Structure and Lattice Dynamics of Bi1−xNdxFeO3 and Bi1−xGdxFeO3 Ceramics near the Morphotropic Phase Boundary
Abstract
:1. Introduction
2. Experimental Section
3. Results
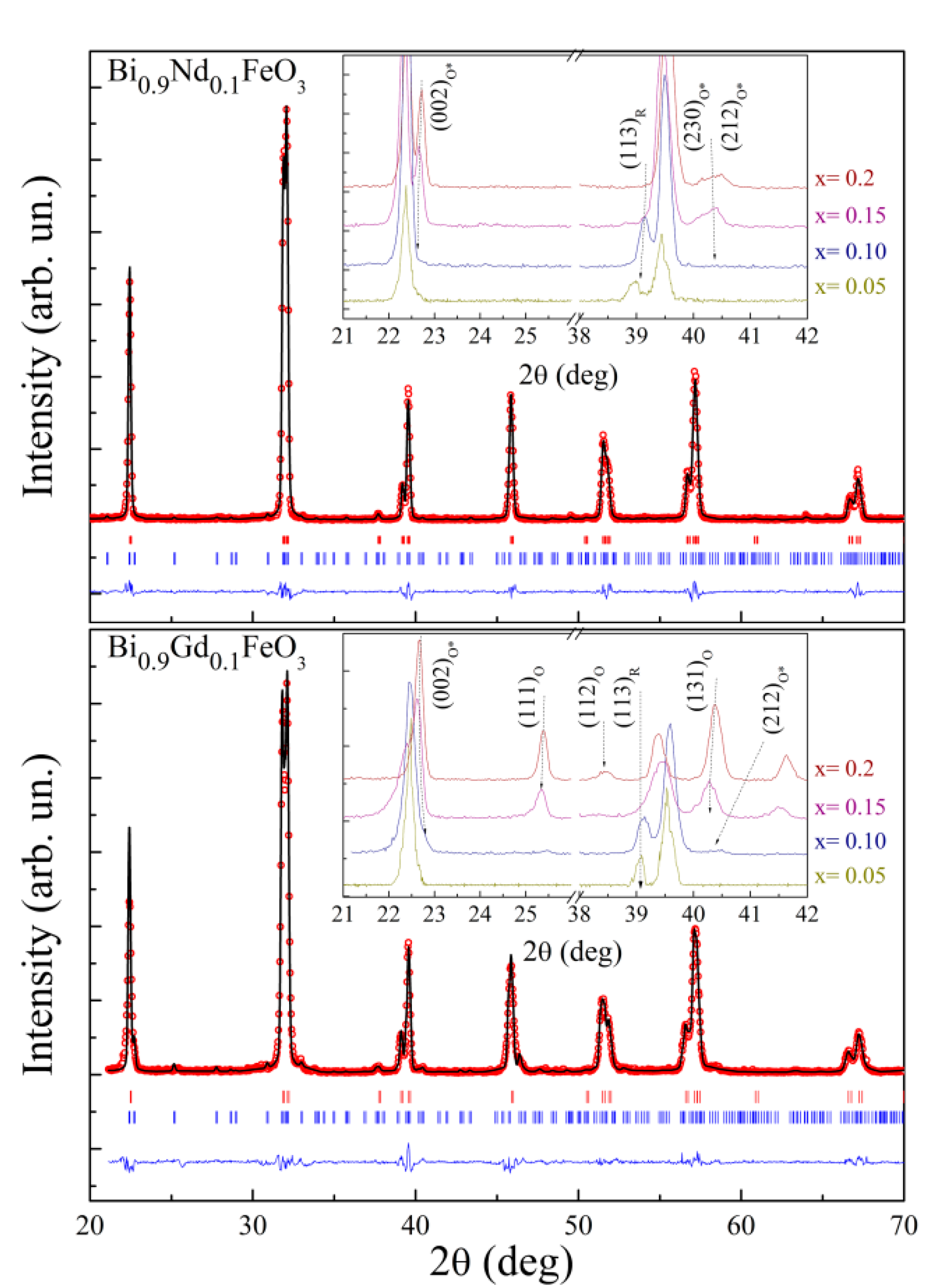
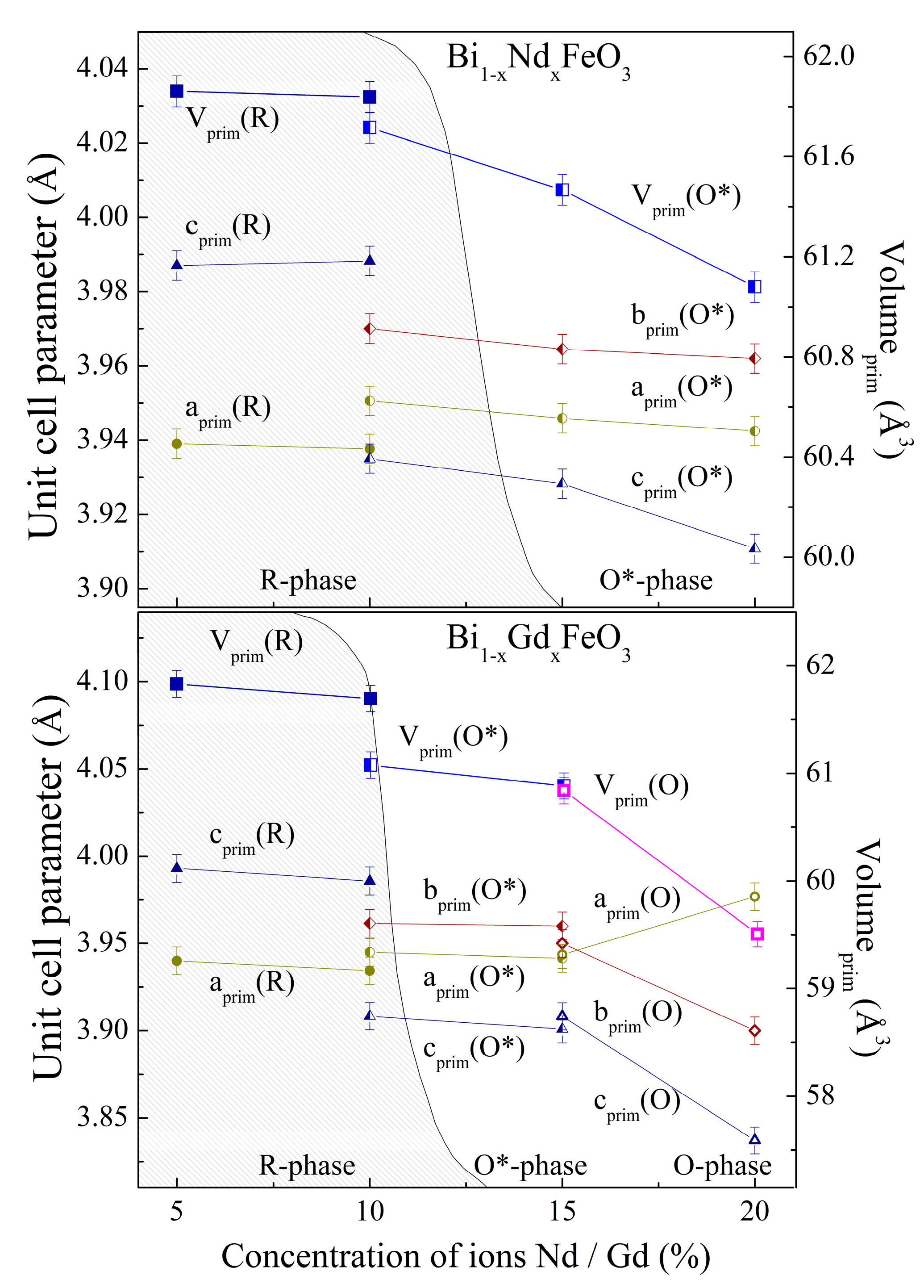
3.1. Analysis of the Crystal Structure by Diffraction Measurements
3.2. FT-IR Spectroscopy Analysis
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Pullar, R.C. Hexagonal ferrites: A review of the synthesis, properties and applications of hexaferrite ceramics. Prog. Mater Sci. 2012, 57, 1191–1334. [Google Scholar] [CrossRef]
- Trukhanov, A.V.; Turchenko, V.O.; Bobrikov, I.A.; Trukhanov, S.V.; Kazakevich, I.S.; Balagurov, A.M. Crystal structure and magnetic properties of the BaFe12−xAlxO19 (x=0.1–1.2) solid solutions. J. Magn. Magn. Mater. 2015, 393, 253–259. [Google Scholar] [CrossRef]
- Trukhanov, A.V.; Kostishyn, V.G.; Panina, L.V.; Korovushkin, V.V.; Turchenko, V.A.; Thakur, P.; Thakur, A.; Yang, Y.; Vinnik, D.A.; Yakovenko, E.S.; et al. Control of electromagnetic properties in substituted M-type hexagonal ferrites. J. Alloys Compd. 2018, 754, 247–256. [Google Scholar] [CrossRef]
- Trukhanov, S.V.; Trukhanov, A.V.; Turchenko, V.A.; Trukhanov, A.V.; Trukhanova, E.L.; Tishkevich, D.I.; Ivanov, V.M.; Zubar, T.I.; Salem, M.; Kostishyn, V.G.; et al. Polarization origin and iron positions in indium doped barium hexaferrites. Ceram. Int. 2018, 44, 290–300. [Google Scholar] [CrossRef]
- Almessiere, M.A.; Trukhanov, A.V.; Slimani, Y.; You, K.Y.; Trukhanov, S.V.; Trukhanova, E.L.; Esa, F.; Sadaqat, A.; Chaudhary, K.; Zdorovets, M.; et al. Correlation Between Composition and Electrodynamics Properties in Nanocomposites Based on Hard/Soft Ferrimagnetics with Strong Exchange Coupling. Nanomaterials 2019, 9, 202. [Google Scholar] [CrossRef]
- Spaldin, N.A.; Ramesh, R. Advances in magnetoelectric multiferroics. Nat. Mater. 2019, 18, 203–212. [Google Scholar] [CrossRef]
- Ghosh, A.; Trujillo, D.P.; Choi, H.; Nakhmanson, S.M.; Alpay, S.P.; Zhu, J.-X. Electronic and Magnetic Properties of Lanthanum and Strontium Doped Bismuth Ferrite: A First-Principles Study. Sci. Rep. 2019, 9, 194. [Google Scholar] [CrossRef] [PubMed]
- Karpinsky, D.V.; Silibin, M.V.; Trukhanov, S.V.; Trukhanov, A.V.; Zhaludkevich, A.L.; Latushka, S.I.; Zhaludkevich, D.V.; Khomchenko, V.A.; Alikin, D.O.; Abramov, A.S.; et al. Peculiarities of the Crystal Structure Evolution of BiFeO3–BaTiO3 Ceramics across Structural Phase Transitions. Nanomaterials 2020, 10, 801. [Google Scholar] [CrossRef]
- Zhang, Y.-j.; Zhang, H.-g.; Yin, J.-h.; Zhang, H.-w.; Chen, J.-l.; Wang, W.-q.; Wu, G.-h. Structural and magnetic properties in Bi1−xRxFeO3 (x=0–1, R=La, Nd, Sm, Eu and Tb) polycrystalline ceramics. J. Magn. Magn. Mater. 2010, 322, 2251–2255. [Google Scholar] [CrossRef]
- Xue, F.; Fu, Q.; Zhou, D.; Tian, Y.; Hu, Y.; Zheng, Z.; Zhou, L. Properties of Bi0.8Ln0.2FeO3 (Ln=La, Gd, Ho) multiferroic ceramics. Ceram. Int. 2015, 41(Part B), 14718–14726. [Google Scholar] [CrossRef]
- Troyanchuk, I.O.; Karpinsky, D.V.; Bushinsky, M.V.; Mantytskaya, O.S.; Tereshko, N.V.; Shut, V.N. Phase transitions, magnetic and piezoelectric properties of rare-earth-substituted BiFeO3 ceramics. J. Am. Ceram. Soc. 2011, 94, 4502–4506. [Google Scholar] [CrossRef]
- Arnold, D.C. Composition-driven structural phase transitions in rare-earth-doped BiFeO3 ceramics: A review. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2015, 62, 62–82. [Google Scholar] [CrossRef] [PubMed]
- Lazenka, V.V.; Zhang, G.; Vanacken, J.; Makoed, I.I.; Ravinski, A.F.; Moshchalkov, V.V. Structural transformation and magnetoelectric behaviour in Bi1−xGdxFeO3 multiferroics. J. Phys. D Appl. Phys. 2012, 45, 125002. [Google Scholar] [CrossRef]
- Godara, P.; Agarwal, A.; Ahlawat, N.; Sanghi, S. Crystal structure, dielectric and magnetic properties of Gd doped BiFeO3 multiferroics. Phys. B 2018, 550, 414–419. [Google Scholar] [CrossRef]
- Rodríguez-Carvajal, J. Recent advances in magnetic structure determination by neutron powder diffraction. Physica B 1993, 192, 55. [Google Scholar] [CrossRef]
- Maleki, H. Photocatalytic activity, optical and ferroelectric properties of Bi0.8Nd0.2FeO3 nanoparticles synthesized by sol-gel and hydrothermal methods. J. Magn. Magn. Mater. 2018, 458, 277–284. [Google Scholar] [CrossRef]
- Dhanya, S.R.; Nair, S.G.; Satapathy, J.; Kumar, N.P. Structural and spectroscopic characterization of bismuth-ferrites. AIP Conf. Proc. 2019, 2166, 020017. [Google Scholar]
- Chen, P.; Xu, X.; Koenigsmann, C.; Santulli, A.C.; Wong, S.S.; Musfeldt, J.L. Size-Dependent Infrared Phonon Modes and Ferroelectric Phase Transition in BiFeO3 Nanoparticles. Nano Lett. 2010, 10, 4526–4532. [Google Scholar] [CrossRef]
- Tuboltsev, V.; Savin, A.; Sakamoto, W.; Hieno, A.; Yogo, T.; Räisänen, J. Spin-glass behavior of nanocrystalline multiferroic bismuth ferrite lead titanate. J. Mater. Chem. 2011, 21, 781–788. [Google Scholar] [CrossRef]
- Gaikwad, V.M.; Acharya, S.A. Investigation of spin phonon coupling in BiFeO3 based system by Fourier transform infrared spectroscopy. J. Appl. Phys. 2013, 114, 193901. [Google Scholar] [CrossRef]
- Komandin, G.A.; Torgashev, V.I.; Volkov, A.A.; Porodinkov, O.E.; Spektor, I.E.; Bush, A.A. Optical properties of BiFeO3 ceramics in the frequency range 0.3–30.0 THz. Phys. Solid State 2010, 52, 734–743. [Google Scholar] [CrossRef]
- Jiang, P.P.; Zhang, X.L.; Chang, P.; Hu, Z.G.; Bai, W.; Li, Y.W.; Chu, J.H. Spin-phonon interactions of multiferroic Bi4Ti3O12-BiFeO3 ceramics: Low-temperature Raman scattering and infrared reflectance spectra investigations. J. Appl. Phys. 2014, 115, 144101. [Google Scholar]
- Karpinsky, D.V.; Pakalniškis, A.; Niaura, G.; Zhaludkevich, D.V.; Zhaludkevich, A.L.; Latushka, S.I.; Silibin, M.; Serdechnova, M.; Garamus, V.M.; Lukowiak, A.; et al. Evolution of the crystal structure and magnetic properties of Sm-doped BiFeO3 ceramics across the phase boundary region. Ceram. Int. 2020, 47, 5399–5406. [Google Scholar] [CrossRef]
- Delfard, N.B.; Maleki, H.; Badizi, A.M.; Taraz, M. Enhanced Structural, Optical, and Multiferroic Properties of Rod-Like Bismuth Iron Oxide Nanoceramics by Dopant Lanthanum. J. Supercond. Novel Magn. 2020, 33, 1207–1214. [Google Scholar] [CrossRef]

| WP: 6a | E | C3 | WP: 18b | E | ||||
| A | 6 | 0 | 0 | A | 18 |
| E | 2C3 (3, ITA) | 3Cv (m1–10, ITA) | |
|---|---|---|---|
| A1 | 1 | 1 | 1 |
| A2 | 1 | 1 | −1 |
| E | 2 | −1 | 0 |
| WP | Ag | Au | B1g | B1u | B2g | B2u | B3g | B3u |
|---|---|---|---|---|---|---|---|---|
| 4a | · | · | · | 3 | · | 3 | · | 3 |
| 4b | · | · | · | 3 | · | 3 | · | 3 |
| 4c | · | · | · | 2 | · | 1 | · | 2 |
| 8d | · | · | · | 3 | · | 3 | · | 3 |
| WP | Ag | Au | B1g | B1u | B2g | B2u | B3g | B3u |
|---|---|---|---|---|---|---|---|---|
| 2a | · | · | · | 1 | · | 2 | · | 2 |
| 2b | · | · | · | 1 | · | 2 | · | 2 |
| 2c | · | · | · | 1 | · | 2 | · | 2 |
| 2d | · | · | · | 1 | · | 2 | · | 2 |
| 4e | · | · | · | 1 | · | 2 | · | 2 |
| 4f | · | · | · | 1 | · | 2 | · | 2 |
| 4g | · | · | · | 1 | · | 2 | · | 2 |
| 4h | · | · | · | 1 | · | 2 | · | 2 |
| 8i | · | · | · | 3 | · | 3 | · | 3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sobol, V.R.; Yanushkevich, K.I.; Latushka, S.I.; Zhaludkevich, D.V.; Nekludov, K.N.; Silibin, M.V.; Sayyed, M.I.; Almousa, N.; Korzun, B.V.; Mazurenko, O.N.; et al. Structure and Lattice Dynamics of Bi1−xNdxFeO3 and Bi1−xGdxFeO3 Ceramics near the Morphotropic Phase Boundary. Magnetochemistry 2022, 8, 103. https://doi.org/10.3390/magnetochemistry8090103
Sobol VR, Yanushkevich KI, Latushka SI, Zhaludkevich DV, Nekludov KN, Silibin MV, Sayyed MI, Almousa N, Korzun BV, Mazurenko ON, et al. Structure and Lattice Dynamics of Bi1−xNdxFeO3 and Bi1−xGdxFeO3 Ceramics near the Morphotropic Phase Boundary. Magnetochemistry. 2022; 8(9):103. https://doi.org/10.3390/magnetochemistry8090103
Chicago/Turabian StyleSobol, Valery R., Kazimir I. Yanushkevich, Siarhei I. Latushka, Dmitry V. Zhaludkevich, Kapiton N. Nekludov, Maxim V. Silibin, M. I. Sayyed, Nouf Almousa, Barys V. Korzun, Olga N. Mazurenko, and et al. 2022. "Structure and Lattice Dynamics of Bi1−xNdxFeO3 and Bi1−xGdxFeO3 Ceramics near the Morphotropic Phase Boundary" Magnetochemistry 8, no. 9: 103. https://doi.org/10.3390/magnetochemistry8090103






