Recent Applications of Benchtop Nuclear Magnetic Resonance Spectroscopy
Abstract
:1. Introduction
2. Applications of Benchtop NMR Spectroscopy
2.1. Foods
2.2. Pharmaceuticals and Drugs
2.3. Polymer Materials
2.4. Monitoring Tools
2.4.1. Process and Reaction Monitoring
2.4.2. Bioprocess Monitoring
2.4.3. Disease Monitoring
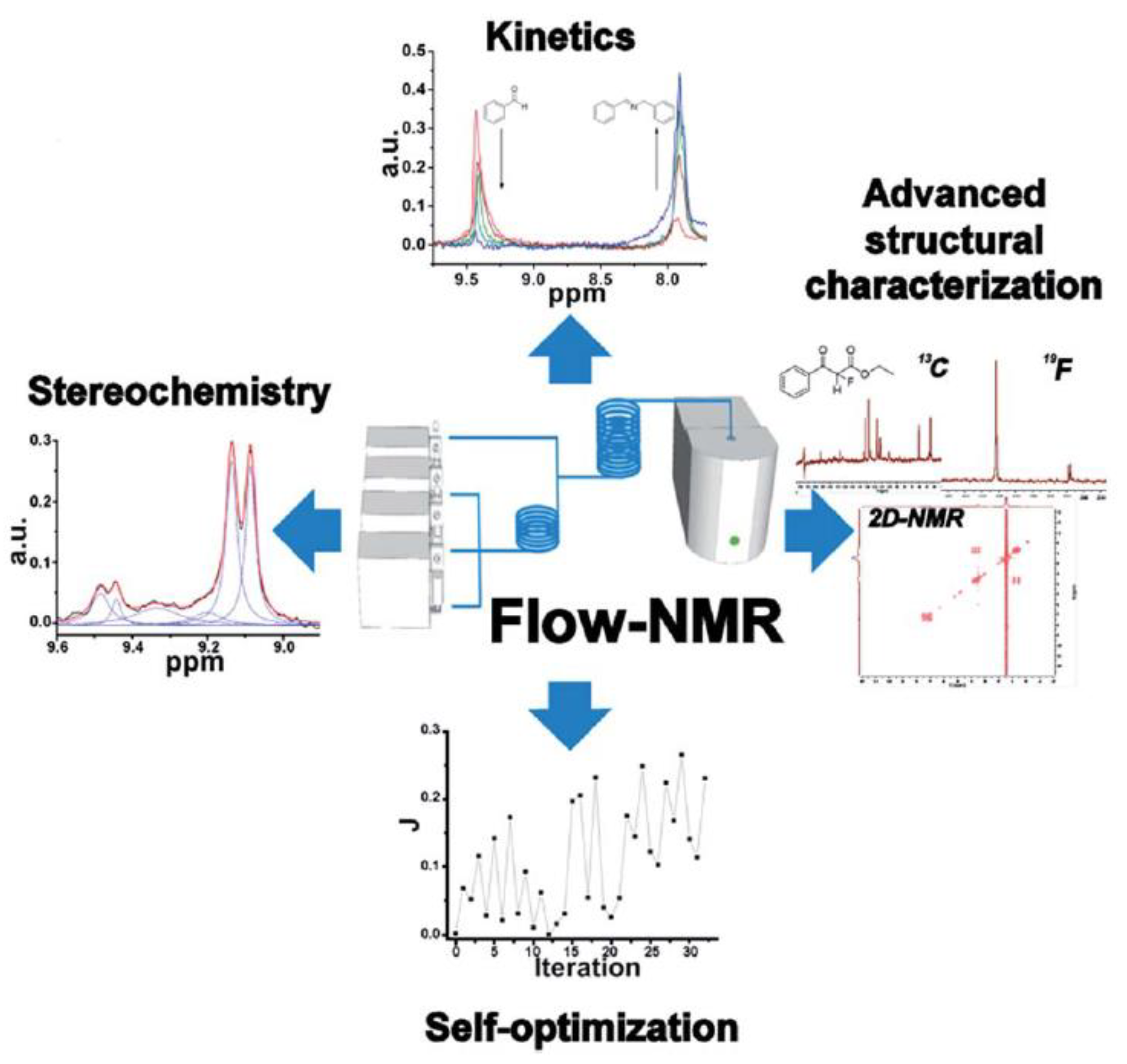
3. Recent Advanced Methodologies in Benchtop NMR Spectroscopy
3.1. Techniques for Sensitivity and Resolution Enhancement
3.2. Multidimensional NMR Spectroscopy
3.3. Heteroatom NMR Spectroscopy
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gunther, H.; Gunther, H. NMR Spectroscopy: Basic Principles, Concepts, and Applications in Chemistry; John Wiley & Sons: Chichester, UK, 1994. [Google Scholar]
- Claridge, T.D. High-Resolution NMR Techniques in Organic Chemistry; Elsevier: Amsterdam, The Netherlands, 2016; Volume 27. [Google Scholar]
- James, T.L. Fundamentals of NMR; Online Textbook; Department of Pharmaceutical Chemistry, University of California: San Francisco, CA, USA, 1998; pp. 1–31. [Google Scholar]
- Blumich, B. Introduction to compact NMR: A review of methods. Trac-Trend Anal. Chem. 2016, 83, 2–11. [Google Scholar] [CrossRef]
- Blümich, B. Low-field and benchtop NMR. J. Magn. Reson. 2019, 306, 27–35. [Google Scholar] [CrossRef] [PubMed]
- Zalesskiy, S.S.; Danieli, E.; Blumich, B.; Ananikov, V.P. Miniaturization of NMR systems: Desktop spectrometers, microcoil spectroscopy, and “NMR on a chip” for chemistry, biochemistry, and industry. Chem. Rev. 2014, 114, 5641–5694. [Google Scholar] [CrossRef] [PubMed]
- Blümich, B.; Singh, K. Desktop NMR and its applications from materials science to organic chemistry. Angew. Chem. Int. Ed. 2018, 57, 6996–7010. [Google Scholar] [CrossRef]
- Grootveld, M.; Percival, B.; Gibson, M.; Osman, Y.; Edgar, M.; Molinari, M.; Mather, M.L.; Casanova, F.; Wilson, P.B. Progress in low-field benchtop NMR spectroscopy in chemical and biochemical analysis. Anal. Chim. Acta 2019, 1067, 11–30. [Google Scholar] [CrossRef] [Green Version]
- Blümich, B.; Perlo, J.; Casanova, F. Mobile single-sided NMR. Prog. Nucl. Magn. Reson. Spectrosc. 2008, 52, 197–269. [Google Scholar] [CrossRef]
- Singh, K.; Blumich, B. NMR spectroscopy with compact instruments. Trac-Trend Anal. Chem. 2016, 83, 12–26. [Google Scholar] [CrossRef]
- Hatzakis, E. Nuclear Magnetic Resonance (NMR) spectroscopy in food science: A comprehensive review. Compr. Rev. Food Sci. F 2019, 18, 189–220. [Google Scholar] [CrossRef] [Green Version]
- Defernez, M.; Colquhoun, I.J. Factors affecting the robustness of metabolite fingerprinting using 1H NMR spectra. Phytochemistry 2003, 62, 1009–1017. [Google Scholar] [CrossRef]
- Sobolev, A.P.; Thomas, F.; Donarski, J.; Ingallina, C.; Circi, S.; Marincola, F.C.; Capitani, D.; Mannina, L. Use of NMR applications to tackle future food fraud issues. Trends Food Sci. Technol. 2019, 91, 347–353. [Google Scholar] [CrossRef] [Green Version]
- McDowell, D.; Defernez, M.; Kemsley, E.K.; Elliott, C.T.; Koidis, A. Low vs. high field 1h Nmr spectroscopy for the detection of adulteration of cold pressed rapeseed oil with refined oils. LWT 2019, 111, 490–499. [Google Scholar] [CrossRef]
- Kim, J.H.; Lee, H.J.; Kwon, K.; Chun, H.S.; Ahn, S.; Kim, B.H. A 43 MHz low-field benchtop 1H nuclear magnetic resonance method to discriminate perilla oil authenticity. J. Oleo Sci. 2018, 67, 507–513. [Google Scholar] [CrossRef] [Green Version]
- Defernez, M.; Wren, E.; Watson, A.D.; Gunning, Y.; Colquhoun, I.J.; Le Gall, G.; Williamson, D.; Kemsley, E.K. Low-field 1H NMR spectroscopy for distinguishing between arabica and robusta ground roast coffees. Food Chem. 2017, 216, 106–113. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Isaac-Lam, M.F. Determination of alcohol content in alcoholic beverages using 45 MHz benchtop NMR spectrometer. Int. J. Spectrosc. 2016, 2016, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Rudszuck, T.; Foerster, E.; Nirschl, H.; Guthausen, G. Low-field NMR for quality control on oils. Magn. Reson. Chem. 2019, 57, 777–793. [Google Scholar] [CrossRef] [PubMed]
- Van Beek, T.A. Low-field benchtop NMR spectroscopy: Status and prospects in natural product analysis. Phytochem. Anal. 2021, 32, 24–37. [Google Scholar] [CrossRef] [Green Version]
- Parker, T.; Limer, E.; Watson, A.D.; Defernez, M.; Williamson, D.; Kemsley, E.K. 60 MHz 1H NMR spectroscopy for the analysis of edible oils. Trends Anal. Chem. 2014, 57, 147–158. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krause, A.; Wu, Y.; Tian, R.; van Beek, T.A. Is low-field NMR a complementary tool to GC-MS in quality control of essential oils? A case study: Patchouli essential oil. Planta Med. 2018, 84, 953–963. [Google Scholar] [CrossRef] [Green Version]
- Gerdova, A.; Defernez, M.; Jakes, W.; Limer, E.; McCallum, C.; Nott, K.; Parker, T.; Rigby, N.; Sagidullin, A.; Watson, A. 60 MHz 1H NMR spectroscopy of triglyceride mixtures. In Magnetic Resonance in Food Science: Defining Food by Magnetic Resonance; Capozzi, F., Laghi, L., Belton., P.S., Eds.; Royal Society of Chemistry: London, UK, 2015; pp. 17–30. [Google Scholar]
- Gunning, Y.; Jackson, A.J.; Colmer, J.; Taous, F.; Philo, M.; Brignall, R.M.; El Ghali, T.; Defernez, M.; Kemsley, E.K. High-throughput screening of argan oil composition and authenticity using benchtop 1H NMR. Magn. Reson. Chem. 2020, 58, 1177–1186. [Google Scholar] [CrossRef] [Green Version]
- Jakes, W.; Gerdova, A.; Defernez, M.; Watson, A.; McCallum, C.; Limer, E.; Colquhoun, I.; Williamson, D.; Kemsley, E. Authentication of beef versus horse meat using 60 MHz 1H NMR spectroscopy. Food Chem. 2015, 175, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Gunning, Y.; Defernez, M.; Watson, A.D.; Beadman, N.; Colquhoun, I.J.; Le Gall, G.; Philo, M.; Garwood, H.; Williamson, D.; Davis, A.P. 16-O-methylcafestol is present in ground roast Arabica coffees: Implications for authenticity testing. Food Chem. 2018, 248, 52–60. [Google Scholar] [CrossRef]
- Soyler, A.; Cikrikci, S.; Cavdaroglu, C.; Bouillaud, D.; Farjon, J.; Giraudeau, P.; Oztop, M.H. Multi-scale benchtop 1H NMR spectroscopy for milk analysis. LWT 2021, 139, 1–10. [Google Scholar] [CrossRef]
- Matviychuk, Y.; Yeo, J.; Holland, D.J. A field-invariant method for quantitative analysis with benchtop NMR. J. Magn. Reson. 2019, 298, 35–47. [Google Scholar] [CrossRef]
- Burkhardtsmaier, P.; Pavlovskaja, K.; Maier, D.; Schäfer, S.; Salat, U.; Schmidt, M.S. Quantitative monitoring of the fermentation process of a barley malt mash by benchtop 1H NMR spectroscopy. Food Anal. Methods 2021, 1–7. [Google Scholar] [CrossRef]
- Santos, P.M.; Pereira-Filho, E.R.; Colnago, L.A. Detection and quantification of milk adulteration using time domain nuclear magnetic resonance (TD-NMR). Microchem. J. 2016, 124, 15–19. [Google Scholar] [CrossRef]
- Bouillaud, D.; Heredia, V.; Castaing-Cordier, T.; Drouin, D.; Charrier, B.; Goncalves, O.; Farjon, J.; Giraudeau, P. Benchtop flow NMR spectroscopy as an online device for the in vivo monitoring of lipid accumulation in microalgae. Algal Res. 2019, 43, 1–6. [Google Scholar] [CrossRef]
- Duffy, J.; Urbas, A.; Niemitz, M.; Lippa, K.; Marginean, I. Differentiation of fentanyl analogues by low-field NMR spectroscopy. Anal. Chim. Acta 2019, 1049, 161–169. [Google Scholar] [CrossRef]
- Pagès, G.; Gerdova, A.; Williamson, D.; Gilard, V.; Martino, R.; Malet-Martino, M. Evaluation of a benchtop cryogen-free low-field 1H NMR spectrometer for the analysis of sexual enhancement and weight loss dietary supplements adulterated with pharmaceutical substances. Anal. Chem. 2014, 86, 11897–11904. [Google Scholar] [CrossRef]
- Wu, N.; Balayssac, S.; Danoun, S.; Malet-Martino, M.; Gilard, V. Chemometric analysis of low-field 1H NMR spectra for unveiling adulteration of slimming dietary supplements by pharmaceutical compounds. Molecules 2020, 25, 193. [Google Scholar] [CrossRef] [Green Version]
- Assemat, G.; Balayssac, S.; Gerdova, A.; Gilard, V.; Caillet, C.; Williamson, D.; Malet-Martino, M. Benchtop low-field 1H Nuclear magnetic resonance for detecting falsified medicines. Talanta 2019, 196, 163–173. [Google Scholar] [CrossRef]
- Santos, A.D.C.; Dutra, L.M.; Menezes, L.R.A.; Santos, M.F.C.; Barison, A. Forensic NMR spectroscopy: Just a beginning of a promising partnership. Trac-Trend Anal. Chem. 2018, 107, 31–42. [Google Scholar] [CrossRef]
- Castaing-Cordier, T.; Ladroue, V.; Besacier, F.; Bulete, A.; Jacquemin, D.; Giraudeau, P.; Farjon, J. High-field and benchtop NMR spectroscopy for the characterization of new psychoactive substances. Forensic Sci. Int. 2021, 321, 1–8. [Google Scholar] [CrossRef]
- Assemat, G.; Dubocq, F.; Balayssac, S.; Lamoureux, C.; Malet-Martino, M.; Gilard, V. Screening of “spice” herbal mixtures: From high-field to low-field proton NMR. Forensic Sci. Int. 2017, 279, 88–95. [Google Scholar] [CrossRef]
- Zhong, Y.; Huang, K.; Luo, Q.; Yao, S.; Liu, X.; Yang, N.; Lin, C.; Luo, X. The application of a desktop NMR spectrometer in drug analysis. Int. J. Anal. Chem. 2018, 2018, 1–7. [Google Scholar] [CrossRef]
- Araneda, J.F.; Chu, T.; Leclerc, M.C.; Riegel, S.D.; Spingarn, N. Quantitative analysis of cannabinoids using benchtop NMR instruments. Anal. Methods-UK 2020, 12, 4853–4857. [Google Scholar] [CrossRef]
- Hussain, J.H.; Gilbert, N.; Costello, A.; Schofield, C.J.; Kemsley, E.K.; Sutcliffe, O.B.; Mewis, R.E. Quantification of MDMA in seized tablets using benchtop 1H NMR spectroscopy in the absence of internal standards. Forensic Chem. 2020, 20, 1–9. [Google Scholar] [CrossRef]
- Keizers, P.H.; Bakker, F.; Ferreira, J.; Wackers, P.F.; van Kollenburg, D.; van der Aa, E.; van Beers, A. Benchtop NMR spectroscopy in the analysis of substandard and falsified medicines as well as illegal drugs. J. Pharm. Biomed. Anal. 2020, 178, 1–10. [Google Scholar] [CrossRef]
- Bogun, B.; Moore, S. 1H and 31P benchtop NMR of liquids and solids used in and/or produced during the manufacture of methamphetamine by the HI reduction of pseudoephedrine/ephedrine. Forensic Sci. Int. 2017, 278, 68–77. [Google Scholar] [CrossRef]
- Gilbert, N.; Mewis, R.E.; Sutcliffe, O.B. Fast & fluorinated—Development and validation of a rapid benchtop NMR approach and other routine screening methods for the detection and quantification of synthesized fluorofentanyl derivatives. Forensic Chem. 2021, 23, 1–12. [Google Scholar] [CrossRef]
- Hulme, M.C.; Hayatbakhsh, A.; Brignall, R.M.; Gilbert, N.; Costello, A.; Schofield, C.J.; Williamson, D.C.; Kemsley, E.K.; Sutcliffe, O.B.; Mewis, R.E. Detection, discrimination and quantification of amphetamine, cathinone and nor-ephedrine regioisomers using benchtop 1H and 19F NMR spectroscopy. Magn. Reson. Chem. 2021, 1–10. [Google Scholar] [CrossRef]
- Antonides, L.H.; Brignall, R.M.; Costello, A.; Ellison, J.; Firth, S.E.; Gilbert, N.; Groom, B.J.; Hudson, S.J.; Hulme, M.C.; Marron, J. Rapid identification of novel psychoactive and other controlled substances using low-field 1H NMR spectroscopy. ACS Omega 2019, 4, 7103–7112. [Google Scholar] [CrossRef] [Green Version]
- Scheirs, J. Compositional and Failure Analysis of Polymers: A Practical Approach; John Wiley & Sons: Hoboken, NJ, USA, 2000. [Google Scholar]
- Ibbett, R.N. NMR Spectroscopy of Polymers; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2012. [Google Scholar]
- Duchowny, A.; Adams, A. Compact NMR spectroscopy for low-cost identification and quantification of PVC plasticizers. Molecules 2021, 26, 1221. [Google Scholar] [CrossRef] [PubMed]
- Singh, K.; Blümich, B. Compact low-field NMR spectroscopy and chemometrics: A tool box for quality control of raw rubber. Polymer 2018, 141, 154–165. [Google Scholar] [CrossRef]
- Chakrapani, S.B.; Minkler, M.J.; Beckingham, B.S. Low-field 1H-NMR spectroscopy for compositional analysis of multicomponent polymer systems. Analyst 2019, 144, 1679–1686. [Google Scholar] [CrossRef]
- Singh, K.; Bluemich, B. Desktop NMR spectroscopy for quality control of raw rubber. Macromol. Symp. 2016, 365, 191–193. [Google Scholar] [CrossRef]
- Minkler, M.J., Jr.; Kim, J.M.; Shinde, V.V.; Beckingham, B.S. Low-field 1H NMR spectroscopy: Factors impacting signal-to-noise ratio and experimental time in the context of mixed microstructure polyisoprenes. Magn. Reson. Chem. 2020, 58, 1168–1176. [Google Scholar] [CrossRef] [PubMed]
- Adams, A. Non-destructive analysis of polymers and polymer-based materials by compact NMR. Magn. Reson. Imaging 2019, 56, 119–125. [Google Scholar] [CrossRef]
- Vargas, M.A.; Cudaj, M.; Hailu, K.; Sachsenheimer, K.; Guthausen, G. Online low-field 1H NMR spectroscopy: Monitoring of emulsion polymerization of butyl acrylate. Macromolecules 2010, 43, 5561–5568. [Google Scholar] [CrossRef]
- Knox, S.T.; Parkinson, S.; Stone, R.; Warren, N.J. Benchtop flow-NMR for rapid online monitoring of RAFT and free radical polymerisation in batch and continuous reactors. Polym. Chem. 2019, 10, 4774–4778. [Google Scholar] [CrossRef]
- Rubens, M.; Van Herck, J.; Junkers, T. Automated Polymer synthesis platform for integrated conversion targeting based on inline benchtop NMR. ACS Macro Lett. 2019, 8, 1437–1441. [Google Scholar] [CrossRef]
- Cudaj, M.; Guthausen, G.; Hofe, T.; Wilhelm, M. Online coupling of size-exclusion chromatography and low-field 1H NMR spectroscopy. Macromol. Chem. Phys. 2012, 213, 1933–1943. [Google Scholar] [CrossRef]
- Höpfner, J.; Ratzsch, K.F.; Botha, C.; Wilhelm, M. Medium resolution 1H-NMR at 62 MHz as a new chemically sensitive online detector for size-exclusion chromatography (SEC–NMR). Macromol. Rapid Commun. 2018, 39, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Botha, C.; Hopfner, J.; Mayerhofer, B.; Wilhelm, M. On-line SEC-MR-NMR hyphenation: Optimization of sensitivity and selectivity on a 62 MHz benchtop NMR spectrometer. Polym. Chem. 2019, 10, 2230–2246. [Google Scholar] [CrossRef] [Green Version]
- Höpfner, J.; Mayerhöfer, B.; Botha, C.; Bouillaud, D.; Farjon, J.; Giraudeau, P.; Wilhelm, M. Solvent suppression techniques for coupling of size exclusion chromatography and 1H NMR using benchtop spectrometers at 43 and 62 MHz. J. Magn. Reson. 2021, 323, 1–13. [Google Scholar] [CrossRef]
- Meyer, K.; Kern, S.; Zientek, N.; Guthausen, G.; Maiwald, M. Process control with compact NMR. TrAC Trends Anal. Chem. 2016, 83, 39–52. [Google Scholar] [CrossRef]
- Guthausen, G.; von Garnier, A.; Reimert, R. Investigation of hydrogenation of toluene to methylcyclohexane in a trickle bed reactor by low-field nuclear magnetic resonance spectroscopy. Appl. Spectrosc. 2009, 63, 1121–1127. [Google Scholar] [CrossRef]
- Danieli, E.; Perlo, J.; Duchateau, A.; Verzijl, G.; Litvinov, V.; Blümich, B.; Casanova, F. On-line monitoring of chemical reactions by using bench-top nuclear magnetic resonance spectroscopy. ChemPhysChem 2014, 15, 3060–3066. [Google Scholar] [CrossRef]
- Goldbach, M.; Danieli, E.; Perlo, J.; Kaptein, B.; Litvinov, V.M.; Blümich, B.; Casanova, F.; Duchateau, A.L. Preparation of Grignard reagents from magnesium metal under continuous flow conditions and on-line monitoring by NMR spectroscopy. Tetrahedron Lett. 2016, 57, 122–125. [Google Scholar] [CrossRef]
- Silva Elipe, M.V.; Milburn, R.R. Monitoring chemical reactions by low-field benchtop NMR at 45 MHz: Pros and cons. Magn. Reson. Chem. 2016, 54, 437–443. [Google Scholar] [CrossRef]
- Ahmed-Omer, B.; Sliwinski, E.; Cerroti, J.P.; Ley, S.V. Continuous processing and efficient in situ reaction monitoring of a hypervalent iodine (III) mediated cyclopropanation using benchtop NMR spectroscopy. Org. Process Res. Dev. 2016, 20, 1603–1614. [Google Scholar] [CrossRef] [Green Version]
- Dalitz, F.; Kreckel, L.; Maiwald, M.; Guthausen, G. Quantitative medium-resolution NMR spectroscopy under non-equilibrium conditions, studied on the example of an esterification reaction. Appl. Magn. Reson. 2014, 45, 411–425. [Google Scholar] [CrossRef]
- Sagmeister, P.; Poms, J.; Williams, J.D.D.; Kappe, C.O. Multivariate analysis of inline benchtop NMR data enables rapid optimization of a complex nitration in flow. React. Chem. Eng. 2020, 5, 677–684. [Google Scholar] [CrossRef] [Green Version]
- Matviychuk, Y.; Steimers, E.; von Harbou, E.; Holland, D.J. Bayesian approach for automated quantitative analysis of benchtop NMR data. J. Magn. Reson. 2020, 319, 1–17. [Google Scholar] [CrossRef]
- Maschmeyer, T.; Prieto, P.L.; Grunert, S.; Hein, J.E. Exploration of continuous-flow benchtop NMR acquisition parameters and considerations for reaction monitoring. Magn. Reson. Chem. 2020, 58, 1234–1248. [Google Scholar] [CrossRef]
- Friebel, A.; Froscher, A.; Munnemann, K.; von Harbou, E.; Hasse, H. In situ measurement of liquid-liquid equilibria by medium field nuclear magnetic resonance. Fluid Phase Equilibr. 2017, 438, 44–52. [Google Scholar] [CrossRef]
- Romero, J.A.; Kazimierczuk, K.; Golowicz, D. Enhancing benchtop NMR spectroscopy by means of sample shifting. Analyst 2020, 145, 7406–7411. [Google Scholar] [CrossRef]
- Nestle, N.; Lim, Z.J.; Böhringer, T.; Abtmeyer, S.; Arenz, S.; Leinweber, F.C.; Weiß, T.; von Harbou, E. Taking compact NMR to monitoring real reactions in large-scale chemical industries—General considerations and learnings from a lab-scale test case. Magn. Reson. Chem. 2020, 58, 1213–1221. [Google Scholar] [CrossRef]
- Sans, V.; Porwol, L.; Dragone, V.; Cronin, L. A self optimizing synthetic organic reactor system using real-time in-line NMR spectroscopy. Chem. Sci. 2015, 6, 1258–1264. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kern, S.; Meyer, K.; Guhl, S.; Grasser, P.; Paul, A.; King, R.; Maiwald, M. Online low-field NMR spectroscopy for process control of an industrial lithiation reaction-automated data analysis. Anal. Bioanal. Chem. 2018, 410, 3349–3360. [Google Scholar] [CrossRef]
- Kern, S.; Wander, L.; Meyer, K.; Guhl, S.; Mukkula, A.R.G.; Holtkamp, M.; Salge, M.; Fleischer, C.; Weber, N.; King, R. Flexible automation with compact NMR spectroscopy for continuous production of pharmaceuticals. Anal. Bioanal. Chem. 2019, 411, 3037–3046. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Picard, B.; Gouilleux, B.; Lebleu, T.; Maddaluno, J.; Chataigner, I.; Penhoat, M.; Felpin, F.X.; Giraudeau, P.; Legros, J. Oxidative neutralization of mustard-gas simulants in an on-board flow device with in-line NMR monitoring. Angew. Chem. Int. Ed. 2017, 56, 7568–7572. [Google Scholar] [CrossRef] [PubMed]
- Friebel, A.; von Harbou, E.; Munnemann, K.; Hasse, H. Online process monitoring of a batch distillation by medium field NMR spectroscopy. Chem. Eng. Sci. 2020, 219, 1–8. [Google Scholar] [CrossRef]
- Kim, K.H.; Choi, J.W.; Kim, C.S.; Jeong, K. Parahydrogen-induced polarization in the hydrogenation of lignin-derived phenols using Wilkinson’s catalyst. Fuel 2019, 255, 1–5. [Google Scholar] [CrossRef]
- Leutzsch, M.; Sederman, A.J.; Gladden, L.F.; Mantle, M.D. In situ reaction monitoring in heterogeneous catalysts by a benchtop NMR spectrometer. Magn. Reson. Imaging 2019, 56, 138–143. [Google Scholar] [CrossRef] [Green Version]
- Claaßen, C.; Mack, K.; Rother, D. Benchtop NMR for online reaction monitoring of the biocatalytic synthesis of aromatic amino alcohols. ChemCatChem 2020, 12, 1190–1199. [Google Scholar] [CrossRef] [Green Version]
- Nantogma, S.; Joalland, B.; Wilkens, K.; Chekmenev, E.Y. Clinical-scale production of nearly pure (>98.5%) parahydrogen and quantification by benchtop NMR spectroscopy. Anal. Chem. 2021, 93, 3594–3601. [Google Scholar] [CrossRef]
- Dalitz, F.; Cudaj, M.; Maiwald, M.; Guthausen, G. Process and reaction monitoring by low-field NMR spectroscopy. Prog. Nucl. Magn. Reson. Spectrosc. 2012, 60, 52–70. [Google Scholar] [CrossRef]
- Mitchell, J.; Gladden, L.F.; Chandrasekera, T.C.; Fordham, E.J. Low-field permanent magnets for industrial process and quality control. Prog. Nucl. Magn. Reson. Spectrosc. 2014, 76, 1–60. [Google Scholar] [CrossRef]
- Gomez, M.V.; de la Hoz, A. NMR reaction monitoring in flow synthesis. Beilstein J. Org. Chem. 2017, 13, 285–300. [Google Scholar] [CrossRef] [Green Version]
- Nelder, J.A.; Mead, R. A simplex method for function minimization. Comput. J. 1965, 7, 308–313. [Google Scholar] [CrossRef]
- Giraudeau, P.; Felpin, F.-X. Flow reactors integrated with in-line monitoring using benchtop NMR spectroscopy. React. Chem. Eng. 2018, 3, 399–413. [Google Scholar] [CrossRef]
- Bouillaud, D.; Farjon, J.; Goncalves, O.; Giraudeau, P. Benchtop NMR for the monitoring of bioprocesses. Magn. Reson. Chem. 2019, 57, 794–804. [Google Scholar] [CrossRef]
- Kreyenschulte, D.; Paciok, E.; Regestein, L.; Blumich, B.; Buchs, J. Online monitoring of fermentation processes via non-invasive low-field NMR. Biotechnol. Bioeng. 2015, 112, 1810–1821. [Google Scholar] [CrossRef] [PubMed]
- Soyler, A.; Bouillaud, D.; Farjon, J.; Giraudeau, P.; Oztop, M.H. Real-time benchtop NMR spectroscopy for the online monitoring of sucrose hydrolysis. LWT Food Sci. Technol. 2020, 118, 1–7. [Google Scholar] [CrossRef]
- Anderssen, K.E.; McCarney, E.R. Online monitoring of enzymatic hydrolysis of marine by-products using benchtop nuclear magnetic resonance spectroscopy. Food Control 2020, 112, 1–10. [Google Scholar] [CrossRef]
- Bouillaud, D.; Drouin, D.; Charrier, B.; Jacquemmoz, C.; Farjon, J.; Giraudeau, P.; Goncalves, O. Using benchtop NMR spectroscopy as an online non-invasive in vivo lipid sensor for microalgae cultivated in photobioreactors. Process Biochem. 2020, 93, 63–68. [Google Scholar] [CrossRef]
- Linck, Y.G.; Killner, M.; Danieli, E.; Blümich, B. Mobile low-field 1H NMR spectroscopy desktop analysis of biodiesel production. Appl. Magn. Reson. 2013, 44, 41–53. [Google Scholar] [CrossRef]
- Killner, M.H.M.; Linck, Y.G.; Danieli, E.; Rohwedder, J.J.R.; Blumich, B. Compact NMR spectroscopy for real-time monitoring of a biodiesel production. Fuel 2015, 139, 240–247. [Google Scholar] [CrossRef] [Green Version]
- Killner, M.; Danieli, E.; Casanova, F.; Rohwedder, J.; Blümich, B. Mobile compact 1H NMR spectrometer promises fast quality control of diesel fuel. Fuel 2017, 203, 171–178. [Google Scholar] [CrossRef]
- Singh, K.; Kumar, S.P.; Blümich, B. Monitoring the mechanism and kinetics of a transesterification reaction for the biodiesel production with low field 1H NMR spectroscopy. Fuel 2019, 243, 192–201. [Google Scholar] [CrossRef]
- Gouilleux, B.; Charrier, B.; Akoka, S.; Giraudeau, P. Gradient-based solvent suppression methods on a benchtop spectrometer. Magn. Reson. Chem. 2017, 55, 91–98. [Google Scholar] [CrossRef]
- Emwas, A.H.; Roy, R.; McKay, R.T.; Tenori, L.; Saccenti, E.; Gowda, G.A.N.; Raftery, D.; Alahmari, F.; Jaremko, L.; Jaremko, M.; et al. NMR spectroscopy for metabolomics research. Metabolites 2019, 9, 123. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wishart, D.S. NMR metabolomics: A look ahead. J. Magn. Reson. 2019, 306, 155–161. [Google Scholar] [CrossRef]
- Holmes, E.; Wilson, I.D.; Nicholson, J.K. Metabolic phenotyping in health and disease. Cell 2008, 134, 714–717. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beckonert, O.; Keun, H.C.; Ebbels, T.M.; Bundy, J.; Holmes, E.; Lindon, J.C.; Nicholson, J.K. Metabolic profiling, metabolomic and metabonomic procedures for NMR spectroscopy of urine, plasma, serum and tissue extracts. Nat. Protoc. 2007, 2, 2692–2703. [Google Scholar] [CrossRef]
- Emwas, A.-H.; Luchinat, C.; Turano, P.; Tenori, L.; Roy, R.; Salek, R.M.; Ryan, D.; Merzaban, J.S.; Kaddurah-Daouk, R.; Zeri, A.C. Standardizing the experimental conditions for using urine in NMR-based metabolomic studies with a particular focus on diagnostic studies: A review. Metabolomics 2015, 11, 872–894. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Percival, B.C.; Grootveld, M.; Gibson, M.; Osman, Y.; Molinari, M.; Jafari, F.; Sahota, T.; Martin, M.; Casanova, F.; Mather, M.L. Low-field, benchtop NMR spectroscopy as a potential tool for point-of-care diagnostics of metabolic conditions: Validation, protocols and computational models. High-Throughput 2019, 8, 2. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leenders, J.; Grootveld, M.; Percival, B.; Gibson, M.; Casanova, F.; Wilson, P.B. Benchtop low-frequency 60 MHz NMR analysis of urine: A comparative metabolomics investigation. Metabolites 2020, 10, 155. [Google Scholar] [CrossRef] [Green Version]
- Edgar, M.; Percival, B.C.; Gibson, M.; Jafari, F.; Grootveld, M. Low-field benchtop NMR spectroscopy as a potential non-stationary tool for point-of-care urinary metabolite tracking in diabetic conditions. Diabetes Res. Clin. Pract. 2021, 171, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Stolz, M.; Schlawne, C.; Hoffmann, J.; Hartmann, V.; Marini, I.; Fritsche, A.; Peter, A.; Bakchoul, T.; Schick, F. Feasibility of precise and reliable glucose quantification in human whole blood samples by 1 tesla benchtop NMR. NMR Biomed. 2020, 33, 1–11. [Google Scholar] [CrossRef]
- Gouilleux, B.; Farjon, J.; Giraudeau, P. Gradient-based pulse sequences for benchtop NMR spectroscopy. J. Magn. Reson. 2020, 319, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Castaing-Cordier, T.; Bouillaud, D.; Bowyer, P.; Goncalves, O.; Giraudeau, P.; Farjon, J. Highly Resolved pure-shift spectra on a compact NMR spectrometer. ChemPhysChem 2019, 20, 736–744. [Google Scholar] [CrossRef]
- Febrian, R.; Ona, W.J.; Araneda, J.F.; Riegel, S.D.; Bracher, P.J. Benchtop NMR spectroscopy of prebiotically-relevant peptide reactions enabled by salt-induced chemical shift dispersion. ACS Earth Space Chem. 2020, 4, 499–505. [Google Scholar] [CrossRef]
- Chae, H.; Min, S.; Jeong, H.J.; Namgoong, S.K.; Oh, S.; Kim, K.; Jeong, K. Organic reaction monitoring of a glycine derivative using signal amplification by reversible exchange-hyperpolarized benchtop nuclear magnetic resonance spectroscopy. Anal. Chem. 2020, 92, 10902–10907. [Google Scholar] [CrossRef] [PubMed]
- Richardson, P.M.; Parrott, A.J.; Semenova, O.; Nordon, A.; Duckett, S.B.; Halse, M.E. SABRE hyperpolarization enables high-sensitivity 1H and 13C benchtop NMR spectroscopy. Analyst 2018, 143, 3442–3450. [Google Scholar] [CrossRef] [Green Version]
- Robinson, A.D.; Richardson, P.M.; Halse, M.E. Hyperpolarised 1H–13C benchtop NMR spectroscopy. Appl. Sci. 2019, 9, 173. [Google Scholar] [CrossRef] [Green Version]
- Semenova, O.; Richardson, P.M.; Parrott, A.J.; Nordon, A.; Halse, M.E.; Duckett, S.B. Reaction monitoring using SABRE-Hyperpolarized Benchtop (1 T) NMR spectroscopy. Anal. Chem. 2019, 91, 6695–6701. [Google Scholar] [CrossRef] [Green Version]
- Tennant, T.; Hulme, M.C.; Robertson, T.B.R.; Sutcliffe, O.B.; Mewis, R.E. Benchtop NMR analysis of piperazine-based drugs hyperpolarised by SABRE. Magn. Reson. Chem. 2020, 58, 1151–1159. [Google Scholar] [CrossRef]
- Gouilleux, B.; Charrier, B.; Akoka, S.; Felpin, F.X.; Rodriguez-Zubiri, M.; Giraudeau, P. Ultrafast 2D NMR on a benchtop spectrometer: Applications and perspectives. Trac-Trend Anal. Chem. 2016, 83, 65–75. [Google Scholar] [CrossRef]
- Singh, K.; Blumich, B. Desktop NMR for structure elucidation and identification of strychnine adulteration. Analyst 2017, 142, 1459–1470. [Google Scholar] [CrossRef]
- Gouilleux, B.; Marchand, J.; Charrier, B.; Remaud, G.S.; Giraudeau, P. High-throughput authentication of edible oils with benchtop Ultrafast 2D NMR. Food Chem. 2018, 244, 153–158. [Google Scholar] [CrossRef] [PubMed]
- Gouilleux, B.; Charrier, B.; Danieli, E.; Dumez, J.N.; Akoka, S.; Felpin, F.X.; Rodriguez-Zubiri, M.; Giraudeau, P. Real-time reaction monitoring by ultrafast 2D NMR on a benchtop spectrometer. Analyst 2015, 140, 7854–7858. [Google Scholar] [CrossRef]
- Friebel, A.; von Harbou, E.; Münnemann, K.; Hasse, H. Reaction monitoring by benchtop NMR spectroscopy using a novel stationary flow reactor setup. Ind. Eng. Chem. Res. 2019, 58, 18125–18133. [Google Scholar] [CrossRef]
- Weidener, D.; Singh, K.; Blumich, B. Synthesis of alpha-fluoro-alpha, beta-unsaturated esters monitored by 1D and 2D benchtop NMR spectroscopy. Magn. Reson. Chem. 2019, 57, 852–860. [Google Scholar] [CrossRef]
- Rehm, T.H.; Hofmann, C.; Reinhard, D.; Kost, H.J.; Lob, P.; Besold, M.; Welzel, K.; Barten, J.; Didenko, A.; Sevenard, D.V.; et al. Continuous-flow synthesis of fluorine-containing fine chemicals with integrated benchtop NMR analysis. React. Chem. Eng. 2017, 2, 315–323. [Google Scholar] [CrossRef]
- Heerah, K.; Waclawek, S.; Konzuk, J.; Longstaffe, J.G. Benchtop 19F NMR spectroscopy as a practical tool for testing of remedial technologies for the degradation of perfluorooctanoic acid, a persistent organic pollutant. Magn. Reson. Chem. 2020, 58, 1160–1167. [Google Scholar] [CrossRef] [PubMed]
- Sakla, R.; Jose, D.A. New fluorinated manganese carbonyl complexes for light controlled carbon monoxide (CO) release and the use of benchtop 19F-NMR spectroscopy. Inorg. Chim. Acta 2021, 516, 1–8. [Google Scholar] [CrossRef]
- Gouilleux, B.; Christensen, N.V.; Malmos, K.G.; Vosegaard, T. Analytical evaluation of low-field 31P NMR spectroscopy for lipid analysis. Anal. Chem. 2019, 91, 3035–3042. [Google Scholar] [CrossRef] [Green Version]
- Araneda, J.F.; Hui, P.; Leskowitz, G.M.; Riegel, S.D.; Mercado, R.; Green, C. Lithium-7 qNMR as a method to quantify lithium content in brines using benchtop NMR. Analyst 2021, 146, 882–888. [Google Scholar] [CrossRef]
- Chighine, K.; Léonce, E.; Boutin, C.; Desvaux, H.; Berthault, P. 129 Xe Ultrafast Z-spectroscopy enables micromolar detection of biosensors on a 1T benchtop spectrometer. Magn. Reson. Discuss. 2021, 1–21. [Google Scholar] [CrossRef]
- Bernard, G.M.; Michaelis, V.K. Lead-207 NMR spectroscopy at 1.4 T: Application of benchtop instrumentation to a challenging I = ½ nucleus. Magn. Reson. Chem. 2020, 58, 1203–1212. [Google Scholar] [CrossRef] [PubMed]

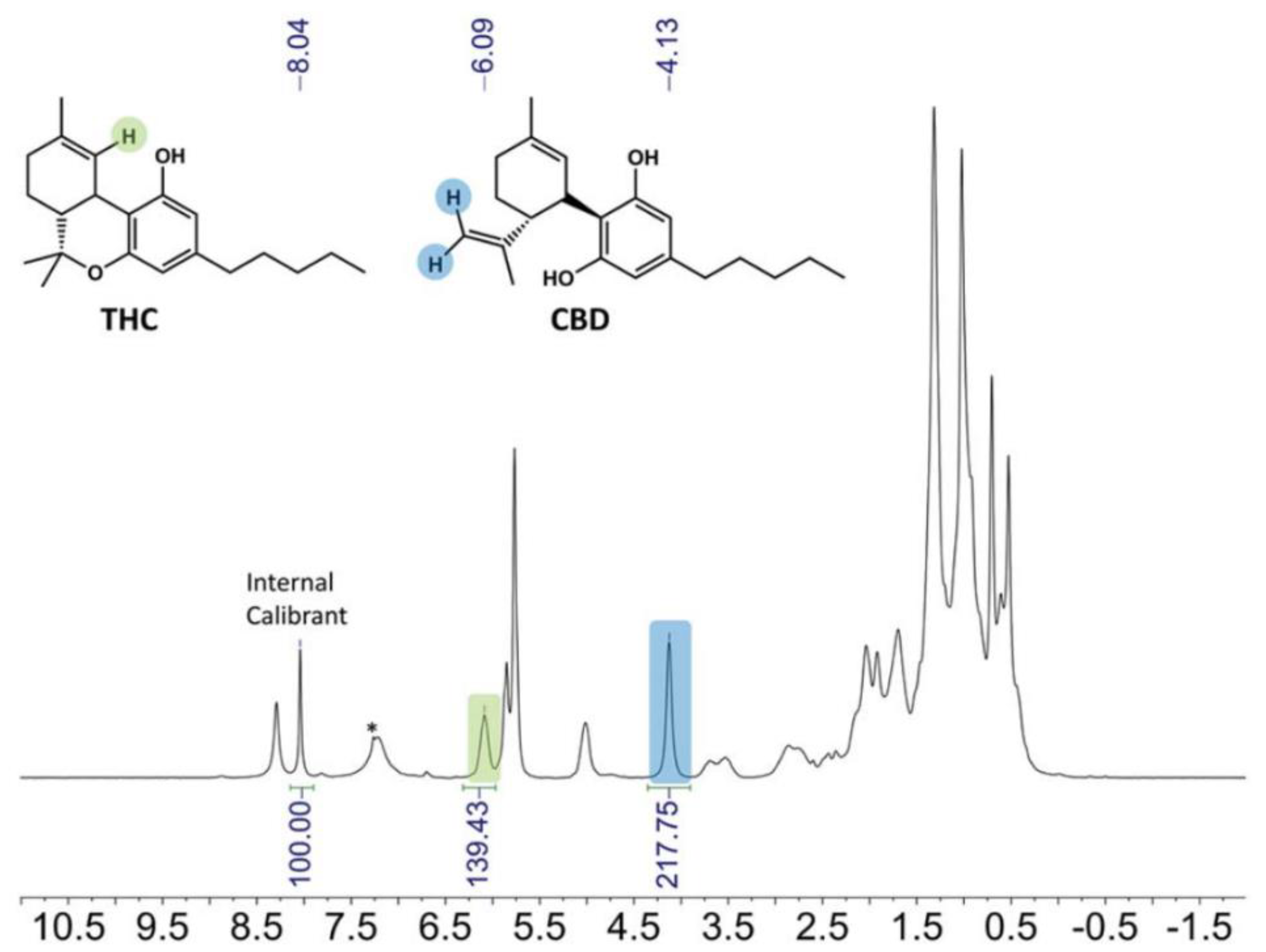
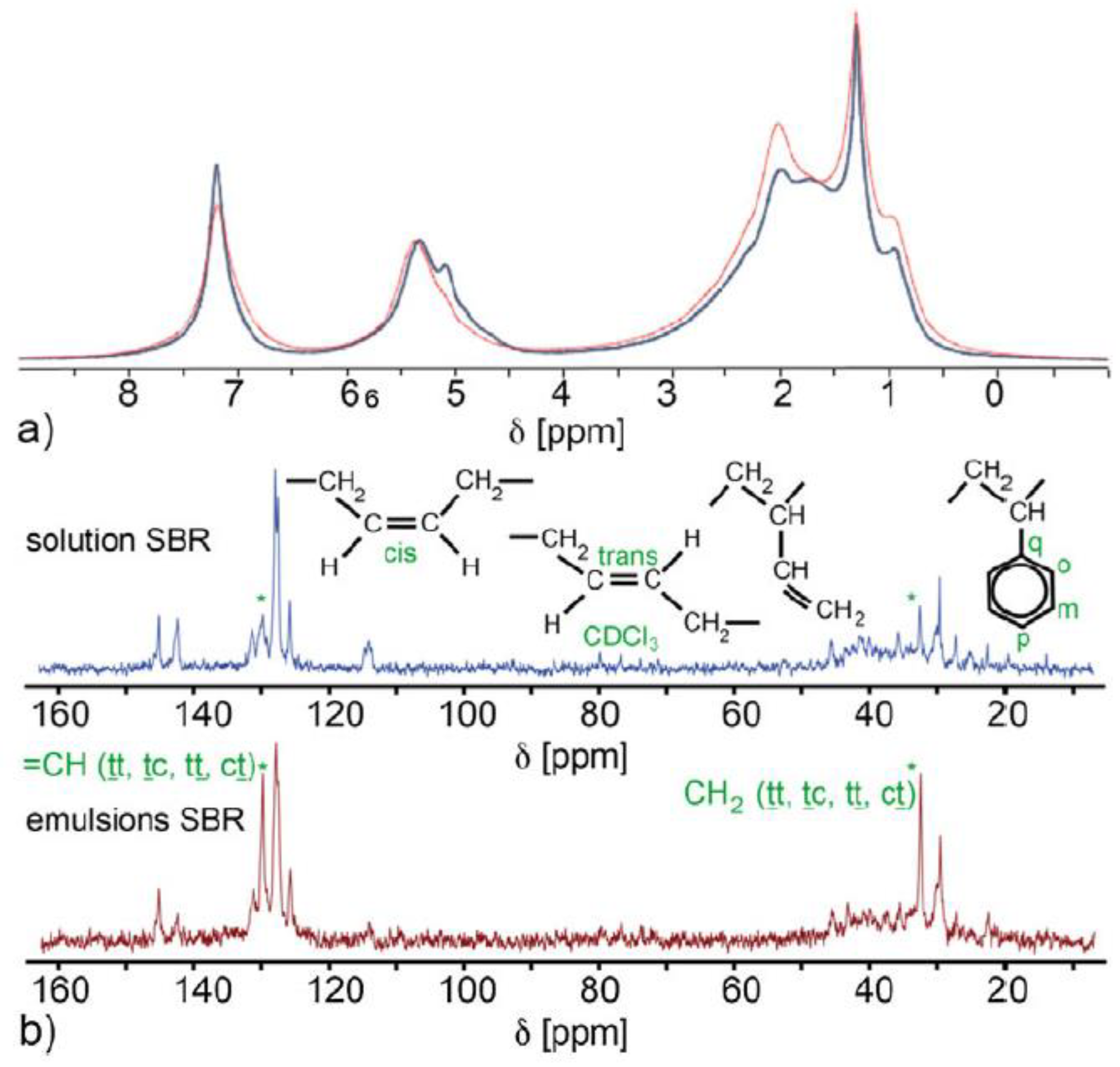
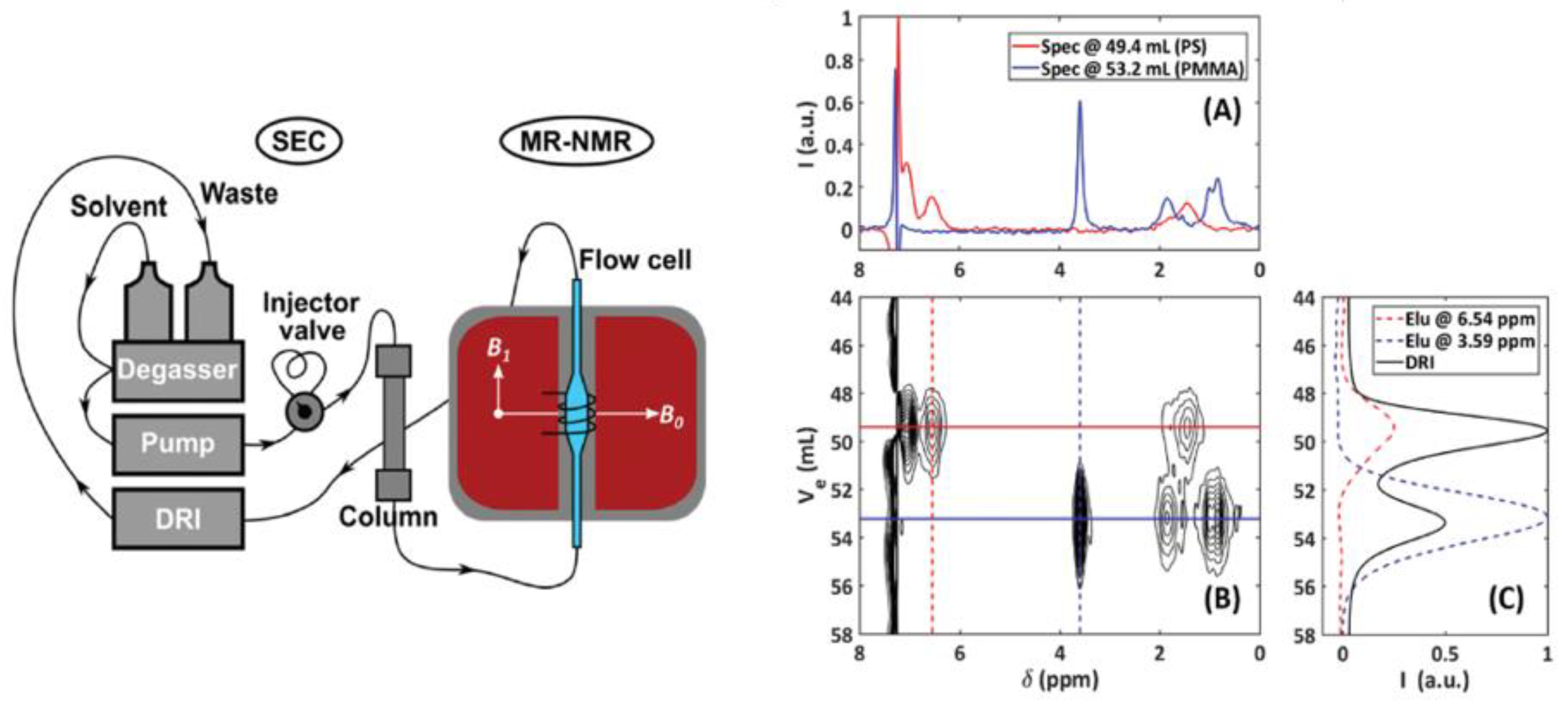

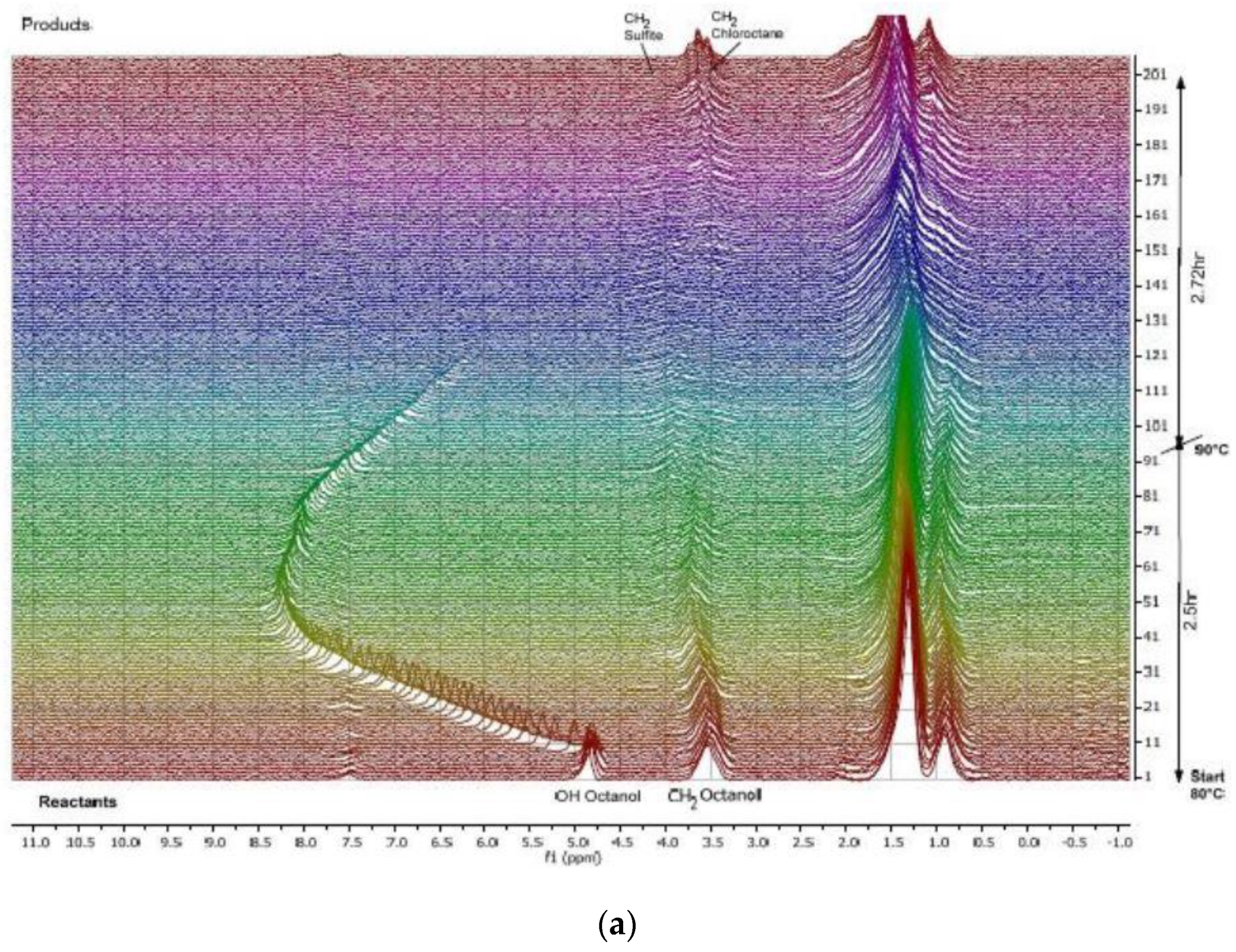
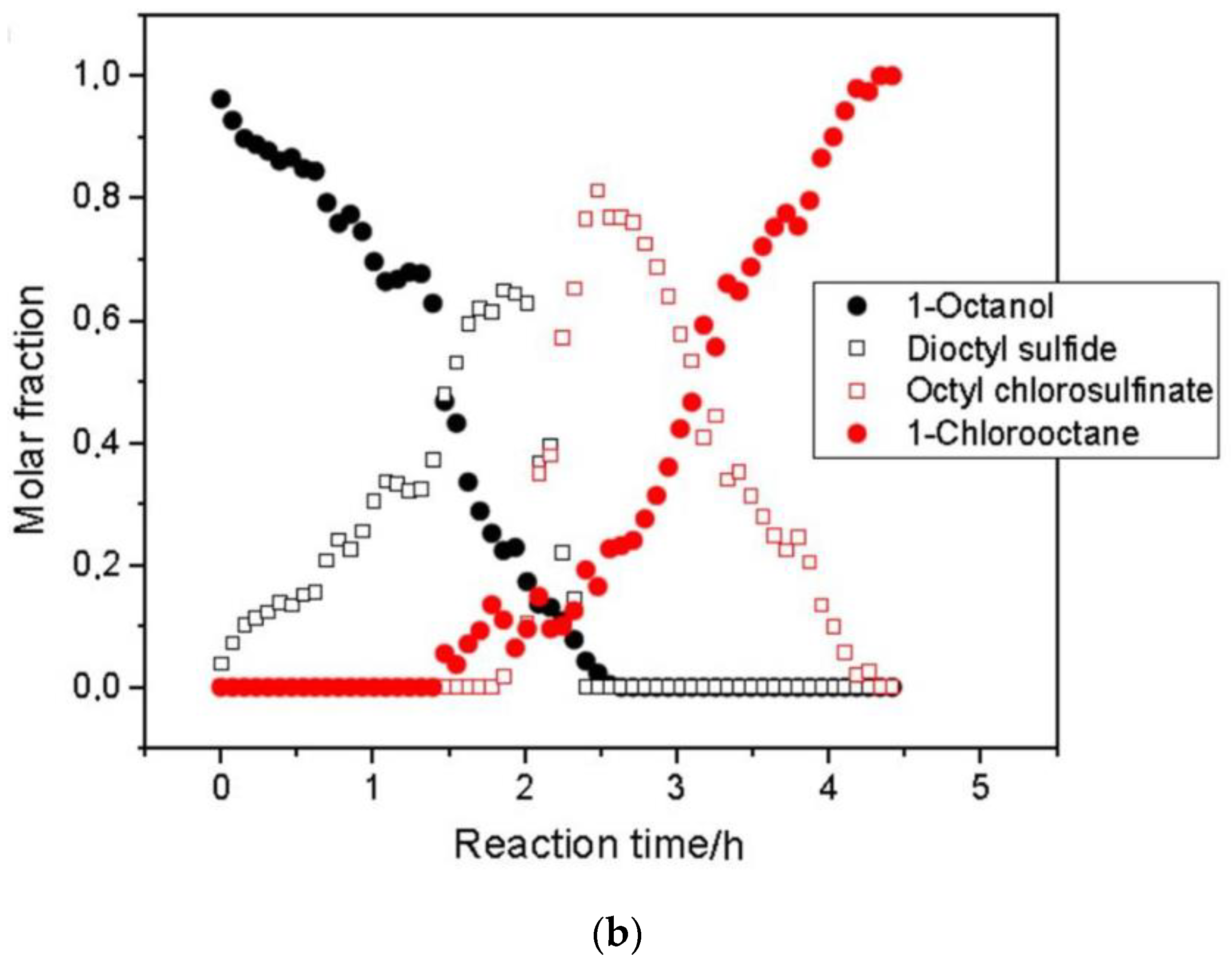

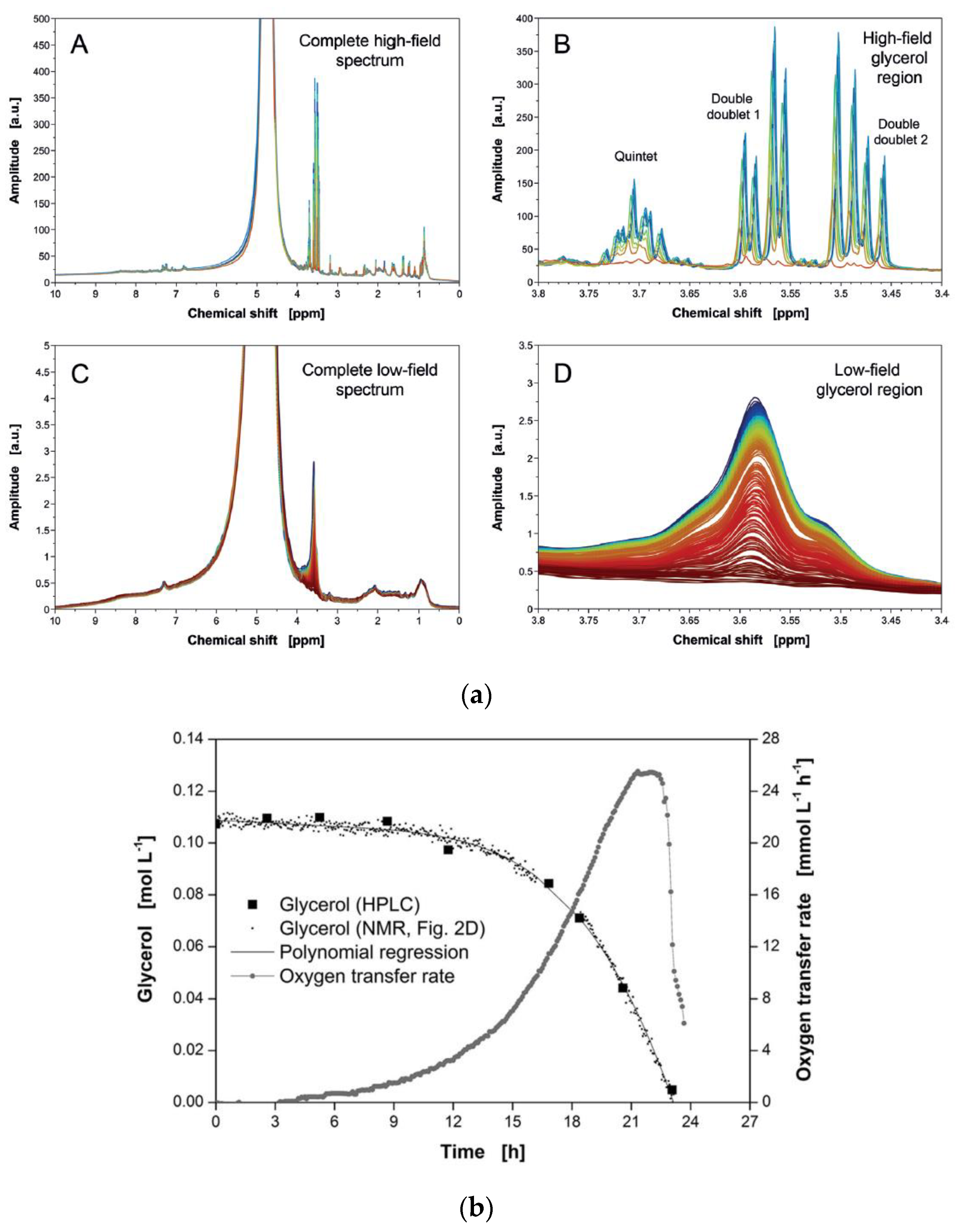
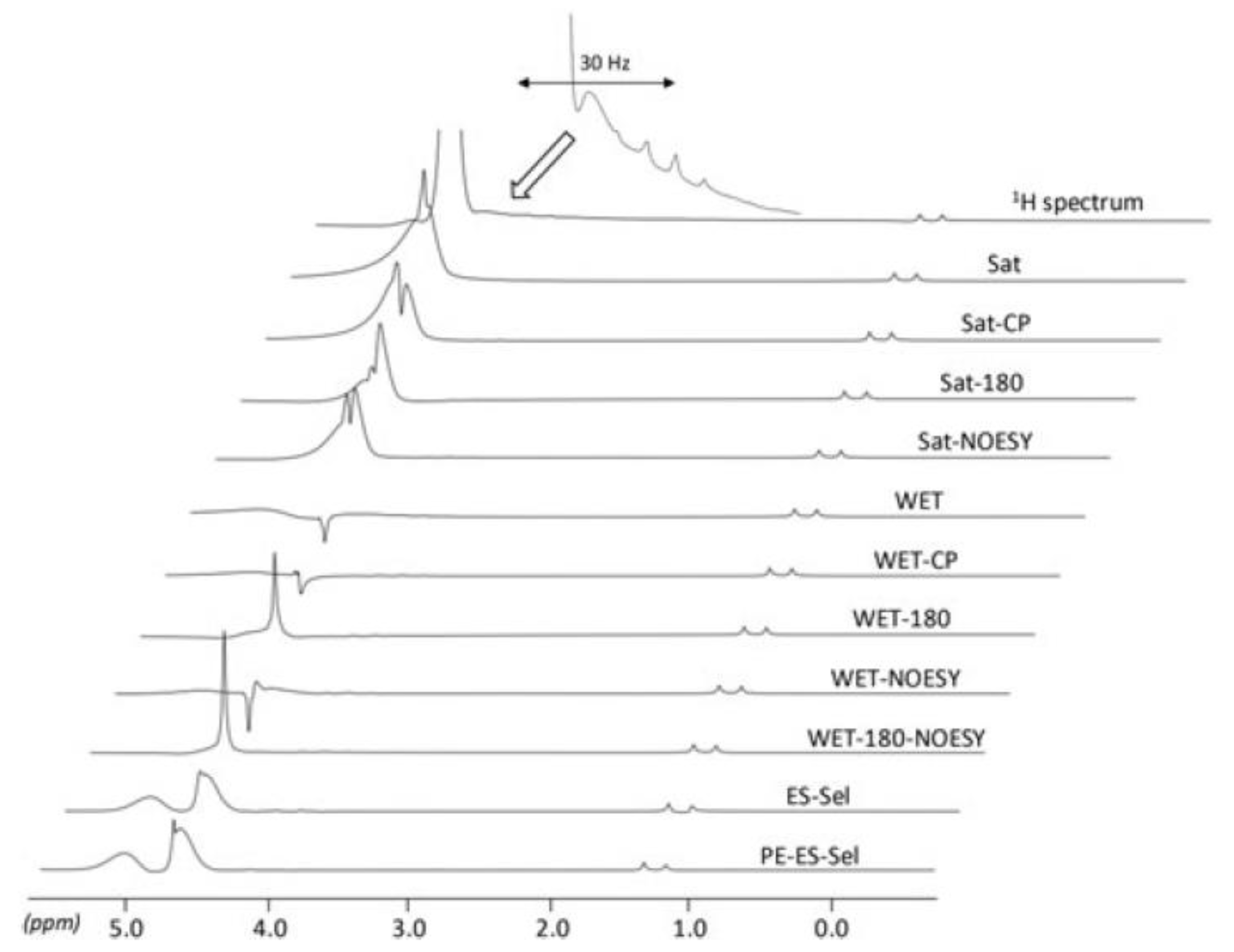
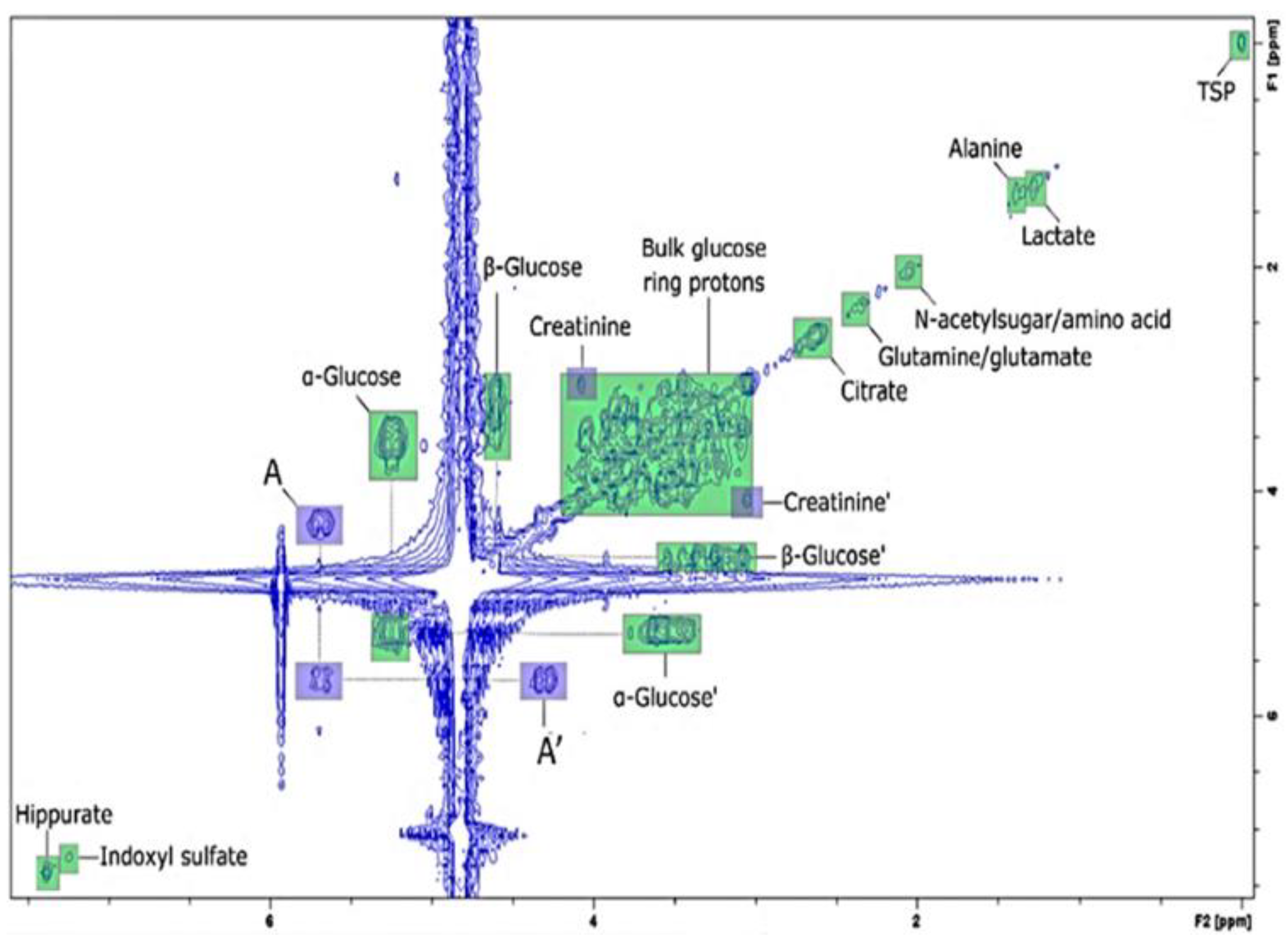
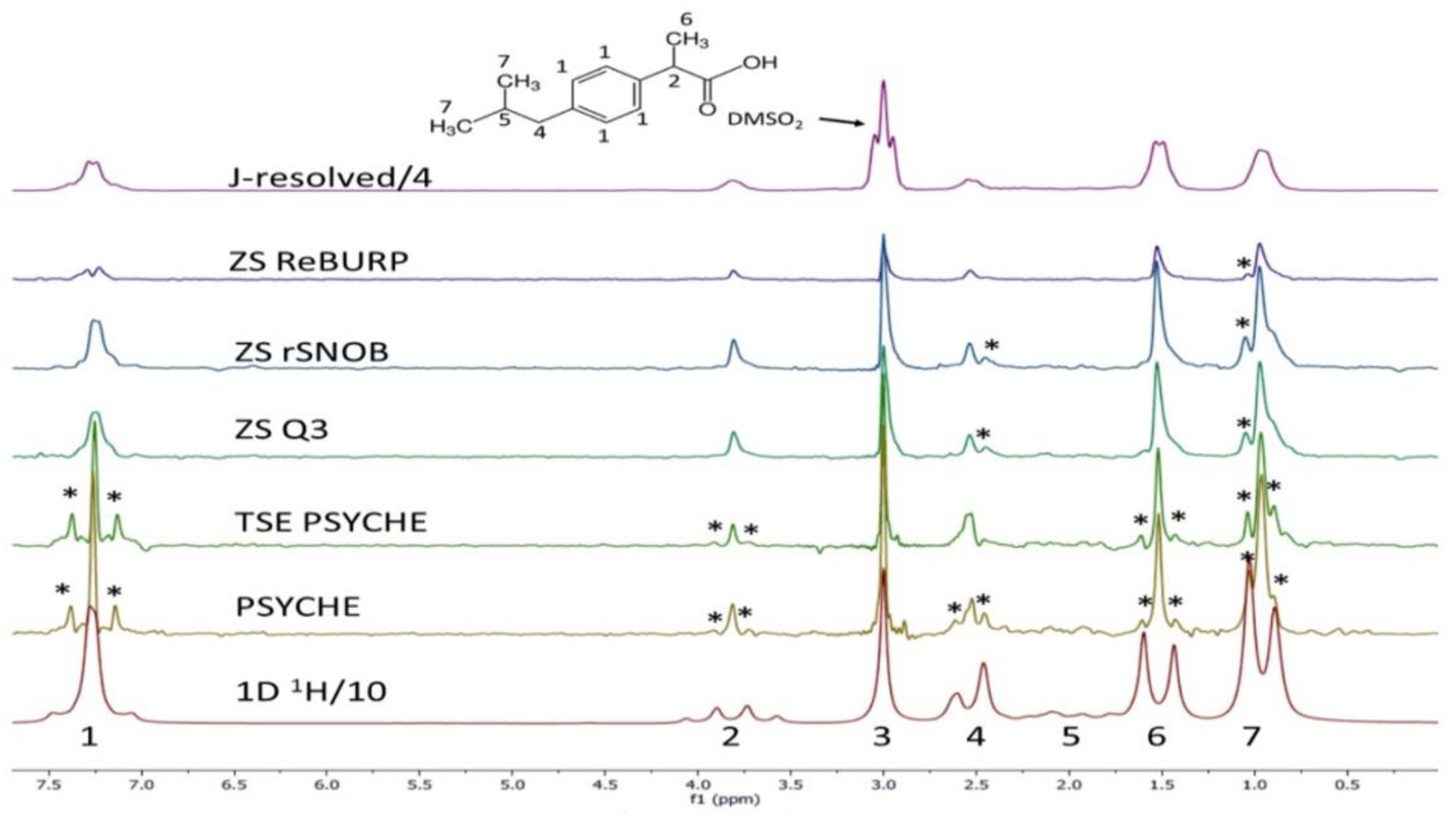

| Food | Analysis Goal | Technique | Ref. | |
|---|---|---|---|---|
| Oils | Olive oil | Adulteration | 60 MHz 1H NMR | [20] |
| Perilla oil | Authenticity | 43 MHz 1H NMR | [15] | |
| Patchouli essential oil | Adulteration | 60 MHz 1H NMR | [21] | |
| Rapeseed oil | Adulteration | 60 MHz 1H NMR | [14] | |
| Argan oil | Authenticity | 60 MHz 1H NMR | [23] | |
| Meat | Beef | Authentication | 60 MHz 1H NMR | [24] |
| Beverages | Coffee | Authenticity | 60 MHz 1H NMR | [16,25] |
| Wine | Alcohol content | 45 MHz 1H NMR | [17] | |
| Milk | Online monitoring Authenticity | 43 MHz 1H NMR | [26] | |
| Juice | Sugar content | 43 MHz 1H NMR | [27] | |
| Grains | Barley | Fermentation | 60 MHz 1H NMR | [28] |
| Substance | Analysis Goal | Technique | Ref. |
|---|---|---|---|
| Sexual enhancement and sliming dietary supplements | Adulteration | 60 MHz 1H NMR | [32] |
| Sliming and dietary supplements | Adulteration | 60 MHz 1H NMR | [33] |
| Antimalarials and erectile dysfunction drugs | Detection of falsified medicines | 60 MHz 1H NMR | [34] |
| Smokable herbal mixture | Screening | 60 MHz 1H NMR | [37] |
| Derivatives of morphine, amphetamine, and ketamine | Screening | 80 MHz 1H NMR | [38] |
| Cannabinoids | Screening Quantitation | 60 MHz 1H NMR | [39] |
| Recreational MDMA/ecstasy | Screening Quantitation | 60 MHz 1H NMR | [40] |
| Substandard, falsified medicines Illegal drugs | Screening Quantitation | 60 MHz 1H NMR | [41] |
| (Pseudo)ephedrine | Identification | 43 MHz 1H NMR 17.5 MHz 31P NMR | [42] |
| Fluorofentanyl derivatives | Screening Quantitation | 60 MHz 1H NMR 54 MHz 19F NMR | [43] |
| Amphetamine, cathinone, norephedrine regioisomers | Screening Quantitation | 60 MHz 1H NMR 54 MHz 19F NMR | [44] |
| Psychoactive substances (cocaine and MDMA) | Identification | 60 MHz 1H NMR | [45] |
| Fentanyl analogues | Screening | 60 MHz 1H NMR | [31] |
| Reaction/Process Details | Technique | Ref. |
|---|---|---|
| Quantitative molecular characterization of copolymer and polymer blends containing polystyrene | 60 MHz 1H NMR | [50] |
| Quality control of SBR rubber | 43 MHz 1H NMR | [49,51] |
| Quantitative characterization of polyisoprene microstructure | 60 MHz 1H NMR | [52] |
| Quantitation of PVC plasticizers | 43 MHz 1H NMR | [48,53] |
| Online monitoring of emulsion polymerization of butyl acrylate | 20 MHz 1H NMR | [54] |
| Online monitoring of RAFT synthesis of poly(dimethyl acrylamide) | 60 MHz 1H NMR | [55] |
| Inline monitoring of polymerization of poly(methyl acrylate) | 60 MHz 1H NMR | [56] |
| Online detector for size-exclusion chromatography | 62 MHz 1H NMR | [57,58,59,60] |
| Reaction/Process Details | Technique | Ref. |
|---|---|---|
| Comparison of offline gas-chromatographic method and online NMR method for monitoring toluene hydrogenation | 20 MHz 1H NMR | [62] |
| Online monitoring of transfer hydrogenation of acetophenone to phenylethanol | 43 MHz 1H NMR | [63] |
| Online monitoring of Grignard reagent preparation using fluid-bed Mg column | 60 MHz 1H NMR | [64] |
| Chemical reaction (Fischer esterification, Suzuki coupling, oxime reaction) monitoring using a flow cell | 45 MHz 1H NMR | [65] |
| In- and online monitoring of hypervalent I(III)–mediated cyclopropanation with solvent switching system | 43 MHz 1H NMR COSY | [66] |
| Continuous monitoring of esterification using bypass method in flow cell to control temperature and pressure | 20 MHz 1H NMR | [67] |
| Inline monitoring of complex nitration in flow using multivariate analysis | 43 MHz 1H NMR | [68] |
| Bayesian approach for automated quantitative analysis using mixtures of alcohols and acetates | 43 MHz 1H NMR | [69] |
| Exploration of data acquisition parameter selection such as flow and mixing behavior of continuous flow system | 60 MHz 1H NMR | [70] |
| Development of sample shifting method | 43 MHz 1H NMR | [71] [72] |
| Development of automation platform based on prototype benchtop NMR spectrometer using alcohol chlorination into corresponding alkyl chloride | 60 MHz 1H NMR | [73] |
| Development of fully automated platform based on inline benchtop NMR spectroscopy (imine formation, electrophilic fluorinations, and Diels–Alder reactions) | 43 MHz 1H NMR | [74] |
| Online monitoring of continuous lab-scale NDPA synthesis with automated data analysis using MATLAB, PLS-R, and indirect hard modeling | 43 MHz 1H NMR | [75] |
| Real-time process monitoring for continuous production of NDPA with NIR in pilot plant | 43 MHz 1H NMR | [76] |
| Inline monitoring of oxidative neutralization of mustard gas simulants | 43 MHz 1H NMR | [77] |
| Online process monitoring of batch distillation | 43 MHz 1H NMR | [78] |
| Inline monitoring of hydrogenation of lignin-derived phenols over Wilkinsons’s catalyst | 60 MHz H NMR | [79] |
| Reaction monitoring of porous heterogeneous catalysts using reduction of 1-octene over Pd/TiO2 as example reaction | 60 MHz 1H NMR T1/T2 NMR relaxometry | [80] |
| Online monitoring of biocatalytic synthesis of aromatic amino alcohols | 60 MHz 1H NMR | [81] |
| Real-time monitoring of highly enriched parahydrogen production | 60 MHz 1H NMR | [82] |
| Reaction/Process Details | Technique | Ref. |
|---|---|---|
| Online monitoring of fermentation processes using bypass system | 43 MHz 1H NMR | [89] |
| Online monitoring of sucrose hydrolysis using water suppression techniques | 43 MHz 1H NMR | [90] |
| Online monitoring of enzymatic hydrolysis of marine byproducts using water suppression techniques | 43 MHz 1H NMR | [91] |
| Online non-invasive in vivo monitoring of lipid production by microalgae | 43 MHz 1H NMR | [30,92] |
| Online monitoring of biodiesel production using transesterification | 43 MHz 1H NMR | [93,94,95,96] |
| Reaction/Process Details | Technique | Ref. |
|---|---|---|
| Biomarkers of diabetic disease such as α-glucose and acetone in human urine | 60 MHz 1H NMR COSY | [103,104] |
| 60 MHz 1H NMR | [105] | |
| Glucose quantitation in human whole blood | 43 MHz 1H NMR | [106] |
| Technique | Ref. |
|---|---|
| Solvent suppression based on gradient pulse sequence | [97,107] |
| Homo-decoupling method using different pure-shift methods | [108] |
| Addition of salts as chemical shift agents to peptide sample | [109] |
| Hyperpolarization methods (SABRE) | [110,111,112,113,114] |
| Reaction/Process Details | Technique | Ref. |
|---|---|---|
| Structural analysis of strychnine | 43 MHz 1H NMR, 13C NMR, DEPT, COSY, HETCOR, HSQC, HMBC, J-resolved spectroscopy | [116] |
| Authentication of edible oil | 43 MHz 1H NMR, COSY | [117] |
| Real-time monitoring of Heck-Matsuda coupling reaction | 43 MHz 1H NMR, COSY | [118] |
| Monitoring of esterification reactions | 43 MHz 1H NMR, COSY, HETCOR, HMBC | [119] |
| Monitoring of α-fluoro-α,β-unsaturated ester synthesis | 43 MHz 1H NMR, COSY, 19F NMR | [120] |
| Heteroatom | Technique | Reaction/Process Details | Ref. |
|---|---|---|---|
| 13C | 43 MHz 1H NMR 13C 1D NMR | Quality control of SBR rubber | [49,51] |
| 43 MHz 1H NMR 13C 1D NMR | Quantitation of m-anisaldehyde and (R)-(+)-limonene | [72] | |
| 43 MHz 1H NMR 13C 1D NMR | Application of SABRE hyperpolarization | [111,112] | |
| 19F | 60 MHz 1H NMR 19F NMR | Detection and quantitation of fluorofentanyl derivatives | [43] |
| 60 MHz 1H NMR 19F NMR | Detection and quantitation of amphetamine, cathinone, norephedrine regioisomers | [44] | |
| 60 MHz 1H NMR 19F NMR | Reaction monitoring of fluorine-containing fine chemicals | [121] | |
| 60 MHz 1H NMR 19F NMR | Degradation of perfluorooctanoic acid | [122] | |
| 43 MHz 1H NMR 19F NMR | Monitoring of carbon monoxide release using fluorinated manganese carbonyl complexes | [123] | |
| 31P | 43 MHz 1H NMR 31P NMR | Identification of (pseudo)ephedrine | [42] |
| 43, 80 MHz 1H NMR 31P NMR 1H-31P TOCSY | Identification and quantitation of phospholipids | [124] | |
| 7Li | 7Li NMR(1.4 T) | Quantitation of lithium in real brine samples | [125] |
| 129Xe | 129Xe NMR(1 T) | Detection of caged Xe as a bioprobe | [126] |
| 207Pb | 207Pb NMR(1.4 T) | Detection of methylammonium lead chloride perovskite | [127] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, H.-Y.; Myoung, S.; Ahn, S. Recent Applications of Benchtop Nuclear Magnetic Resonance Spectroscopy. Magnetochemistry 2021, 7, 121. https://doi.org/10.3390/magnetochemistry7090121
Yu H-Y, Myoung S, Ahn S. Recent Applications of Benchtop Nuclear Magnetic Resonance Spectroscopy. Magnetochemistry. 2021; 7(9):121. https://doi.org/10.3390/magnetochemistry7090121
Chicago/Turabian StyleYu, Hyo-Yeon, Sangki Myoung, and Sangdoo Ahn. 2021. "Recent Applications of Benchtop Nuclear Magnetic Resonance Spectroscopy" Magnetochemistry 7, no. 9: 121. https://doi.org/10.3390/magnetochemistry7090121






