefańczyk, O.; Pinkowicz, D.; Chorąży, S.; Podgajny, R.; Sieklucka, B. The impact of ligands upon topology and functionality of octacyanidometallate-based assemblies. Coord. Chem. Rev. 2012, 256, 1946–1971. [Google Scholar] [CrossRef]
- Wang, X.-Y.; Avendaño, C.; Dunbar, K.R. Malecular magnetic materials based on 4d and 5d transition metals. Chem. Soc. Rev. 2011, 40, 3213–3238. [Google Scholar] [CrossRef] [PubMed]
- Entley, W.R.; Girolami, G.S. High-temperature molecular magnets based on cyanovanadate building blocks: Spontaneous magnetization at 230 K. Science 1995, 268, 397–402. [Google Scholar] [CrossRef] [PubMed]
- Imoto, K.; Takemura, M.; Tokoro, H.; Ohkoshi, S. A Cyano-Bridged Vanadium-Niobium Bimetal Assembly Exhibiting a High Curie Temperature of 210 K. Eur. J. Inorg. Chem. 2012, 2649–2652. [Google Scholar] [CrossRef]
- Mizuno, M.; Ohkoshi, S.; Hashimoto, K. Electrochemical Synthesis of High-Tc, Colored, Magnetic Thin Films Composed of Vanadium(II/III)-Chromium(II) Hexacyanochromate(III). Adv. Mater. 2000, 12, 1955–1958. [Google Scholar] [CrossRef]
- Ohkoshi, S.; Mizuno, M.; Hung, G.J.; Hashimoto, K. Magnetooptical Effects of Room Temperature Molecular-Based Magnetic Films Composed of Vanadium Hexacyanochromates. J. Phys. Chem. B 2000, 104, 9365–9367. [Google Scholar] [CrossRef]
- Ferlay, S.; Mallah, T.; Ouahés, R.; Veillet, P.; Verdaguer, M. A room-temperature organometallic magnet based on Prossian blue. Nature 1995, 378, 701–703. [Google Scholar] [CrossRef]
- Holmes, S.M.; Girolami, G.S. Sol-Gel Synthesis of KVII[CrIII(CN)6]·2H2O: A Crystalline Molecule-Based Magnet with a Magnetic Ordering Temperature above 100 °C. J. Am. Chem. Soc. 1999, 121, 5593–5594. [Google Scholar] [CrossRef]
- Hatlevik, Ø.; Buschmann, W.E.; Zhang, J.; Manson, J.L.; Miller, J.S. Enhancement of the Magnetic Ordering Temperature and Air Stability of a Mixed Valent Vanadium Hexacyanochromate(III) Magnet to 99 °C (372 K). Adv. Mater. 1999, 11, 914–918. [Google Scholar] [CrossRef]
- Ohkoshi, S.-I.; Iyoda, T.; Fujishima, A.; Hashimoto, K. Magnetic properties of mixed ferro-ferrimagnets composed of Prussian blue analogs. Phys. Rev. B 1997, 56, 11642–11652. [Google Scholar] [CrossRef]
- Pinkowicz, D.; Podgajny, R.; Pełka, R.; Nitek, W.; Bałanda, M.; Makarewicz, M.; Czapla, M.; Żukowski, J.; Kapusta, C.; Zając, D.; et al. Iron(II)-octacyanoniobate(IV) ferromagnet with TC 43 K. Dalton Trans. 2009, 7771–7777. [Google Scholar] [CrossRef] [PubMed]
- Handzlik, G.; Sieklucka, B.; Pinkowicz, D. Cross-linking of cyanide magnetic coordination polymers by rational insertion of formate, cyanide or azide. Dalton Trans. 2018. [Google Scholar] [CrossRef] [PubMed]
- Herrera, J.M.; Franz, P.; Podgajny, R.; Pilkington, M.; Biner, M.; Decurtins, S.; Stoeckli-Evans, H.; Neels, A.; Grade, R.; Dromzee, Y.; et al. Three-dimensional bimetallic octacyanidometalates [MIV{(μ-CN)4MnII(H2O)2}2·4H2O]n (M=Nb, Mo, W): Synthesis, single-crystal X-ray diffraction and magnetism. C. R. Chim. 2008, 11, 1192. [Google Scholar] [CrossRef]
- Alvarez, S.; Alemany, P.; Casanova, D.; Cirera, J.; Llunell, M.; Avnir, D. Shape maps and polyhedral interconversion paths in transition metal chemistry. Coord. Chem. Rev. 2005, 249, 1693–1708. [Google Scholar] [CrossRef]
- Handzlik, G.; Magott, M.; Sieklucka, B.; Pinkowicz, D. Alternative Synthetic Route to Potassium Octacyanidoniobate(IV) and Its Molybdenum Congener. Eur. J. Inorg. Chem. 2016, 30, 4872–4877. [Google Scholar] [CrossRef]
- Sheldrick, G.M. A short history of SHELX. Acta Crystallogr. Sect. A: Found. Crystallogr. 2008, 64, 112–122. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. Sect. C: Struct. Chem. 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Llunell, M.; Casanova, D.; Cirera, J.; Alemany, P.; Alvarez, S. SHAPE v. 2.1; University of Barcelona: Barcelona, Spain, 2013. [Google Scholar]

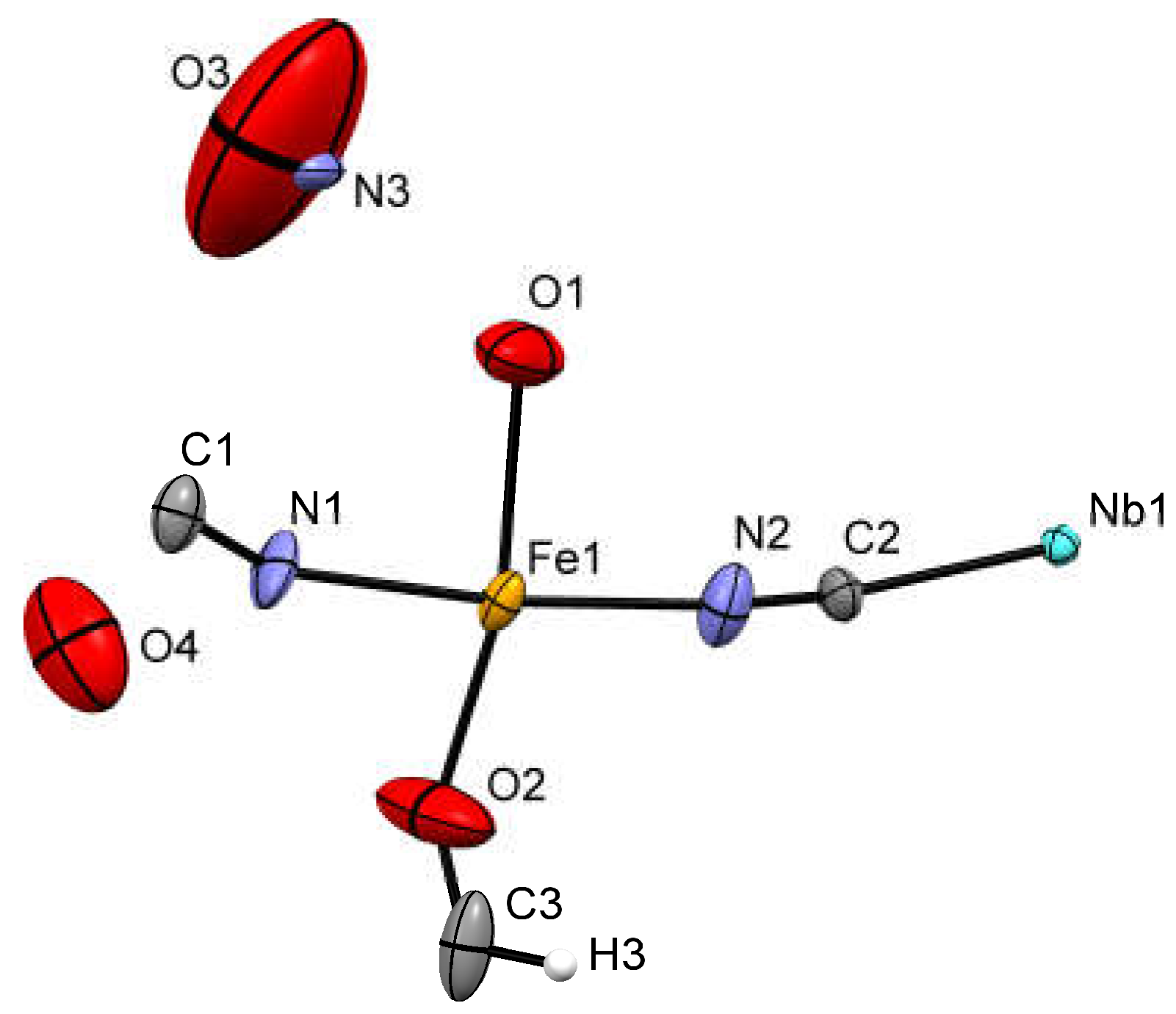






| Formula | C9HFe2N9NbO7 |
| Temperature, K | 296(2) |
| λ, Å | 0.71073 Å |
| Molecular weight, g/mol | 551.80 |
| Crystallographic system | tetragonal |
| Space group | I4cm |
| Unit cell | a = 11.8782(12) Å b = 13.2770(14) Å |
| Volume V, Å3 | 1873.3(4) |
| Z | 4 |
| Density ρcalc, g/cm3 | 1.957 |
| F (000) | 1068 |
| θ, deg | 3.4–28.0 |
| Abs. coeff. µ, mm−1 | 2.18 |
| Data/parameters /restrains | 1211/81/8 |
| R [F2 > 2σ(F2)] | 0.032 |
| wR (F2) | 0.064 |
| GOF on F2 | 1.103 |
| Flack parameter | 0.472(25) |
| max/min resid. density, e· Å−3 | 1.13/−0.59 |
| Reflections collected | 13274 |
| Unique reflections | 1211 |
| Rint | 0.048 |
| Completeness, % | 99.6 |
| Atom Names | Fe2Nb | Fe2NbHCOO |
|---|---|---|
| Nb···Fe | 5.409 5.508 | 5.461 5.480 |
| Nb···Feav | 5.459 | 5.471 |
| Fe···Fe | 6.609(1) | 6.141(1) |
| Fe-NCN | 2.144(2) 2.175(2) | 2.119(10) 2.171(10) |
| Fe-N-C | 154.5(2) 167.5(2) | 157.8(11) 170.0(9) |
| Nb-C-N | 174.6(2) 176.7(2) | 173.0(10) 178.4(11) |
| Fe-Oaq | 2.099(3) 2.130(3) | 2.191(9) |
| Fe-OHCOO | - | 1.977(9) |
| NbIV | Square antiprism (SAPR-8) | 0.192 | 0.113 |
| FeII | Octahedron (OC-6) | 0.161 | 0.562 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Handzlik, G.; Pinkowicz, D. How to Make a Better Magnet? Insertion of Additional Bridging Ligands into a Magnetic Coordination Polymer. Magnetochemistry 2018, 4, 41. https://doi.org/10.3390/magnetochemistry4030041
Handzlik G, Pinkowicz D. How to Make a Better Magnet? Insertion of Additional Bridging Ligands into a Magnetic Coordination Polymer. Magnetochemistry. 2018; 4(3):41. https://doi.org/10.3390/magnetochemistry4030041
Chicago/Turabian StyleHandzlik, Gabriela, and Dawid Pinkowicz. 2018. "How to Make a Better Magnet? Insertion of Additional Bridging Ligands into a Magnetic Coordination Polymer" Magnetochemistry 4, no. 3: 41. https://doi.org/10.3390/magnetochemistry4030041





