Magnetic Force Microscopy in Physics and Biomedical Applications
Abstract
1. Introduction
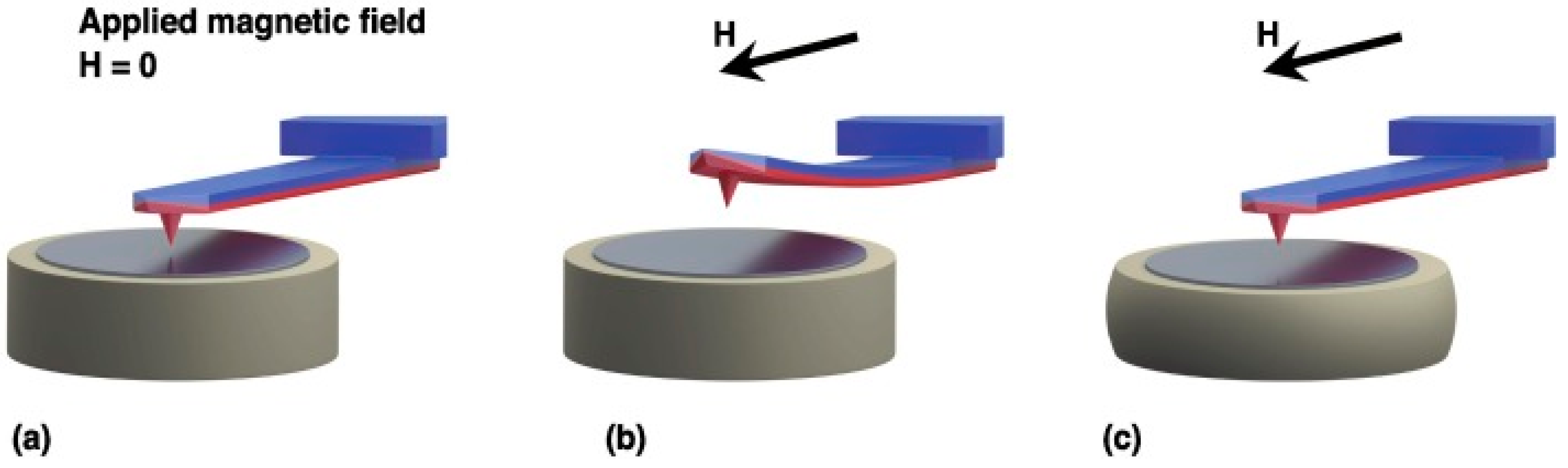
2. Principle of Magnetic Force Microscopy
3. Aspects of Magnetic Force Microscopy for Various Engineering Fields
3.1. MFM Design Ideas Applied in Magnetostrictive Thin Films
3.2. MFM for IC Current Used in Conductors and Superconductors
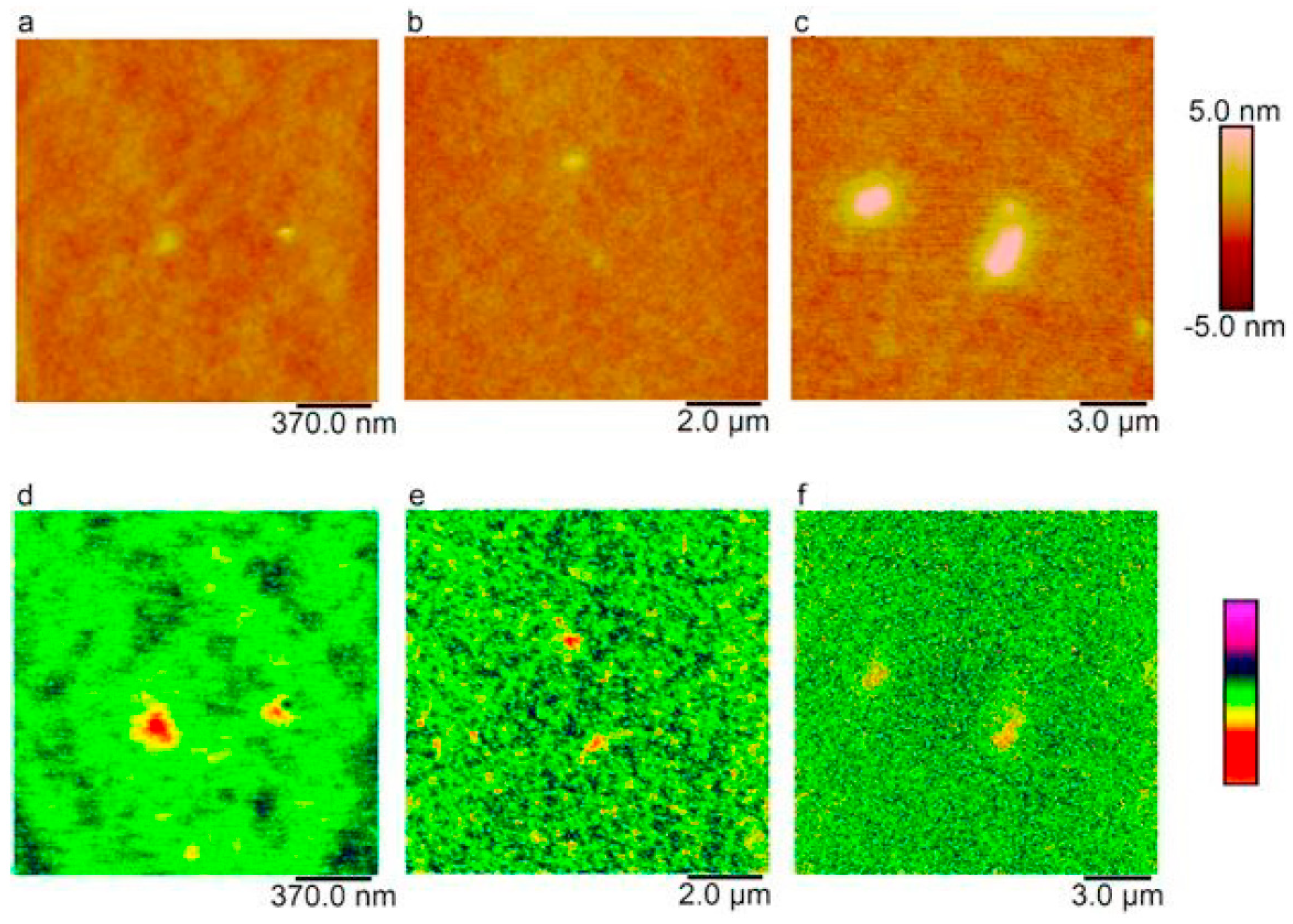
3.3. MFM Measurement for Magnetic and Non-Magnetic Particles Reinforced Matrix
4. Magnetic Force Microscopy for Bio-/Medical Applications
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Suits, J.C.; Geiss, R.H.; Lin, C.J.; Rugar, D.; Bell, A.E. Lorentz Microscopy of Micron-Sized Laser-Written Magnetic Domains in TbFe. Appl. Phys. Lett. 1986, 49, 419–421. [Google Scholar] [CrossRef]
- Stupakiewicz, A.; Chizhik, A.; Tekielak, M.; Zhukov, A.; Gonzalez, J.; Maziewski, A. Direct Imaging of the Magnetization Reversal in Microwires Using All-MOKE Microscopy. Rev. Sci. Instrum. 2014, 85, 103702. [Google Scholar] [CrossRef]
- Morales, J.R.; Amos, N.; Khizroev, S.; Garay, J.E. Magneto-Optical Faraday Effect in Nanocrystalline Oxides. J. Appl. Phys. 2011, 109, 093110. [Google Scholar] [CrossRef]
- Patterson, W.C.; Garraud, N.; Shorman, E.E.; Arnold, D.P. A Magneto-Optical Microscope for Quantitative Measurement of Magnetic Microstructures. Rev. Sci. Instrum. 2015, 86, 094704. [Google Scholar] [CrossRef] [PubMed]
- Corredor, E.C.; Kuhrau, S.; Kloodt-Twesten, F.; Frömter, R.; Oepen, H.P. SEMPA Investigation of the Dzyaloshinskii-Moriya Interaction in the Single, Ideally Grown Co/Pt (111) Interface. Phys. Rev. B 2017, 96, 060410. [Google Scholar] [CrossRef]
- Koike, K.; Hayakawa, K. Scanning Electron Microscope Observation of Magnetic Domains Using Spin-Polarized Secondary Electrons. Jpn. J. Appl. Phys. 1984, 23, L187. [Google Scholar] [CrossRef]
- Schneider, C.M. Perspectives in Element-Specific Magnetic Domain Imaging. J. Magn. Magn. Mater. 1996, 156, 94–98. [Google Scholar] [CrossRef]
- Okabayashi, J.; Miura, Y.; Munekata, H. Anatomy of Interfacial Spin-Orbit Coupling in Co/Pd Multilayers Using X-Ray Magnetic Circular Dichroism and First-Principles Calculations. Sci. Rep. 2018, 8, 8303. [Google Scholar] [CrossRef] [PubMed]
- Van der Laan, G.; Figueroa, A.I. X-ray Magnetic Circular Dichroism—A Versatile Tool to Study Magnetism. Coord. Chem. Rev. 2014, 277, 95–129. [Google Scholar] [CrossRef]
- Züger, O.; Rugar, D. Magnetic Resonance Detection and Imaging Using Force Microscope Techniques. J. Appl. Phys. 1994, 75, 6211–6216. [Google Scholar] [CrossRef]
- Toda, M.; Ono, T. Three-Dimensional Imaging of Electron Spin Resonance-Magnetic Resonance Force Microscopy at Room Temperature. J. Magn. Reson. 2021, 330, 107045. [Google Scholar] [CrossRef] [PubMed]
- Mamin, H.J.; Poggio, M.; Degen, C.L.; Rugar, D. Nuclear Magnetic Resonance Imaging with 90-NM Resolution. Nat. Nanotechnol. 2007, 2, 301–306. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, A.; Wiesendanger, R. Magnetic Sensitive Force Microscopy. Nano Today 2008, 3, 28–39. [Google Scholar] [CrossRef]
- Schoenherr, P.; Giraldo, L.M.; Lilienblum, M.; Trassin, M.; Meier, D.; Fiebig, M. Magnetoelectric Force Microscopy on Antiferromagnetic 180° Domains in Cr2O3. Materials 2017, 10, 1051. [Google Scholar] [CrossRef]
- Gutierrez, J.; Raes, B.; Silhanek, A.V.; Li, L.J.; Zhigadlo, N.D.; Karpinski, J.; Tempere, J.; Moshchalkov, V.V. Scanning Hall Probe Microscopy of Unconventional Vortex Patterns in the Two-Gap MgB2 Superconductor. Phys. Rev. B 2012, 85, 094511. [Google Scholar] [CrossRef]
- Shaw, G.; Kramer, R.B.G.; Dempsey, N.M.; Hasselbach, K. A Scanning Hall Probe Microscope for High Resolution, Large Area, Variable Height Magnetic Field Imaging. Rev. Sci. Instrum. 2016, 87, 113702. [Google Scholar] [CrossRef]
- Rondin, L.; Tetienne, J.P.; Hingant, T.; Roch, J.F.; Maletinsky, P.; Jacques, V. Magnetometry with Nitrogen-Vacancy Defects in Diamond. Rep. Prog. Phys. 2014, 77, 056503. [Google Scholar] [CrossRef]
- Aharonovich, I.; Neu, E. Diamond Nanophotonics. Adv. Opt. Mater. 2014, 2, 911–928. [Google Scholar] [CrossRef]
- Bitter, F. On Inhomogeneities in the Magnetization of Ferromagnetic Materials. Phys. Rev. 1931, 38, 1903. [Google Scholar] [CrossRef]
- Israel, C.; Hyun, C.; de Lozanne, A.; Phark, S.; Khim, Z.G. Compact Variable-Temperature Magnetic Force Microscope with Optical Access and Lateral Cantilever Positioning. Rev. Sci. Instrum. 2006, 77, 023704. [Google Scholar] [CrossRef]
- Yang, J.; Yang, I.; Kim, Y.W.; Shin, D.; Jeong, J.; Wulferding, D.; Yeom, H.W.; Kim, J. Construction of a 3He Magnetic Force Microscope with a Vector Magnet. Rev. Sci. Instrum. 2016, 87, 023704. [Google Scholar] [CrossRef] [PubMed]
- Nazaretski, E.; Graham, K.S.; Thompson, J.D.; Wright, J.A.; Pelekhov, D.V.; Hammel, P.C.; Movshovich, R. Design of a Variable Temperature Scanning Force Microscope. Rev. Sci. Instrum. 2009, 80, 083704. [Google Scholar] [CrossRef] [PubMed]
- De Lozanne, A. Application of Magnetic Force Microscopy in Nanomaterials Characterization. Microsc. Res. Tech. 2006, 69, 550–562. [Google Scholar] [CrossRef] [PubMed]
- Kazakova, O.; Puttock, R.; Barton, C.; Corte-León, H.; Jaafar, M.; Neu, V.; Asenjo, A. Frontiers of Magnetic Force Microscopy. J. Appl. Phys. 2019, 125, 060901. [Google Scholar] [CrossRef]
- Howland, R.; Benatar, L. A Practical Guide: To Scanning Probe Microscopy. In Park Scientific Instruments; Symanski, C., Emerson, L., Leckenby, J., Eds.; ThermoMicroscopes: Sunnyvale, CA, USA, 1996. [Google Scholar]
- Binnig, G.; Rohrer, H.; Gerber, C.; Weibel, E. Surface Studies by Scanning Tunneling Microscopy. Phys. Rev. Lett. 1982, 49, 57. [Google Scholar] [CrossRef]
- Binnig, G.; Rohrer, H. In Touch with Atoms. Rev. Mod. Phys. 1999, 71, S324. [Google Scholar] [CrossRef]
- Binnig, G.; Quate, C.F.; Gerber, C. Atomic Force Microscopy. Phys. Rev. Lett. 1986, 56, 930. [Google Scholar] [CrossRef]
- Zhong, Q.; Inniss, D.; Kjoller, K.; Elings, V.B. Fractured Polymer/Silica Fiber Surface Studied by Tapping Mode Atomic Force Microscopy. Surf. Sci. Lett. 1993, 290, L688–L692. [Google Scholar]
- Garcıa, R.; Perez, R. Dynamic Atomic Force Microscopy Methods. Surf. Sci. Rep. 2002, 47, 197–301. [Google Scholar] [CrossRef]
- Schneiderbauer, M.; Wastl, D.; Giessibl, F.J. qPlus Magnetic Force Microscopy in Frequency-Modulation Mode with Millihertz Resolution. Beilstein J. Nanotechnol. 2012, 3, 174–178. [Google Scholar] [CrossRef]
- Zhao, X.; Schwenk, J.; Mandru, A.O.; Penedo, M.; Baćani, M.; Marioni, M.A.; Hug, H.J. Magnetic Force Microscopy with Frequency-Modulated Capacitive Tip–Sample Distance Control. New J. Phys. 2018, 20, 013018. [Google Scholar] [CrossRef]
- Hrouzek, M. Feedback Control in an Atomic Force Microscope Used as a Nano-Manipulator. Acta Polytech. 2005, 45, 65–69. [Google Scholar] [CrossRef]
- Jaafar, M.; Iglesias-Freire, O.; Serrano-Ramón, L.; Ibarra, M.R.; de Teresa, J.M.; Asenjo, A. Distinguishing Magnetic and Electrostatic Interactions by a Kelvin Probe Force Microscopy–Magnetic Force Microscopy Combination. Beilstein J. Nanotechnol. 2011, 2, 552–560. [Google Scholar] [CrossRef] [PubMed]
- Martin, Y.; Wickramasinghe, H.K. Magnetic Imaging by ‘‘Force Microscopy’’ with 1000 Å Resolution. Appl. Phys. Lett. 1987, 50, 1455–1457. [Google Scholar] [CrossRef]
- Saenz, J.J.; Garcia, N.; Grütter, P.; Meyer, E.; Heinzelmann, H.; Wiesendanger, R.; Rosenthaler, L.; Hidber, H.R.; Güntherodt, H.J. Observation of Magnetic Forces by the Atomic Force Microscope. J. Appl. Phys. 1987, 62, 4293–4295. [Google Scholar] [CrossRef]
- Hobbs, P.C.; Abraham, D.W.; Wickramasinghe, H.K. Magnetic Force Microscopy with 25 NM Resolution. Appl. Phys. Lett. 1989, 55, 2357–2359. [Google Scholar] [CrossRef]
- Seo, Y.; Cadden-Zimansky, P.; Chandrasekhar, V. Low-Temperature High-Resolution Magnetic Force Microscopy Using a Quartz Tuning Fork. Appl. Phys. Lett. 2005, 87, 103103. [Google Scholar] [CrossRef]
- Babic, B.; Hsu, M.T.; Gray, M.B.; Lu, M.; Herrmann, J. Mechanical and Electrical Characterization of Quartz Tuning Fork Force Sensors. Sens. Actuators A 2015, 223, 167–173. [Google Scholar] [CrossRef]
- Grutter, P.; Rugar, D.; Mamin, H.J.; Castillo, G.; Lambert, S.E.; Lin, C.-J.; Valletta, R.M. Batch Fabricated Sensors for Magnetic Force Microscopy. Appl. Phys. Lett. 1990, 57, 1820–1822. [Google Scholar] [CrossRef]
- Babcock, K.; Elings, V.; Dugas, M.; Loper, S. Optimization of Thin-Film Tips for Magnetic Force Microscopy. IEEE Trans. Magn. 1994, 30, 4503–4505. [Google Scholar] [CrossRef]
- Gehanno, V.; Marty, A.; Gilles, B.; Samson, Y. Magnetic Domains in Epitaxial Ordered FePd (001) Thin Films with Perpendicular Magnetic Anisotropy. Phys. Rev. B 1997, 55, 12552. [Google Scholar] [CrossRef]
- Nguyen, G.D.; Lee, J.; Berlijn, T.; Zou, Q.; Hus, S.M.; Park, J.; Gai, Z.; Lee, C.; Li, A.P. Visualization and Manipulation of Magnetic Domains in the Quasi-Two-Dimensional Material Fe3GeTe2. Phys. Rev. B 2018, 97, 014425. [Google Scholar] [CrossRef]
- Vokoun, D.; Svatuška, M.; Olejníček, J.; Kohout, M.; Drahokoupil, J.; Rameš, M.; Vejpravová, J.; Mantlíková, A.; Fekete, L.; Kopeček, J.; et al. Ni–TiO2 Nanocomposite Films and Their Magnetic Properties. Phys. B Condens. Matter 2016, 503, 44–50. [Google Scholar] [CrossRef]
- Vokoun, D.; Vronka, M.; Rameš, M.; Olejníček, J.; Kohout, M.; Heczko, O. Ni Nanoparticles in TiO2 Films and Their Magnetic Properties. Phys. B Condens. Matter 2020, 578, 411862. [Google Scholar] [CrossRef]
- Naibert, T.R.; Polshyn, H.; Garrido-Menacho, R.; Durkin, M.; Wolin, B.; Chua, V.; Mondragon-Shem, I.; Hughes, T.; Mason, N.; Budakian, R. Imaging and Controlling Vortex Dynamics in Mesoscopic Superconductor–Normal-Metal–Superconductor Arrays. Phys. Rev. B 2021, 103, 224526. [Google Scholar] [CrossRef]
- Shinjo, T.; Okuno, T.; Hassdorf, R.; Shigeto, K.; Ono, T. Magnetic Vortex Core Observation in Circular Dots of Permalloy. Science 2000, 289, 930–932. [Google Scholar] [CrossRef]
- Fert, A.; Reyren, N.; Cros, V. Magnetic Skyrmions: Advances in Physics and Potential Applications. Nat. Rev. Mater. 2017, 2, 17031. [Google Scholar] [CrossRef]
- Meng, K.Y.; Ahmed, A.S.; Baćani, M.; Mandru, A.O.; Zhao, X.; Bagués, N.; Esser, B.D.; Flores, J.; McComb, D.W.; Hug, H.J.; et al. Observation of Nanoscale Skyrmions in SrIrO3/SrRuO3 Bilayers. Nano Lett. 2019, 19, 3169–3175. [Google Scholar] [CrossRef]
- Medvedkin, G.A.; Ishibashi, T.; Nishi, T.; Hayata, K.; Hasegawa, Y.; Sato, K. Room Temperature Ferromagnetism in Novel Diluted Magnetic Semiconductor Cd1-xMnxGeP2. Jpn. J. Appl. Phys. 2000, 39, L949. [Google Scholar] [CrossRef]
- Straka, L.; Fekete, L.; Heczko, O. Antiphase Boundaries in Bulk Ni-Mn-Ga Heusler Alloy Observed by Magnetic Force Microscopy. Appl. Phys. Lett. 2018, 113, 172901. [Google Scholar] [CrossRef]
- Hug, H.J.; Stiefel, B.; Van Schendel, P.J.A.; Moser, A.; Hofer, R.; Martin, S.; Güntherodt, H.J.; Porthun, S.; Abelmann, L.; Lodder, J.C.; et al. Quantitative Magnetic Force Microscopy on Perpendicularly Magnetized Samples. J. Appl. Phys. 1998, 83, 5609–5620. [Google Scholar] [CrossRef]
- Lohau, J.; Kirsch, S.; Carl, A.; Dumpich, G.; Wassermann, E.F. Quantitative Determination of Effective Dipole and Monopole Moments of Magnetic Force Microscopy Tips. J. Appl. Phys. 1999, 86, 3410–3417. [Google Scholar] [CrossRef]
- Bian, K.; Gerber, C.; Heinrich, A.J.; Müller, D.J.; Scheuring, S.; Jiang, Y. Scanning Probe Microscopy. Nat. Rev. Methods Primers 2021, 1, 36. [Google Scholar] [CrossRef]
- Wadas, A.; Hug, H.J. Models for the Stray Field from Magnetic Tips Used in Magnetic Force Microscopy. J. Appl. Phys. 1992, 72, 203–206. [Google Scholar] [CrossRef]
- Wadas, A.; Grütter, P. Theoretical Approach to Magnetic Force Microscopy. Phys. Rev. B 1989, 39, 12013. [Google Scholar] [CrossRef] [PubMed]
- Vokoun, D.; Beleggia, M. Forces between Arrays of Permanent Magnets of Basic Geometric Shapes. J. Magn. Magn. Mater. 2014, 350, 174–178. [Google Scholar] [CrossRef]
- Hartmann, U. Magnetic Force Microscopy. Annu. Rev. Mater. Sci. 1999, 29, 53–87. [Google Scholar] [CrossRef]
- Stachiv, I.; Fang, T.-H.; Chen, T.-H. Micro-/Nanosized Cantilever Beams and Mass Sensors under Applied Axial Tensile/Compressive Force Vibrating in Vaccum and Viscous Fluid. AIP Adv. 2015, 5, 117140. [Google Scholar] [CrossRef]
- Pethica, J.B.; Oliver, W.C. Tip Surface Interactions in STM and AFM. Phys. Scr. 1987, 1987, 61–66. [Google Scholar] [CrossRef]
- Ueyama, H.; Sugawara, Y.; Morita, S. Stable Operation Mode for Dynamic Noncontact Atomic Force Microscopy. Appl. Phys. A 1998, 66, S295–S297. [Google Scholar] [CrossRef]
- Osiander, R.; Ecelberger, S.A.; Givens, A.B.; Wickenden, D.K.; Murphy, J.C.; Kistenmacher, T.J. A Micromechanical-Based Magnetostrictive Magnetometer. Appl. Phys. Lett. 1996, 69, 2930. [Google Scholar] [CrossRef]
- Ludwig, A.; Quandt, E. Giant Magnetostrictive Thin Films for Applications in Micromechanical Systems (Invited). J. Appl. Phys. 2000, 87, 4691. [Google Scholar] [CrossRef]
- Coïsson, M.; Hüttenes, W.; Cialone, M.; Barrera, G.; Celegato, F.; Rizzi, P.; Barber, Z.H.; Tiberto, P. Measurement of Thin Film Magnetostriction Using Field-Dependent Atomic Force Microscopy. Appl. Surf. Sci. 2020, 525, 146514. [Google Scholar] [CrossRef]
- Klokholm, E. The Measurement of Magnetostriction in Ferromagnetic Thin Films. IEEE Trans. Magn. 1976, 6, 819–821. [Google Scholar] [CrossRef]
- Harin, E.V.; Sheftel, E.N.; Krikunov, A.I. Atomic Force Microscopy Measurements of Magnetostriction of Soft-Magnetic Films. Solid State Phenom. 2012, 190, 179–182. [Google Scholar] [CrossRef]
- Xie, S.H.; Liu, X.Y.; Zhou, Y.C.; Li, J.Y. Correlation of Magnetic Domains and Magnetostrictive Strains in Terfenol-D via Magnetic Force Microscopy. J. Appl. Phys. 2011, 109, 063911. [Google Scholar] [CrossRef]
- Goiriena-Goikoetxea, M.; Guslienko, K.Y.; Rouco, M.; Orue, I.; Berganza, E.; Jaafar, M.; García-Arribas, A. Magnetization Reversal in Circular Vortex Dots of Small Radius. Nanoscale 2017, 9, 11269–11278. [Google Scholar] [CrossRef]
- Sasaki, J.; Matsubara, F. Circular Phase of a Two-Dimensional Ferromagnet with Dipolar Interactions. J. Phys. Soc. Jpn. 1997, 66, 2138. [Google Scholar] [CrossRef][Green Version]
- Cowburn, R.P.; Koltsov, D.K.; Adeyeye, A.O.; Welland, M.E.; Tricker, D.M. Single-Domain Circular Nanomagnets. Phys. Rev. Lett. 1999, 83, 1042. [Google Scholar] [CrossRef]
- Volodin, A.P.; Marchevsky, M.V. Magnetic Force Microscopy Investigations of Suprconductors. Ultramicroscopy 1992, 42–44, 757–763. [Google Scholar] [CrossRef]
- Berte, R.; Hartman, U.; Heiden, C. Scanning Tunneling Microscopy of the Abrikosov Flux Lattice with Ferromagnetic Probes. Appl. Phys. Lett. 1990, 57, 2351. [Google Scholar] [CrossRef]
- Grutter, P.; Jung, T.; Heinzelmann, H.; Meyer, E.; Hidber, H.-R.; Guntherodt, H.-J. 10-nm Resolution by Magnetic Force Microscopy on FeNdB. J. Appl. Phys. 1990, 67, 1437. [Google Scholar] [CrossRef][Green Version]
- Krivcov, A.; Schneider, J.; Junkers, T.; Mobius, H. Magnetic Force Microscopy of in a Polymer Matrix Embedded Single Magnetic Nano Particles. Phys. Status Solidi A 2019, 216, 1800753. [Google Scholar] [CrossRef]
- Sarma, L.; Aomoa, N.; Sarmah, T.; Sarma, S.; Srinivasan, A.; Sharma, G.; Gupta, A.; Reddy, V.R.; Satpati, B.; Srivastava, D.N.; et al. Synthesis of Finest Superparamagnetic Carbon-Encapsulated Magnetic Nanoparticles by a Plasma Expansion Method for Biomedical Applications. J. Alloys Compd. 2018, 749, 768. [Google Scholar] [CrossRef]
- Nguyen, D.; Pham, B.T.T.; Huynh, V.; Kim, B.J.; Pham, N.T.H.; Bickley, S.A.; Jones, S.K.; Serelis, A.; Davey, T.; Such, C.; et al. Monodispersed Polymer Encapsulated Superparamagnetic Iron Oxide Nanoparticles for Cell Labeling. Polymer 2016, 106, 238. [Google Scholar] [CrossRef]
- Schreiber, S.; Savla, M.; Pelekhov, D.V.; Iscru, D.F.; Selcu, C.; Hammel, P.C.; Agarwal, G. Magnetic Force Microscopy of Superparamagnetic Nanoparticles. Small 2008, 4, 270. [Google Scholar] [CrossRef]
- Rasa, M.; Philipse, A.P. Scanning Probe Microscopy on Magnetic Colloidal Particles. J. Magn. Magn. Mater. 2002, 252, 101–103. [Google Scholar] [CrossRef][Green Version]
- Chapman, J.N. The Investigation of Magnetic Domain Structures in Thin Foils by Electron Microscopy. J. Phys. D Appl. Phys. 1984, 17, 623. [Google Scholar] [CrossRef]
- Chen, C.T.; Idzerda, Y.U.; Lin, H.-J.; Meigs, G.; Chaiken, A.; Prinz, G.A.; Ho, G.H. Element-Specific Magnetic Hysteresis as a Means for Studying Heteromagnetic Multilayers. Phys. Rev. B. 1993, 48, 642. [Google Scholar] [CrossRef]
- Passeri, D.; Dong, C.; Angeloni, L.; Pantanella, F.; Natalizi, T.; Berlutti, F. Thickness Measurement of Soft Thin Films on Periodically Patterned Magnetic Substrates by Phase Difference Magnetic Force Microscopy. Ultramicroscopy 2014, 136, 96–106. [Google Scholar] [CrossRef]
- Newacheck, S.; Huynh, N.U.; Youssef, G. Colossal Crystal in P3HT:PCBM Blends for Enhanced Organic Magnetism. Cryst. Growth Des. 2021, 21, 5300–5305. [Google Scholar] [CrossRef]
- Nocera, T.M.; Zeng, Y.; Agarwal, G. Distinguishing Ferritin from Apoferritin Using Magnetic Force Microscopy. Nanotechnology 2014, 25, 461001. [Google Scholar] [CrossRef]
- Zhang, N.; Yu, X.; Xie, J.; Xu, H. New Insights into the Role of Ferritin in Iron Homeostasis and Neurodegenerative Diseases. Mol. Neurobiol. 2021, 58, 2812–2823. [Google Scholar] [CrossRef] [PubMed]
- Peng, D.; Chen, C.A.; Ruhela, D.; Li, Y.; Regan, R.F. Deferoxamine Deconditioning Increases Neuronal Vulnerability to Hemoglobin. Exp. Cell. Res. 2020, 390, 111926. [Google Scholar] [CrossRef] [PubMed]
- Palombarini, F.; Di Fabio, E.; Boffi, A.; Macone, A.; Bonamore, A. Ferritin Nanocages for Protein Delivery to Tumor Cells. Molecules 2020, 25, 825. [Google Scholar] [CrossRef]
- Stachiv, I.; Fang, T.-H.; Jeng, Y.-R. Mass Detection in Viscous Fluid Utilizing Vibrating Micro- and Nanomechanical Mass Sensors under Applied Axial Tensile Force. Sensors 2015, 15, 19351–19368. [Google Scholar] [CrossRef]
- Stachiv, I.; Gan, L.; Kuo, C.-Y.; Sittner, P.; Sevecek, O. Mass Spectrometry of Heavy Analytes and Large Biological Aggregates by Monitoring Changes in the Quality Factor of Nanomechanical Resonator in Air. ACS Sens. 2020, 5, 2128–2135. [Google Scholar] [CrossRef]
- Sifford, J.; Walsch, K.J.; Tong, S.; Bao, G.; Agarwal, G. Indirect Magnetic Force Microscopy. Nanoscale Adv. 2019, 1, 2348–2355. [Google Scholar] [CrossRef]
- Moya, C.; Iglesias-Freire, O.; Perez, N.; Batlle, X.; Labarta, A.; Asenjo, A. Direct Imaging of the Magnetic Polarity and Reversal Mechanism in Individual Fe3-XO4 Nanoparticles. Nanoscale 2015, 7, 8110–8114. [Google Scholar] [CrossRef]
- Pinilla-Cienfuegos, E.; Mañas-Valero, S.; Forment-Aliaga, A.; Coronado, E. Switching the Magnetic Vortex Core in a Single Nanoparticle. ACS Nano 2016, 10, 1764–1770. [Google Scholar] [CrossRef]
- Li, R.-N.; Da, X.-H.; Li, X.; Lu, Y.-S.; Gu, F.-F.; Liu, Y. Functionalized Magnetic Nanoparticles for Drug Delivery in Tumor Therapy. Chinese Phys. B 2021, 30, 017502. [Google Scholar] [CrossRef]
- Clavo, B.; Rodríguez-Esparragón, F.; Rodríguez-Abreu, D.; Martínez-Sánchez, G.; Llontop, P.; Aguiar-Bujanda, D.; Fernández-Pérez, L.; Santana-Rodríguez, N. Modulation of Oxidative Stress by Ozone Therapy in the Prevention and Treatment of Chemotherapy-Induced Toxicity: Review and Prospects. Antioxidants 2019, 8, 588. [Google Scholar] [CrossRef] [PubMed]
- Oun, R.; Moussa, Y.E.; Wheate, N.J. The Side Effects of Platinum-Based Chemotherapy Drugs: A Review for Chemists. Dalton Trans. 2018, 47, 6645–6653. [Google Scholar] [CrossRef] [PubMed]
- Patel, H.; Panchal, D.R.; Patel, U.; Brahmbhatt, T.; Suthar, M. Matrix Type Drug Delivery System: A Review. J. Pharmaceut. Sci. Biosci. Res. 2011, 1, 143–151. [Google Scholar]
- Aslam, H.; Shukrullah, S.; Naz, M.Y.; Fatima, H.; Hussain, H.; Ullah, S.; Assiri, M.A. Current and Future Perspectives of Multifunctional Magnetic Nanoparticles Based Controlled Drug Delivery Systems. J. Drug Deliv. Sci. Technol. 2022, 67, 102946. [Google Scholar] [CrossRef]
- Ngema, L.M.; Adeyemi, S.A.; Marimuthu, T.; Choonara, Y.E. A Review on Engineered Magnetic Nanoparticles in Non-Small-Cell Lung Carcinoma Targeted Therapy. Int. J. Pharm. 2021, 606, 120870. [Google Scholar] [CrossRef]
- Mody, V.V.; Singh, A.; Wesley, B. Basics of Magnetic Nanoparticles for Their Application in the Field of Magnetic Fluid Hyperthermia. Eur. J. Nanomed. 2013, 5, 11–21. [Google Scholar] [CrossRef]
- Veiseh, O.; Gunn, J.; Zhang, M. Design and Fabrication of Magnetic Nanoparticles for Targeted Drug Delivery and Imaging. Adv. Drug Deliv. Rev. 2010, 62, 284–304. [Google Scholar] [CrossRef]
- Mukhortova, Y.R.; Pryadko, A.S.; Chernozem, R.V.; Pariy, I.O.; Akoulina, E.A.; Demianova, I.V.; Zharkova, I.I.; Ivanov, Y.F.; Wagner, D.V.; Bonartsev, A.P.; et al. Fabrication and Characterization of a Magnetic Biocomposite of Magnetite Nanoparticles and Reduced Graphene Oxide for Biomedical Applications. Nano-Struct. Nano-Objects 2022, 29, 100843. [Google Scholar] [CrossRef]
- Frantellizzi, V.; Conte, M.; Pontico, M.; Pani, A.; Pani, R.; De Vincentis, G. New Frontiers in Molecular Imaging with Superparamagnetic Iron Oxide Nanoparticles (SPIONs): Efficacy, Toxicity, and Future Applications. Nucl. Med. Mol. Imag. 2020, 54, 65–80. [Google Scholar] [CrossRef]
- Batlle, X.; Moya, C.; Escoda-Torroella, M.; Iglesias, O.; Rodrigez, A.F.; Labarta, A. Magnetic Nanoparticles: From the Nanostructure to the Physical Properties. J. Magn. Magn. Mater. 2022, 543, 168594. [Google Scholar] [CrossRef]
- Cordova, G.; Lee, B.Y.; Leonenko, Z. Magnetic Force Microscopy for Nanoparticle Characterization. Nano World J. 2016, 2, 10–14. [Google Scholar] [CrossRef]
- Park, J.; An, K.; Hwang, Y.; Park, J.-G.; Noh, H.-J.; Kim, J.-Y.; Park, J.-H.; Hwang, N.-M.; Hyeon, T. Ultra-Large-Scale Syntheses of Monodisperse Nanocrystals. Nat. Mater. 2004, 3, 891–895. [Google Scholar] [CrossRef]
- Biehl, P.; Von der Lühe, M.; Dutz, S.; Schacher, F.H. Synthesis, Characterization, and Applications of Magnetic Nanoparticles Featuring Polyzwitterionic Coatings. Polymers 2018, 10, 91. [Google Scholar] [CrossRef]
- Nocera, T.M.; Chen, J.; Murray, C.B.; Agarwal, G. Magnetic Anisotropy Considerations in Magnetic Force Microscopy Studies of Single Super-Paramagnetic Nanoparticle. Nanotechnology 2012, 23, 495704. [Google Scholar] [CrossRef]
- Fuentes-García, J.A.; Diaz-Cano, A.I.; Guillen-Cervantes, A.; Santoyo-Salazar, J. Magnetic Domain Interactions of Fe3O4 Nanoparticles Embedded in a SiO2 Matrix. Sci. Rep. 2018, 8, 5096. [Google Scholar] [CrossRef]
- Li, Y.; Liu, Q.; Hess, A.J.; Mi, S.; Liu, X.; Chen, Z.; Xie, Y.; Smalyukh, I.I. Programmable Ultralight Magnets via Orientation Arrangement of Ferromagnetic Nanoparticles with Aerosol Hosts. ACS Nano 2019, 13, 13875–13883. [Google Scholar] [CrossRef]
- Cianchetti, M.; Laschi, C.; Menciassi, A.; Dario, P. Biomedical Applications of Soft Robotics. Nat. Rev. Mater. 2018, 3, 143–153. [Google Scholar] [CrossRef]
- Sievers, S.; Braun, K.-F.; Eberbeck, D.; Gustafsson, S.; Olsson, E.; Schumacher, H.W.; Sieger, U. Quantitative Measurement of the Magnetic Moment of Individual Magnetic Nanoparticles by Magnetic Force Microscopy. Small 2012, 8, 2675–2679. [Google Scholar] [CrossRef] [PubMed]
- Krivcov, A.; Junkers, T.; Mobius, H. Understanding of Electrostatic and Magnetic Forces in Magnetic Force Microscopy: Towards Single Superparamagnetic Nanoparticle Resolution. J. Phys. Commun. 2018, 2, 075019. [Google Scholar] [CrossRef]
- Araujo, J.F.D.F.; Tahir; Arsalami, S.; Freire, F.L., Jr.; Mariotto, G.; Cremona, M.; Mendoza, L.A.F.; Luz-Lima, C.; Zaman, Q.; Del Rosso, T.; et al. Novel Scanning Magnetic Microscopy Method for the Characterization of Magnetic Nanoparticles. J. Mag. Mag. Mater. 2020, 499, 166300. [Google Scholar] [CrossRef]
- Socoliuc, V.; Peddis, D.; Petrenko, V.I.; Avdeev, M.V.; Susan-Resiga, D.; Szabó, T.; Turcu, R.; Tombácz, E.; Vékás, L. Magnetic Nanoparticle Systems for Nanomedicine—A Materials Science Perspective. Magnetochemistry 2020, 6, 2. [Google Scholar] [CrossRef]
- Nyamjav, D.; Kinsella, J.M.; Ivanisevic, A. Magnetic Nanowires with DNA Cores: A Magnetic Force Microscopy. Appl. Phys. Lett. 2005, 86, 093107. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vokoun, D.; Samal, S.; Stachiv, I. Magnetic Force Microscopy in Physics and Biomedical Applications. Magnetochemistry 2022, 8, 42. https://doi.org/10.3390/magnetochemistry8040042
Vokoun D, Samal S, Stachiv I. Magnetic Force Microscopy in Physics and Biomedical Applications. Magnetochemistry. 2022; 8(4):42. https://doi.org/10.3390/magnetochemistry8040042
Chicago/Turabian StyleVokoun, David, Sneha Samal, and Ivo Stachiv. 2022. "Magnetic Force Microscopy in Physics and Biomedical Applications" Magnetochemistry 8, no. 4: 42. https://doi.org/10.3390/magnetochemistry8040042
APA StyleVokoun, D., Samal, S., & Stachiv, I. (2022). Magnetic Force Microscopy in Physics and Biomedical Applications. Magnetochemistry, 8(4), 42. https://doi.org/10.3390/magnetochemistry8040042







