Effect of Ligand Substitution on Zero-Field Slow Magnetic Relaxation in Mononuclear Dy(III) β-Diketonate Complexes with Phenanthroline-Based Ligands
Abstract
:1. Introduction
2. Results and Discussion
2.1. Crystal Structure of 1 and 2
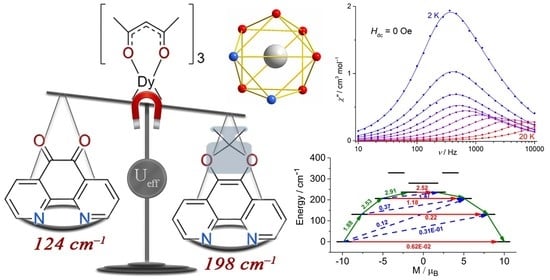
2.2. Magnetic Properties
| Parameter | 1 | 2 | ||
|---|---|---|---|---|
| Hdc, Oe | 0 | 1000 | 0 | 1000 |
| Ueff, cm−1 | 198(2) | 197(1) | 124(10) | 123(6) |
| τ0, s | 1.1(2)·10−11 | 1.5(1)·10−11 | 4.9(9)·10−9 | 4.5(7)·10−9 |
| CRaman, s−1 K−n | 8.0(9)·10−3 | 1.21(8)·10−4 | 1.3(6)·10−2 | 4.3(8)·10−3 |
| nRaman | 5.3(2) | 6.65(2) | 4.4(7) | 4.4(9) |
| τQTM, s | 4.8(4)·10−4 | - | 2.9(6)·10−3 | - |
2.3. Quantum Chemical Calculations
3. Conclusions
4. Experimental Details
4.1. Materials and Methods
4.2. Synthesis
4.2.1. Synthesis of [Dy(acac)3(dmdophen)] (1)
4.2.2. Synthesis of [Dy(acac)3(phendione)]·CH3CN (2)
4.3. X-ray Data Collection and Structure Refinement
4.4. Magnetic Measurements
4.5. Computational Details
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Zabala-Lekuona, A.; Seco, J.M.; Colacio, E. Single-Molecule Magnets: From Mn12-ac to dysprosium metallocenes, a travel in time. Coord. Chem. Rev. 2021, 441, 213984. [Google Scholar] [CrossRef]
- Gebrezgiabher, M.; Bayeh, Y.; Gebretsadik, T.; Gebreslassie, G.; Elemo, F.; Thomas, M.; Linert, W. Lanthanide-Based Single-Molecule Magnets Derived from Schiff Base Ligands of Salicylaldehyde Derivatives. Inorganics 2020, 8, 66. [Google Scholar] [CrossRef]
- Perlepe, P.S.; Maniaki, D.; Pilichos, E.; Katsoulakou, E.; Perlepes, S.P. Smart Ligands for Efficient 3d-, 4d- and 5d-Metal Single-Molecule Magnets and Single-Ion Magnets. Inorganics 2020, 8, 39. [Google Scholar] [CrossRef]
- Baldoví, J.J.; Cardona-Serra, S.; Clemente-Juan, J.M.; Coronado, E.; Gaita-Ariño, A.; Palii, A. Rational Design of Single-Ion Magnets and Spin Qubits Based on Mononuclear Lanthanoid Complexes. Inorg. Chem. 2012, 51, 12565–12574. [Google Scholar] [CrossRef]
- Aldoshin, S.M.; Korchagin, D.V.; Palii, A.V.; Tsukerblat, B.S. Some new trends in the design of single molecule magnets. Pure Appl. Chem. 2017, 89, 1119–1143. [Google Scholar] [CrossRef]
- Zhang, P.; Yun-Nan, G.; Jinkui, T. Recent advances in dysprosium-based single molecule magnets: Structural overview and synthetic strategies. Coord. Chem. Rev. 2013, 257, 1728–1763. [Google Scholar] [CrossRef]
- Dey, A.; Kalita, P.; Chandrasekhar, V. Lanthanide(III)-Based Single-Ion Magnets. ACS Omega 2018, 3, 9462–9475. [Google Scholar] [CrossRef]
- Blachowicz, T.; Ehrmann, A. New Materials and Effects in Molecular Nanomagnets. Appl. Sci. 2021, 11, 7510. [Google Scholar] [CrossRef]
- Bazhenova, T.A.; Kopotkov, V.A.; Korchagin, D.V.; Manakin, Y.V.; Zorina, L.V.; Simonov, S.V.; Yakushev, I.A.; Mironov, V.S.; Vasiliev, A.N.; Maximova, O.V.; et al. A Series of Novel Pentagonal-Bipyramidal Erbium(III) Complexes with Acyclic Chelating N3O2 Schiff-Base Ligands: Synthesis, Structure, and Magnetism. Molecules 2021, 26, 6908. [Google Scholar] [CrossRef]
- Zhang, S.; Mo, W.; Zhang, J.; Zhang, Z.; Yin, B.; Hu, D.; Chen, S. Regulation of Substituent Effects on Configurations and Magnetic Performances of Mononuclear DyIII Single-Molecule Magnets. Inorg. Chem. 2019, 58, 15330–15343. [Google Scholar] [CrossRef]
- Shao, D.; Yang, J.; Yang, X.; Tian, Z. An Azido-Bridged Dysprosium Chain Complex Showing Zero-field Slow Magnetic Relaxation. Chem Asian J. 2021, 16, 3331–3335. [Google Scholar] [CrossRef]
- Shao, D.; Tang, W.-J.; Ruan, Z.; Yang, X.; Shi, L.; Wei, X.-Q.; Tian, Z.; Kumari, K.; Singh, S.K. Water-driven reversible switching of single-ion magnetism and proton conduction in a dysprosium sulfonate. Inorg. Chem. Front. 2022. [Google Scholar] [CrossRef]
- Groom, C.R.; Bruno, I.J.; Lightfoot, M.P.; Ward, S.C. The Cambridge Structural Database. Acta Cryst. B 2016, 72, 171–179. [Google Scholar] [CrossRef] [PubMed]
- Tong, Y.Z.; Gao, C.; Wang, Q.-L.; Wang, B.-W.; Gao, S.; Cheng, P.; Liao, D.-Z. Two mononuclear single molecule magnets derived from dysprosium(iii) and tmphen (tmphen = 3,4,7,8-tetramethyl-1,10-phenanthroline). Dalton Trans. 2015, 44, 9020–9026. [Google Scholar] [CrossRef]
- Guo, M.; Wu, J.; Cador, O.; Lu, J.; Yin, B.; Le Guennic, B.; Tang, J. Manipulating the Relaxation of Quasi-D4d Dysprosium Compounds through Alternation of the O-Donor Ligands. Inorg. Chem. 2018, 57, 4534–4542. [Google Scholar] [CrossRef]
- Chen, G.-J.; Gao, C.-Y.; Tian, J.-L.; Tang, J.; Gu, W.; Liu, X.; Yan, S.-P.; Liao, D.-Z.; Cheng, P. Coordination-perturbed single-molecule magnet behaviour of mononuclear dysprosium complexes. Dalton Trans. 2011, 40, 5579–5583. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.-J.; Guo, Y.-N.; Tian, J.-L.; Tang, J.; Gu, W.; Liu, X.; Yan, S.-P.; Cheng, P.; Liao, D.-Z. Enhancing Anisotropy Barriers of Dysprosium(III) Single-Ion Magnets. Chem. Eur. J. 2012, 18, 2484–2487. [Google Scholar] [CrossRef]
- Chen, G.-J.; Zhou, Y.; Jin, G.-X.; Dong, Y.-B. [Dy(acac)3(dppn)]·C2H5OH: Construction of a single-ion magnet based on the square-antiprism dysprosium(III) ion. Dalton Trans. 2014, 43, 16659–16665. [Google Scholar] [CrossRef] [PubMed]
- Aravena, D.; Ruiz, E. Shedding Light on the Single-Molecule Magnet Behavior of Mononuclear DyIII Complexes. Inorg. Chem. 2013, 52, 13770–13778. [Google Scholar] [CrossRef] [PubMed]
- Chilton, N.F.; Collison, D.; McInnes, E.J.L.; Winpenny, R.E.P.; Soncini, A. An electrostatic model for the determination of magnetic anisotropy in dysprosium complexes. Nat. Commun. 2013, 4, 2551. [Google Scholar] [CrossRef] [Green Version]
- Yao, X.; Yan, P.; An, G.; Shi, C.; Li, Y.; Li, G. Single-ion magnets with D4d symmetry based on electron-donating β-diketonate Dy(III) complexes. New J. Chem. 2018, 42, 8438–8444. [Google Scholar] [CrossRef]
- Bi , Y.; Guo, Y.-N.; Zhao, L.; Guo, Y.; Lin, S.-Y.; Jiang, S.-D.; Tang, J.; Wang, B.-W.; Gao, S. Capping Ligand Perturbed Slow Magnetic Relaxation in Dysprosium Single-Ion Magnets. Chem. Eur. J. 2011, 17, 12476–12481. [Google Scholar] [CrossRef]
- Wang, Y.-L.; Ma, Y.; Yang, X.; Tang, J.; Cheng, P.; Wang, Q.-L.; Li, L.-C.; Liao, D.-Z. Syntheses, Structures, and Magnetic and Luminescence Properties of a New DyIII-Based Single-Ion Magnet. Inorg. Chem. 2013, 52, 7380–7386. [Google Scholar] [CrossRef]
- Cen, P.; Liu, X.; Ferrando-Soria, J.; Zhang, Y.-Q.; Xie, G.; Chen, S.; Pardo, E. Capping N-Donor Ligands Modulate the Magnetic Dynamics of DyIII β-Diketonate Single-Ion Magnets with D4d Symmetry. Chem. Eur. J. 2019, 25, 3884–3892. [Google Scholar] [CrossRef] [PubMed]
- Cen, P.; Wang, M.; Ma, X.; Chen, L.; Zhang, Y.-Q.; Li, Y.; Tian, D.; Liu, X. Coordination microenvironment perturbed single-ion magnet behavior in a β-diketone Dy(III) complex. CrystEngComm 2020, 22, 6856–6863. [Google Scholar] [CrossRef]
- Llunell, M.; Casanova, D.; Cirera, J.; Alemany, P.; Alvarez, S. SHAPE. version 2.1. 2013. [Google Scholar]
- Casanova, D.; Llunell, M.; Alemany, P.; Alvarez, S. The rich stereochemistry of eight-vertex polyhedra: A continuous shape measures study. Chem. Eur. J. 2005, 11, 1479–1494. [Google Scholar] [CrossRef]
- AlDamen, M.A.; Cardona-Serra, S.; Clemente-Juan, J.M.; Coronado, E.; Gaita-Ariño, A.; Martí-Gastaldo, C.; Luis, F.; Montero, O. Mononuclear Lanthanide Single Molecule Magnets Based on the Polyoxometalates [Ln(W5O18)2]9− and [Ln(β2-SiW11O39)2]13− (LnIII = Tb, Dy, Ho, Er, Tm, and Yb). Inorg. Chem. 2009, 48, 3467–3479. [Google Scholar] [CrossRef]
- Sorace, L.; Benelli, C.; Gatteschi, D. Lanthanides in molecular magnetism: Old tools in a new field. Chem. Soc. Rev. 2011, 40, 3092–3104. [Google Scholar] [CrossRef]
- Yureva, E.A.; Korchagin, D.V.; Anichkin, A.A.; Shilov, G.V.; Babeshkin, K.A.; Efimov, N.N.; Palii, A.V.; Aldoshin, S.M. Evidence for zero-field slow magnetic relaxation in a Co(ii) complex with a pseudo-tetrahedral N2I2 environment. Dalton Trans. 2022, 51, 11916–11921. [Google Scholar] [CrossRef]
- Gavrikov, A.V.; Koroteev, P.S.; Efimov, N.N.; Dobrokhotova, Z.V.; Ilyukhin, A.B.; Kostopoulos, A.K.; Ariciu, A.-M.; Novotortsev, V.M. Novel mononuclear and 1D-polymeric derivatives of lanthanides and (η 6-benzoic acid)tricarbonylchromium: Synthesis, structure and magnetism. Dalton Trans. 2017, 46, 3369–3380. [Google Scholar] [CrossRef] [PubMed]
- Gavrikov, A.V.; Efimov, N.N.; Dobrokhotova, Z.V.; Ilyukhin, A.B.; Vasilyev, P.N.; Novotortsev, V.M. Novel mononuclear Ln complexes with pyrazine-2-carboxylate and acetylacetonate co-ligands: Remarkable single molecule magnet behavior of a Yb derivative. Dalton Trans. 2017, 46, 11806–11816. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Mo, W.J.; Yin, B.; Zhang, G.N.; Yang, D.S.; Lü, X.Q.; Chen, S.P. The slow magnetic relaxation regulated by the coordination, configuration and intermolecular dipolar field in two mononuclear DyIII single-molecule magnets (SMMs). Dalton Trans. 2018, 47, 12393–12405. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.-D.; Wang, B.-W.; Su, G.; Wang, Z.-M.; Gao, S. A Mononuclear Dysprosium Complex Featuring Single-Molecule-Magnet Behavior. Angew. Chem. Int. Ed. 2010, 49, 7448–7451. [Google Scholar] [CrossRef]
- Habib, F.; Lin, P.-H.; Long, J.; Korobkov, I.; Wernsdorfer, W.; Murugesu, M. The Use of Magnetic Dilution to Elucidate the Slow Magnetic Relaxation Effects of a Dy2 Single-Molecule Magnet. J. Am. Chem. Soc. 2011, 133, 8830–8833. [Google Scholar] [CrossRef]
- Galangau, O.; Flores Gonzalez, J.; Montigaud, V.; Dorcet, V.; le Guennic, B.; Cador, O.; Pointillart, F. Dysprosium Single-Molecule Magnets Involving 1,10-Phenantroline-5,6-dione Ligand. Magnetochemistry 2020, 6, 19. [Google Scholar] [CrossRef] [Green Version]
- Galván, I.F.; Vacher, M.; Alavi, A.; Angeli, C.; Aquilante, F.; Autschbach, J.; Bao, J.J.; Bokarev, S.I.; Bogdanov, N.A.; Carlson, R.K.; et al. OpenMolcas: From Source Code to Insight. J. Chem. Theory Comput. 2019, 15, 5925–5964. [Google Scholar] [CrossRef]
- Aquilante, F.; Autschbach, J.; Baiardi, A.; Battaglia, S.; Borin, V.A.; Chibotaru, L.F.; Conti, I.; De Vico, L.; Delcey, M.; Galván, I.F.; et al. Modern quantum chemistry with [Open]Molcas. J. Chem. Phys. 2020, 152, 214117. [Google Scholar] [CrossRef]
- Kong, M.; Feng, X.; Li, J.; Hu, Z.-B.; Wang, J.; Song, X.-J.; Jing, Z.-Y.; Zhang, Y.-Q.; Song, Y. Structurally modulated single-ion magnets of mononuclear β-diketone dysprosium(III) complexes. Dalton Trans. 2020, 49, 14931–14940. [Google Scholar] [CrossRef]
- Xi, J.; Ma, X.; Cen, P.; Wu, Y.; Zhang, Y.-Q.; Guo, Y.; Yang, J.; Chen, L.; Liu, X. Regulating the magnetic dynamics of mononuclear β-diketone Dy(III) single-molecule magnets through the substitution effect on capping N-donor coligands. Dalton Trans. 2021, 50, 2102–2111. [Google Scholar] [CrossRef]
- Cen, P.-P.; Zhang, S.; Liu, X.-Y.; Song, W.-M.; Zhang, Y.-Q.; Xie, G.; Chen, S.-P. Electrostatic Potential Determined Magnetic Dynamics Observed in Two Mononuclear β-Diketone Dysprosium(III) Single-Molecule Magnets. Inorg. Chem. 2017, 56, 3644–3656. [Google Scholar] [CrossRef] [PubMed]
- Ungur, L.; Chibotaru, L. Strategies toward High-Temperature Lanthanide-Based Single-Molecule Magnets. Inorg. Chem. 2016, 556, 10043–10056. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.-S.; Han, T.; Zhai, Y.-Q.; Reta, D.; Chilton, N.F.; Winpenny, R.E.P.; Zheng, Y.-Z. A Study of Magnetic Relaxation in Dysprosium(III) Single Molecule Magnets. Chem. Eur. J. 2020, 26, 5893–5902. [Google Scholar] [CrossRef] [PubMed]
- Briganti, M.; Santanni, F.; Tesi, L.; Totti, F.; Sessoli, R.; Lunghi, A. A Complete Ab Initio View of Orbach and Raman Spin–Lattice Relaxation in a Dysprosium Coordination Compound. J. Am. Chem. Soc. 2021, 143, 13633–13645. [Google Scholar] [CrossRef] [PubMed]
- Gagliardi, L.; Lindh, R.; Karlstrom, G. Local properties of quantum chemical systems: The LoProp approach. J. Chem. Phys. 2004, 121, 4494. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pfeffer, M.G.; Zedler, L.; Kupfer, S.; Paul, M.; Schwalbe, M.; Peuntinger, K.; Guldi, D.M.; Guthmuller, J.; Popp, J.; Graefe, S.; et al. Tuning of photocatalytic activity by creating a tridentate coordination sphere for palladium. Dalton Trans. 2014, 43, 11676–11686. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Frey, J.; Kraus, T.; Heitz, V.; Sauvage, J.-P. Synthesis of a bis-macrocycle containing two back-to-back rigidly connected 1,10-phenanthroline units as a central core and its incorporation in a handcuff-like catenane. Chem. Eur. J. 2007, 13, 7584–7594. [Google Scholar] [CrossRef]
- Voloshin, A.I.; Shavaleev, N.M.; Kazakov, V.P. Chemiluminescence of praseodymium (III), neodymium (III) and ytterbium (III) β-diketonates in solution excited from 1,2-dioxetane decomposition and singlet-singlet energy transfer from ketone to rare-earth β-diketonates. J. Luminescence 2000, 91, 49–58. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Cryst. C 2015, 71, 3–8. [Google Scholar] [CrossRef] [Green Version]
- Chibotaru, L.F.; Ungur, L. Ab initio calculation of anisotropic magnetic properties of complexes. I. Unique definition of pseudospin Hamiltonians and their derivation. J. Chem. Phys. 2012, 137, 064112. [Google Scholar] [CrossRef]








| Compound 1 | Ueff | Ref. |
|---|---|---|
| [Dy(acac)3(H2O)2] | 45.9 cm−1 (66.1 K) | [34] |
| [Dy(acac)3(phen)] | 44.4 cm−1 (63.8 K) | [16] |
| [Dy(acac)3(dpq)] | 94.5 cm−1 (136 K) | [17] |
| [Dy(acac)3(dppz)] | 130 cm−1 (187 K) | [17] |
| [Dy(acac)3(dppn)] | 28.9 cm−1 (37.2 K) | [18] |
| [Dy(acac)3(tmphen)] | 90.9 cm−1 (130.4 K) | [14] |
| [Dy(acac)3(lz)] | 112 cm−1 (162 K) | [15] |
| [Dy(hfac)3(phendione)] 2 | 83 cm−1 (119 K) | [36] |
| [Dy(acac)3(phendione)] | 124 cm−1 (178 K) | This work, complex 2 |
| [Dy(acac)3(dmdophen)] | 198 cm−1 (284 K) | This work, complex 1 |
| KD | 1 | 2 | ||||||
|---|---|---|---|---|---|---|---|---|
| Energy | gx | gy | gz | Energy | gx | gy | gz | |
| 1 | 0.0 | 0.015 | 0.022 | 19.438 | 0.0 | 0.011 | 0.016 | 19.138 |
| 2 | 130.5 | 0.514 | 0.789 | 15.862 | 115.1 | 0.191 | 0.255 | 15.601 |
| 3 | 206.4 | 2.316 | 4.452 | 10.287 | 184.5 | 0.198 | 0.373 | 12.578 |
| 4 | 236.5 | 2.859 | 5.488 | 10.271 | 224.3 | 4.489 | 6.262 | 8.314 |
| 5 | 279.5 | 1.262 | 1.543 | 13.633 | 263.7 | 2.717 | 3.348 | 12.569 |
| 6 | 328.6 | 0.057 | 0.079 | 18.791 | 313.4 | 0.537 | 0.746 | 18.581 |
| 7 | 420.5 | 0.075 | 0.116 | 19.206 | 402.6 | 0.027 | 0.057 | 19.047 |
| 8 | 563.1 | 0.014 | 0.029 | 19.693 | 546.3 | 0.005 | 0.011 | 19.703 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gorshkov, E.V.; Korchagin, D.V.; Yureva, E.A.; Shilov, G.V.; Zhidkov, M.V.; Dmitriev, A.I.; Efimov, N.N.; Palii, A.V.; Aldoshin, S.M. Effect of Ligand Substitution on Zero-Field Slow Magnetic Relaxation in Mononuclear Dy(III) β-Diketonate Complexes with Phenanthroline-Based Ligands. Magnetochemistry 2022, 8, 151. https://doi.org/10.3390/magnetochemistry8110151
Gorshkov EV, Korchagin DV, Yureva EA, Shilov GV, Zhidkov MV, Dmitriev AI, Efimov NN, Palii AV, Aldoshin SM. Effect of Ligand Substitution on Zero-Field Slow Magnetic Relaxation in Mononuclear Dy(III) β-Diketonate Complexes with Phenanthroline-Based Ligands. Magnetochemistry. 2022; 8(11):151. https://doi.org/10.3390/magnetochemistry8110151
Chicago/Turabian StyleGorshkov, Egor V., Denis V. Korchagin, Elena A. Yureva, Gennadii V. Shilov, Mikhail V. Zhidkov, Alexei I. Dmitriev, Nikolay N. Efimov, Andrew V. Palii, and Sergey M. Aldoshin. 2022. "Effect of Ligand Substitution on Zero-Field Slow Magnetic Relaxation in Mononuclear Dy(III) β-Diketonate Complexes with Phenanthroline-Based Ligands" Magnetochemistry 8, no. 11: 151. https://doi.org/10.3390/magnetochemistry8110151