Impact of Silica-Modification and Oxidation on the Crystal Structure of Magnetite Nanoparticles
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Fe3O4
2.3. Synthesis of Fe3O4-APTES MNPs
2.4. Oxidation of MNPs
2.5. Characteristics of the NPs Microstructure
3. Results and Discussion
4. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhao, X.; Xu, S.; Jiang, Y.; Wang, C.; Rehman, S.U.; Ji, S.; Wang, J.; Tao, T.; Xu, H.; Chen, R.; et al. BSA-magnetite nanotorpedo for safe and efficient delivery of chemotherapy drugs. Chem. Eng. J. 2023, 454, 140440. [Google Scholar] [CrossRef]
- Hsu, C.-H.; Yu, Y.-S.; Gu, Y.; Wu, K.C.-W. Modification of magnetite-doped NH2-MIL-100(Fe) with aliphatic C8 carbon chain for feasible protein purification in reversed-phase mode. Sep. Purif. Technol. 2022, 288, 120528. [Google Scholar] [CrossRef]
- Prilepskii, A.Y.; Kalnin, A.Y.; Fakhardo, A.F.; Anastasova, E.I.; Nedorezova, D.D.; Antonov, G.A.; Vinogradov, V.V. Cationic Magnetite Nanoparticles for Increasing siRNA Hybridization Rates. Nanomaterials 2020, 10, 1018. [Google Scholar] [CrossRef] [PubMed]
- German, S.V.; Navolokin, N.A.; Kuznetsova, N.R.; Zuev, V.V.; Inozemtseva, O.A.; Anis’Kov, A.A.; Volkova, E.K.; Bucharskaya, A.B.; Maslyakova, G.N.; Fakhrullin, R.F.; et al. Liposomes loaded with hydrophilic magnetite nanoparticles: Preparation and application as contrast agents for magnetic resonance imaging. Colloids Surf. B Biointerfaces 2015, 135, 109–115. [Google Scholar] [CrossRef] [PubMed]
- Thomas, N.; Dionysiou, D.D.; Pillai, S.C. Heterogeneous Fenton catalysts: A review of recent advances. J. Hazard. Mater. 2020, 404, 124082. [Google Scholar] [CrossRef] [PubMed]
- Estelrich, J.; Escribano, E.; Queralt, J.; Busquets, M.A. Iron Oxide Nanoparticles for Magnetically-Guided and Magnetically-Responsive Drug Delivery. Int. J. Mol. Sci. 2015, 16, 8070–8101. [Google Scholar] [CrossRef] [PubMed]
- Revia, R.A.; Zhang, M. Magnetite nanoparticles for cancer diagnosis, treatment, and treatment monitoring: Recent advances. Mater. Today 2016, 19, 157–168. [Google Scholar] [CrossRef]
- Yue-Jian, C.; Juan, T.; Fei, X.; Jia-Bi, Z.; Ning, G.; Yi-Hua, Z.; Ye, D.; Liang, G. Synthesis, self-assembly, and characterization of PEG-coated iron oxide nanoparticles as potential MRI contrast agent. Drug Dev. Ind. Pharm. 2010, 36, 1235–1244. [Google Scholar] [CrossRef]
- Masoudi, A.; Hosseini, H.R.M.; Shokrgozar, M.A.; Ahmadi, R.; Oghabian, M.A. The effect of poly(ethylene glycol) coating on colloidal stability of superparamagnetic iron oxide nanoparticles as potential MRI contrast agent. Int. J. Pharm. 2012, 433, 129–141. [Google Scholar] [CrossRef]
- Anuchina, M.M.; Pankratov, D.A.; Abroskin, D.P.; Kulikova, N.A.; Gabbasova, D.T.; Matorin, D.N.; Volkov, D.S.; Perminova, I.V. Estimating the Toxicity and Biological Availability for Interaction Products of Metallic Iron and Humic Substances. Mosc. Univ. Soil Sci. Bull. 2019, 74, 193–198. [Google Scholar] [CrossRef]
- Dwivedi, A.D.; Ma, L.Q. Biocatalytic Synthesis Pathways, Transformation, and Toxicity of Nanoparticles in the Environment. Crit. Rev. Environ. Sci. Technol. 2014, 44, 1679–1739. [Google Scholar] [CrossRef]
- Lin, D.; Tian, X.; Wu, F.; Xing, B. Fate and Transport of Engineered Nanomaterials in the Environment. J. Environ. Qual. 2010, 39, 1896–1908. [Google Scholar] [CrossRef]
- Lowry, G.V.; Gregory, K.B.; Apte, S.C.; Lead, J.R. Transformations of Nanomaterials in the Environment. Environ. Sci. Technol. 2012, 46, 6893–6899. [Google Scholar] [CrossRef]
- Mitrano, D.M.; Motellier, S.; Clavaguera, S.; Nowack, B. Review of nanomaterial aging and transformations through the life cycle of nano-enhanced products. Environ. Int. 2015, 77, 132–147. [Google Scholar] [CrossRef]
- Carpenter, E.E.; Calvin, S.; Stroud, R.M.; Harris, V.G. Passivated Iron as Core−Shell Nanoparticles. Chem. Mater. 2003, 15, 3245–3246. [Google Scholar] [CrossRef]
- Wang, C.M.; Baer, D.R.; Thomas, L.E.; Amonette, J.E.; Antony, J.; Qiang, Y.; Duscher, G. Void formation during early stages of passivation: Initial oxidation of iron nanoparticles at room temperature. J. Appl. Phys. 2005, 98, 094308. [Google Scholar] [CrossRef]
- Pratt, A.; Lari, L.; Hovorka, O.; Shah, A.; Woffinden, C.; Tear, S.P.; Binns, C.; Kröger, R. Enhanced oxidation of nanoparticles through strain-mediated ionic transport. Nat. Mater. 2013, 13, 26–30. [Google Scholar] [CrossRef]
- Thompson, M.D.K. The Reaction Between Iron and Water in the Absence of Oxygen. Trans. Electrochem. Soc. 1940, 78, 251–257. [Google Scholar] [CrossRef]
- Reinsch, B.C.; Forsberg, B.; Penn, R.L.; Kim, C.S.; Lowry, G.V. Chemical Transformations during Aging of Zerovalent Iron Nanoparticles in the Presence of Common Groundwater Dissolved Constituents. Environ. Sci. Technol. 2010, 44, 3455–3461. [Google Scholar] [CrossRef]
- Filip, J.; Karlický, F.; Marušák, Z.; Lazar, P.; Černík, M.; Otyepka, M.; Zbořil, R. Anaerobic Reaction of Nanoscale Zerovalent Iron with Water: Mechanism and Kinetics. J. Phys. Chem. C 2014, 118, 13817–13825. [Google Scholar] [CrossRef]
- Dong, H.; Zhao, F.; Zeng, G.; Tang, L.; Fan, C.; Zhang, L.; Zeng, Y.; He, Q.; Xie, Y.; Wu, Y. Aging study on carboxymethyl cellulose-coated zero-valent iron nanoparticles in water: Chemical transformation and structural evolution. J. Hazard. Mater. 2016, 312, 234–242. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Dong, H.; Zeng, G.; Tang, L.; Jiang, Z.; Zhang, C.; Deng, J.; Zhang, L.; Zhang, Y. The interactions between nanoscale zero-valent iron and microbes in the subsurface environment: A review. J. Hazard. Mater. 2017, 321, 390–407. [Google Scholar] [CrossRef] [PubMed]
- Dong, H.; Zhao, F.; He, Q.; Xie, Y.; Zeng, Y.; Zhang, L.; Tang, L.; Zeng, G. Physicochemical transformation of carboxymethyl cellulose-coated zero-valent iron nanoparticles (nZVI) in simulated groundwater under anaerobic conditions. Sep. Purif. Technol. 2017, 175, 376–383. [Google Scholar] [CrossRef]
- Greenlee, L.F.; Torrey, J.D.; Amaro, R.L.; Shaw, J.M. Kinetics of Zero Valent Iron Nanoparticle Oxidation in Oxygenated Water. Environ. Sci. Technol. 2012, 46, 12913–12920. [Google Scholar] [CrossRef] [PubMed]
- Liu, A.; Liu, J.; Zhang, W.-X. Transformation and composition evolution of nanoscale zero valent iron (nZVI) synthesized by borohydride reduction in static water. Chemosphere 2015, 119, 1068–1074. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.-S.; Kim, T.; Ahn, J.-Y.; Hwang, K.-Y.; Park, J.-Y.; Lim, T.-T.; Hwang, I. Aging characteristics and reactivity of two types of nanoscale zero-valent iron particles (FeBH and FeH2) in nitrate reduction. Chem. Eng. J. 2012, 197, 16–23. [Google Scholar] [CrossRef]
- Sun, G.; Fenglian, F.; Bing, T. Fate of metal-EDTA complexes during ferrihydrite aging: Interaction of metal-EDTA and iron oxides. Chemosphere. 2022, 291, 132791. [Google Scholar] [CrossRef] [PubMed]
- Dong, H.; Jiang, Z.; Deng, J.; Zhang, C.; Cheng, Y.; Hou, K.; Zhang, L.; Tang, L.; Zeng, G. Physicochemical transformation of Fe/Ni bimetallic nanoparticles during aging in simulated groundwater and the consequent effect on contaminant removal. Water Res. 2018, 129, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Salazar, J.S.; Perez, L.; de Abril, O.; Phuoc, L.T.; Ihiawakrim, D.; Vazquez, M.; Greneche, J.-M.; Begin-Colin, S.; Pourroy, G. Magnetic Iron Oxide Nanoparticles in 10−40 nm Range: Composition in Terms of Magnetite/Maghemite Ratio and Effect on the Magnetic Properties. Chem. Mater. 2011, 23, 1379–1386. [Google Scholar] [CrossRef]
- Jungcharoen, P.; Pédrot, M.; Choueikani, F.; Pasturel, M.; Hanna, K.; Heberling, F.; Tesfa, M.; Marsac, R. Probing the effects of redox conditions and dissolved Fe2+ on nanomagnetite stoichiometry by wet chemistry, XRD, XAS and XMCD. Environ. Sci. Nano 2021, 8, 2098–2107. [Google Scholar] [CrossRef]
- Al-Hakkani, M.F.; Gouda, G.A.; Hassan, S.H. A review of green methods for phyto-fabrication of hematite (α-Fe2O3) nanoparticles and their characterization, properties, and applications. Heliyon 2021, 7, e05806. [Google Scholar] [CrossRef] [PubMed]
- Schwaminger, S.P.; Bauer, D.; Fraga-García, P.; Wagner, F.E.; Berensmeier, S. Oxidation of magnetite nanoparticles: Impact on surface and crystal properties. CrystEngComm 2017, 19, 246–255. [Google Scholar] [CrossRef]
- Vikram, S.; Dhakshnamoorthy, M.; Vasanthakumari, R.; Rajamani, A.R.; Rangarajan, M.; Tsuzuki, T. Tuning the Magnetic Properties of Iron Oxide Nanoparticles by a Room-Temperature Air-Atmosphere (RTAA) Co-Precipitation Method. J. Nanosci. Nanotechnol. 2015, 15, 3870–3878. [Google Scholar] [CrossRef] [PubMed]
- Tombácz, E.; Illés, E.; Majzik, A.; Jedlovszky-Hajdú, A.; Rideg, N.; Szekeres, M. Ageing in the inorganic nanoworld: Example of magnetite nanoparticles in aqueous medium. Croat. Chem. Acta 2007, 83, 513–515. [Google Scholar]
- Lei, C.; Zhang, L.; Yang, K.; Zhu, L.; Lin, D. Toxicity of iron-based nanoparticles to green algae: Effects of particle size, crystal phase, oxidation state and environmental aging. Environ. Pollut. 2016, 218, 505–512. [Google Scholar] [CrossRef] [PubMed]
- Saei, A.; Yazdani, M.; Lohse, S.E.; Bakhtiary, Z.; Serpooshan, V.; Ghavami, M.; Asadian, M.; Mashaghi, S.; Dreaden, E.; Mashaghi, A.; et al. Nanoparticle Surface Functionality Dictates Cellular and Systemic Toxicity. Chem. Mater. 2017, 29, 6578–6595. [Google Scholar] [CrossRef]
- Liu, J.; Louie, S.M.; Zhao, J.; Gao, X.; Hu, Y. Aggregation of varied organic coated magnetite nanoparticles: Adsorbed mass and thickness of coatings and interactions with natural organic matter. Sci. Total Environ. 2022, 831, 154976. [Google Scholar] [CrossRef] [PubMed]
- Schwaminger, S.P.; Schwarzenberger, K.; Gatzemeier, J.; Lei, Z.; Eckert, K. Magnetically Induced Aggregation of Iron Oxide Nanoparticles for Carrier Flotation Strategies. ACS Appl. Mater. Interfaces 2021, 13, 20830–20844. [Google Scholar] [CrossRef] [PubMed]
- Suk, J.S.; Xu, Q.; Kim, N.; Hanes, J.; Ensign, L.M. PEGylation as a strategy for improving nanoparticle-based drug and gene delivery. Adv. Drug Deliv. Rev. 2016, 99, 28–51. [Google Scholar] [CrossRef] [PubMed]
- Najahi-Missaoui, W.; Arnold, R.D.; Cummings, B.S. Safe Nanoparticles: Are We There Yet? Int. J. Mol. Sci. 2020, 22, 385. [Google Scholar] [CrossRef] [PubMed]
- Abbas, M.; Rao, B.P.; Islam, N.; Naga, S.; Takahashi, M.; Kim, C. Highly stable- silica encapsulating magnetite nanoparticles (Fe3O4/SiO2) synthesized using single surfactantless- polyol process. Ceram. Int. 2014, 40, 1379–1385. [Google Scholar] [CrossRef]
- Gdula, K.; Gładysz-Płaska, A.; Cristóvão, B.; Ferenc, W.; Skwarek, E. Amine-functionalized magnetite-silica nanoparticles as effective adsorbent for removal of uranium(VI) ions. J. Mol. Liq. 2019, 290, 111217. [Google Scholar] [CrossRef]
- Mokhtar, H.I.; Abdel-Salam, R.A.; Hadad, G.M. A nanocomposite of silica coated magnetite nanoparticles and aniline-anthranilic acid co-polymeric nanorods with improved stability and selectivity for fluoroquinolones dispersive micro solid phase extraction. J. Chromatogr. B 2022, 1206, 123350. [Google Scholar] [CrossRef]
- Ahmad, I.; Siddiqui, W.A.; Ahmad, T. Synthesis and characterization of molecularly imprinted magnetite nanomaterials as a novel adsorbent for the removal of heavy metals from aqueous solution. J. Mater. Res. Technol. 2019, 8, 4239–4252. [Google Scholar] [CrossRef]
- Dayana, I.; Sembiring, T.; Tetuko, A.P.; Sembiring, K.; Maulida, N.; Cahyarani, Z.; Setiadi, E.A.; Asri, N.S.; Ginting, M.; Sebayang, P. The effect of tetraethyl orthosilicate (TEOS) additions as silica precursors on the magnetite nano-particles (Fe3O4) properties for the application of ferro-lubricant. J. Mol. Liq. 2019, 294, 111557. [Google Scholar] [CrossRef]
- Turrina, C.; Oppelt, A.; Mitzkus, M.; Berensmeier, S.; Schwaminger, S.P. Silica-coated superparamagnetic iron oxide nanoparticles: New insights into the influence of coating thickness on the particle properties and lasioglossin binding. MRS Commun. 2022, 12, 632–639. [Google Scholar] [CrossRef]
- Zhang, B.; Wang, Y.; Zhang, J.; Qiao, S.; Fan, Z.; Wan, J.; Chen, K. Well-defined 3-Aminopropyltriethoxysilane functionalized magnetite nanoparticles and their adsorption performance for partially hydrolyzed polyacrylamide from aqueous solution. Colloids Surf. A Physicochem. Eng. Asp. 2020, 586, 124288. [Google Scholar] [CrossRef]
- Mokkarat, A.; Kruanetr, S.; Sakee, U. One-step continuous flow synthesis of aminopropyl silica-coated magnetite nanoparticles. J. Saudi Chem. Soc. 2022, 26, 101506. [Google Scholar] [CrossRef]
- Szultka-Młyńska, M.; Al-Suod, H.; Buszewski, B. Inorganic oxide and chemically bonded sorbents. In Solid-Phase Extraction; Elsevier: Amsterdam, The Netherlands, 2020; pp. 37–54. [Google Scholar] [CrossRef]
- Nnadozie, E.C.; Ajibade, P.A. Data for experimental and calculated values of the adsorption of Pb(II) and Cr(VI) on APTES functionalized magnetite biochar using Langmuir, Freundlich and Temkin equations. Data Brief 2020, 32, 106292. [Google Scholar] [CrossRef]
- Kilic, A.; Karatas, M.E.; Beyazsakal, L.; Okumus, V. Preparation and spectral studies of boronate ester modified magnetite iron nanoparticles (Fe3O4@APTES-B) as a new type of biological agents. J. Mol. Liq. 2022, 361, 119602. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhu, Y.; Dai, R.; Zhang, Y.; Wang, H.; Li, J. Enhanced photocytotoxicity induced by a platinum diimine complex employing amine-functionalized magnetite-silica nanocomposites as delivery vehicles. Photodiagn. Photodyn. Ther. 2018, 23, 50–54. [Google Scholar] [CrossRef] [PubMed]
- Taufiq, A.; Nikmah, A.; Hidayat, A.; Sunaryono, S.; Mufti, N.; Hidayat, N.; Susanto, H. Synthesis of magnetite/silica nanocomposites from natural sand to create a drug delivery vehicle. Heliyon 2020, 6, e03784. [Google Scholar] [CrossRef] [PubMed]
- Ognjanović, M.; Stanković, D.M.; Jaćimović, Ž.K.; Kosović-Perutović, M.; Dojčinović, B.; Antić, B. The effect of surface-modifier of magnetite nanoparticles on electrochemical detection of dopamine and heating efficiency in magnetic hyperthermia. J. Alloy. Compd. 2021, 884, 161075. [Google Scholar] [CrossRef]
- Kermanian, M.; Sadighian, S.; Naghibi, M.; Khoshkam, M. PVP Surface-protected silica coated iron oxide nanoparticles for MR imaging application. J. Biomater. Sci. Polym. Ed. 2021, 32, 1356–1369. [Google Scholar] [CrossRef]
- Pomogailo, A.D.; Kydralieva, K.A.; Zaripova, A.A.; Muratov, V.S.; Dzhardimalieva, G.I.; Pomogailo, S.I.; Golubeva, N.D.; Jorobekova, S.J. Magnetoactive Humic-Based Nanocomposites. Macromol. Symp. 2011, 304, 18–23. [Google Scholar] [CrossRef]
- Stöber, W.; Fink, A.; Bohn, E. Controlled growth of monodisperse silica spheres in the micron size range. J. Colloid Interface Sci. 1968, 26, 62–69. [Google Scholar] [CrossRef]
- Ozmen, M.; Can, K.; Arslan, G.; Tor, A.; Cengeloglu, Y.; Ersoz, M. Adsorption of Cu(II) from aqueous solution by using modified Fe3O4 magnetic nanoparticles. Desalination 2010, 254, 162–169. [Google Scholar] [CrossRef]
- Savitzky, A.; Golay, M.J.E. Smoothing and Differentiation of Data by Simplified Least Squares Procedures. Anal. Chem. 1964, 36, 1627–1639. [Google Scholar] [CrossRef]
- Steinier, J.; Termonia, Y.; Deltour, J. Smoothing and differentiation of data by simplified least square procedure. Anal. Chem. 1972, 44, 1906–1909. [Google Scholar] [CrossRef]
- Landa, P.; Vankova, R.; Andrlova, J.; Hodek, J.; Marsik, P.; Storchova, H.; White, J.C.; Vanek, T. Nanoparticle-specific changes in Arabidopsis thaliana gene expression after exposure to ZnO, TiO2, and fullerene soot. J. Hazard. Mater. 2012, 241-242, 55–62. [Google Scholar] [CrossRef]
- Balzar, D.; Audebrand, N.; Daymond, M.; Fitch, A.; Hewat, A.; Langford, J.I.; Le Bail, A.; Louër, D.; Masson, O.; McCowan, C.N.; et al. Size–strain line-broadening analysis of the ceria round-robin sample. J. Appl. Crystallogr. 2004, 37, 911–924. [Google Scholar] [CrossRef]
- Toby, B.H. R factors in Rietveld analysis: How good is good enough? Powder Diffr. 2006, 21, 67–70. [Google Scholar] [CrossRef]
- Bondarenko, L.S.; Pankratov, D.A.; Dzeranov, A.A.; Dzhardimalieva, G.I.; Streltsova, A.N.; Zarrelli, M.; Kydralieva, K.A. A simple method for quantification of nonstoichiometric magnetite nanoparticles using conventional X-ray diffraction technique. Mendeleev Commun. 2022, 32, 642–644. [Google Scholar] [CrossRef]
- Gorski, C.A.; Scherer, M.M. Determination of nanoparticulate magnetite stoichiometry by Mossbauer spectroscopy, acidic dissolution, and powder X-ray diffraction: A critical review. Am. Miner. 2010, 95, 1017–1026. [Google Scholar] [CrossRef]
- Frison, R.; Cernuto, G.; Cervellino, A.; Zaharko, O.; Colonna, G.M.; Guagliardi, A.; Masciocchi, N. Magnetite–Maghemite Nanoparticles in the 5–15 nm Range: Correlating the Core–Shell Composition and the Surface Structure to the Magnetic Properties. A Total Scattering Study. Chem. Mater. 2013, 25, 4820–4827. [Google Scholar] [CrossRef]
- Gallagher, K.J.; Feitknecht, W.; Mannweiler, U. Mechanism of Oxidation of Magnetite to γ-Fe2O3. Nature 1968, 217, 1118–1121. [Google Scholar] [CrossRef]
- Bondarenko, L.; Illés, E.; Tombácz, E.; Dzhardimalieva, G.; Golubeva, N.; Tushavina, O.; Adachi, Y.; Kydralieva, K. Fabrication, Microstructure and Colloidal Stability of Humic Acids Loaded Fe3O4/APTES Nanosorbents for Environmental Applications. Nanomaterials 2021, 11, 1418. [Google Scholar] [CrossRef]
- Li, S.; Wang, J.; Qu, W.; Cheng, J.; Lei, Y.; Liu, M.; Wang, D. Epoxy/Nano-SiO2 Anticorrosion Coatings Synthesized by Different Molar Ratio of Tetraethyl orthosilicate (TEOS) and Tetramethyl orthosilicate (TMOS). Int. J. Electrochem. Sci. 2019, 14, 11641–11650. [Google Scholar] [CrossRef]
- Kim, W.; Suh, C.-Y.; Cho, S.-W.; Roh, K.-M.; Kwon, H.; Song, K.; Shon, I.-J. A new method for the identification and quantification of magnetite–maghemite mixture using conventional X-ray diffraction technique. Talanta 2012, 94, 348–352. [Google Scholar] [CrossRef]
- Pankratov, D.A.; Anuchina, M.M. Nature-inspired synthesis of magnetic non-stoichiometric Fe3O4 nanoparticles by oxidative in situ method in a humic medium. Mater. Chem. Phys. 2019, 231, 216–224. [Google Scholar] [CrossRef]
- Pankratov, D.A.; Anuchina, M.M.; Spiridonov, F.M.; Krivtsov, G.G. Fe3–δO4 Nanoparticles Synthesized in the Presence of Natural Polyelectrolytes. Crystallogr. Rep. 2020, 65, 393–397. [Google Scholar] [CrossRef]
- Pankratov, D.A. Mössbauer study of oxo derivatives of iron in the Fe2O3-Na2O2 system. Inorg. Mater. 2013, 50, 82–89. [Google Scholar] [CrossRef]
- Oh, S.J.; Cook, D.C.; Townsend, H.E. Characterization of Iron Oxides Commonly Formed as Corrosion Products on Steel. Hyperfine Interact. 1998, 112, 59–66. [Google Scholar] [CrossRef]
- Klygach, D.S.; Vakhitov, M.G.; Pankratov, D.A.; Zherebtsov, D.A.; Tolstoguzov, D.S.; Raddaoui, Z.; El Kossi, S.; Dhahri, J.; Vinnik, D.A.; Trukhanov, A.V. MCC: Specific of preparation, correlation of the phase composition and electrodynamic properties. J. Magn. Magn. Mater. 2021, 526, 167694. [Google Scholar] [CrossRef]
- Chernavskii, P.A.; Kazantsev, R.V.; Pankina, G.V.; Pankratov, D.A.; Maksimov, S.V.; Eliseev, O.L. Unusual Effect of Support Carbonization on the Structure and Performance of Fe/Mgal 2 o 4 Fischer–Tropsch Catalyst. Energy Technol. 2020, 9, 2000877. [Google Scholar] [CrossRef]
- Jones, D.H.; Srivastava, K.K.P. Many-state relaxation model for the Mössbauer spectra of superparamagnets. Phys. Rev. B 1986, 34, 7542–7548. [Google Scholar] [CrossRef]
- Nadeem, K.; Krenn, H.; Traussnig, T.; Würschum, R.; Szabó, D.; Letofsky-Papst, I. Effect of dipolar and exchange interactions on magnetic blocking of maghemite nanoparticles. J. Magn. Magn. Mater. 2011, 323, 1998–2004. [Google Scholar] [CrossRef]
- Adolphi, N.L.; Huber, D.L.; Bryant, H.C.; Monson, T.C.; Fegan, D.L.; Lim, J.; Trujillo, J.E.; Tessier, T.E.; Lovato, D.M.; Butler, K.S.; et al. Characterization of single-core magnetite nanoparticles for magnetic imaging by SQUID relaxometry. Phys. Med. Biol. 2010, 55, 5985–6003. [Google Scholar] [CrossRef] [PubMed]
- Klencsár, Z.; Ábrahám, A.; Szabó, L.; Szabó, E.G.; Stichleutner, S.; Kuzmann, E.; Homonnay, Z.; Tolnai, G. The effect of preparation conditions on magnetite nanoparticles obtained via chemical co-precipitation. Mater. Chem. Phys. 2018, 223, 122–132. [Google Scholar] [CrossRef]
- Kulikova, N.A.; Polyakov, A.Y.; Lebedev, V.; Abroskin, D.P.; Volkov, D.S.; Pankratov, D.; Klein, O.I.; Senik, S.V.; Sorkina, T.; Garshev, A.V.; et al. Key Roles of Size and Crystallinity of Nanosized Iron Hydr(oxides) Stabilized by Humic Substances in Iron Bioavailability to Plants. J. Agric. Food Chem. 2017, 65, 11157–11169. [Google Scholar] [CrossRef]
- Marcuello, C.; Chambel, L.; Rodrigues, M.S.; Ferreira, L.P.; Cruz, M.M. Magnetotactic Bacteria: Magnetism Beyond Magnetosomes. IEEE Trans. NanoBiosci. 2018, 17, 555–559. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Si, Z.; Lei, Y.; Tang, J.; Wang, S.; Su, S.; Song, S.; Zhao, L.; Zhang, H. Direct hydrothermal synthesis of single-crystalline triangular Fe3O4 nanoprisms. CrystEngComm 2010, 12, 2060–2063. [Google Scholar] [CrossRef]
- Komogortsev, S.V.; Stolyar, S.V.; Chekanova, L.A.; Yaroslavtsev, R.N.; Bayukov, O.A.; Velikanov, D.A.; Volochaev, M.N.; Eroshenko, P.E.; Iskhakov, R.S. Square plate shaped magnetite nanocrystals. J. Magn. Magn. Mater. 2021, 527, 167730. [Google Scholar] [CrossRef]

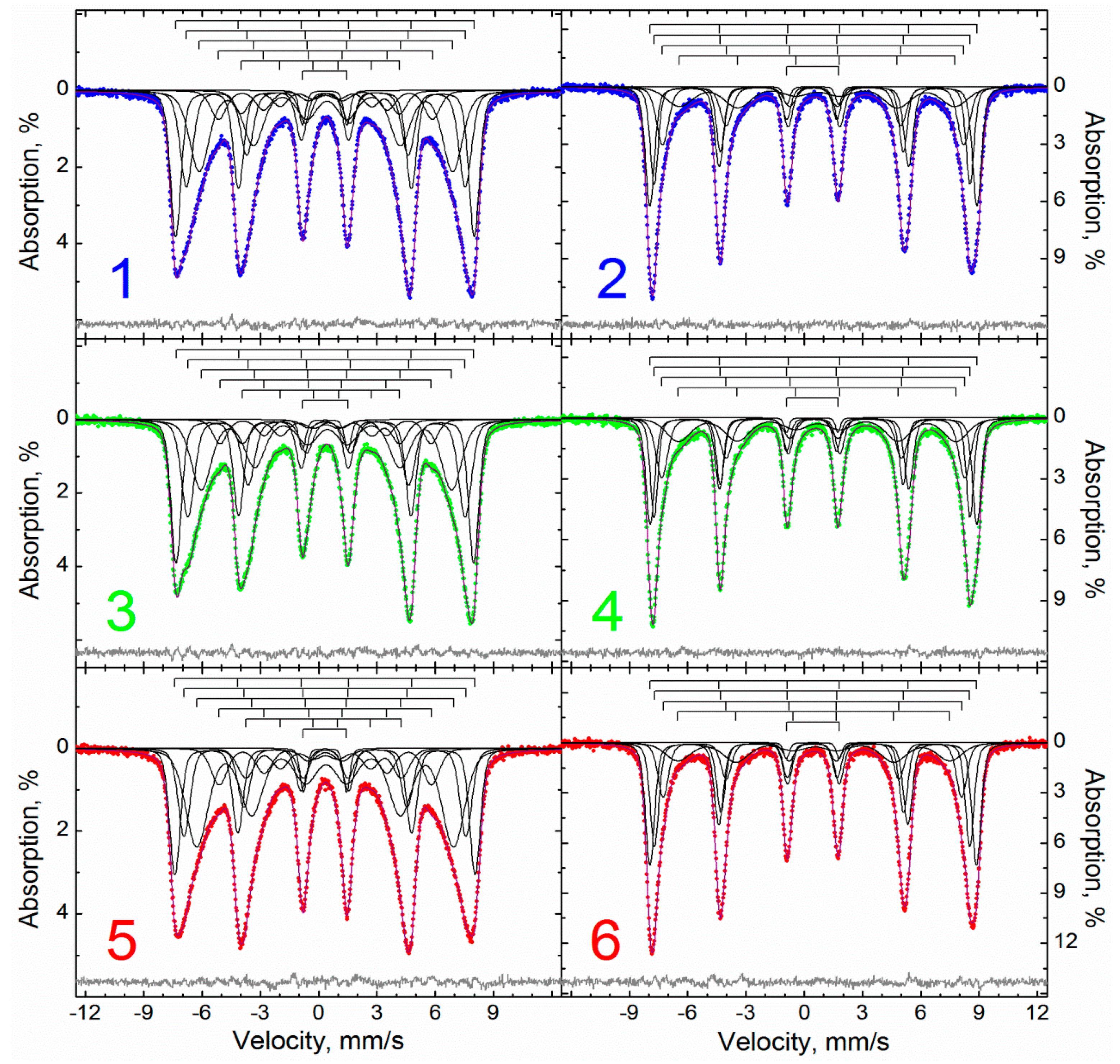
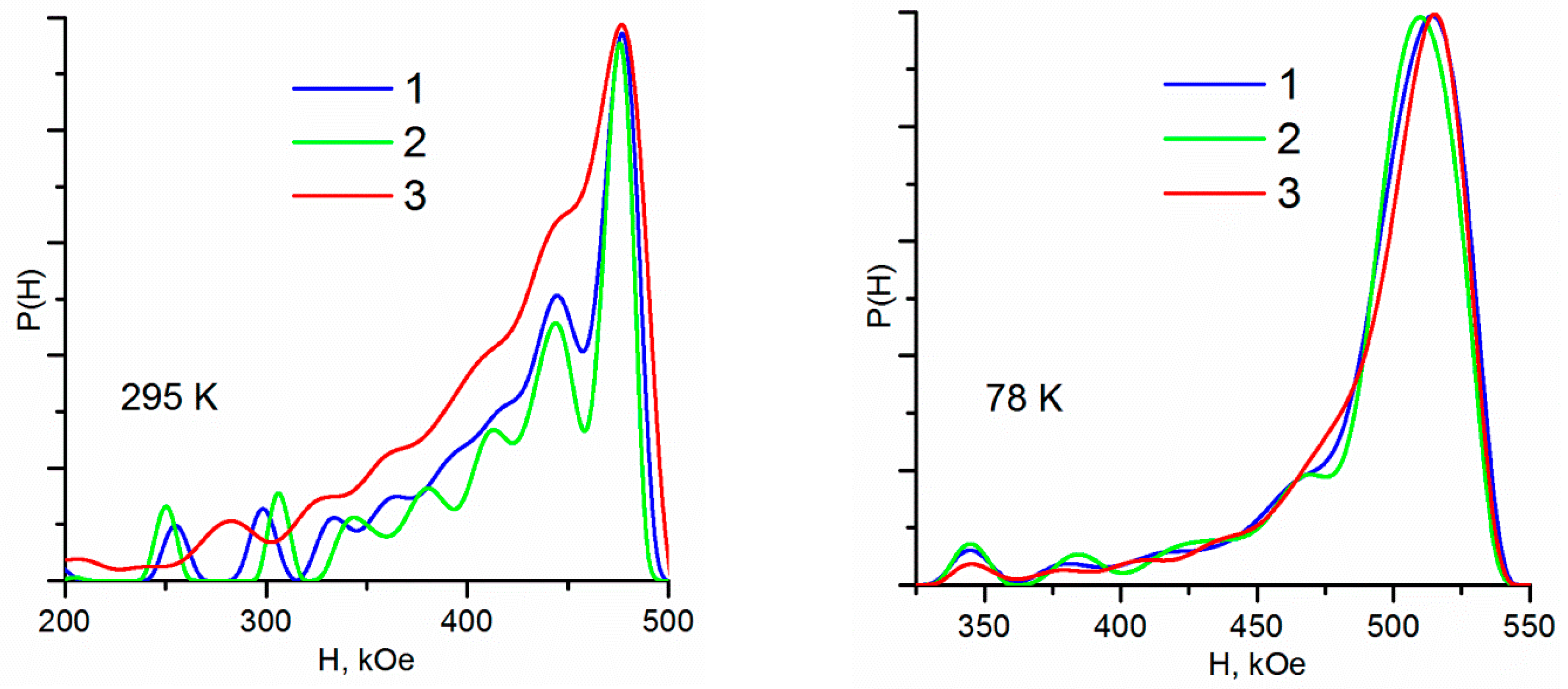
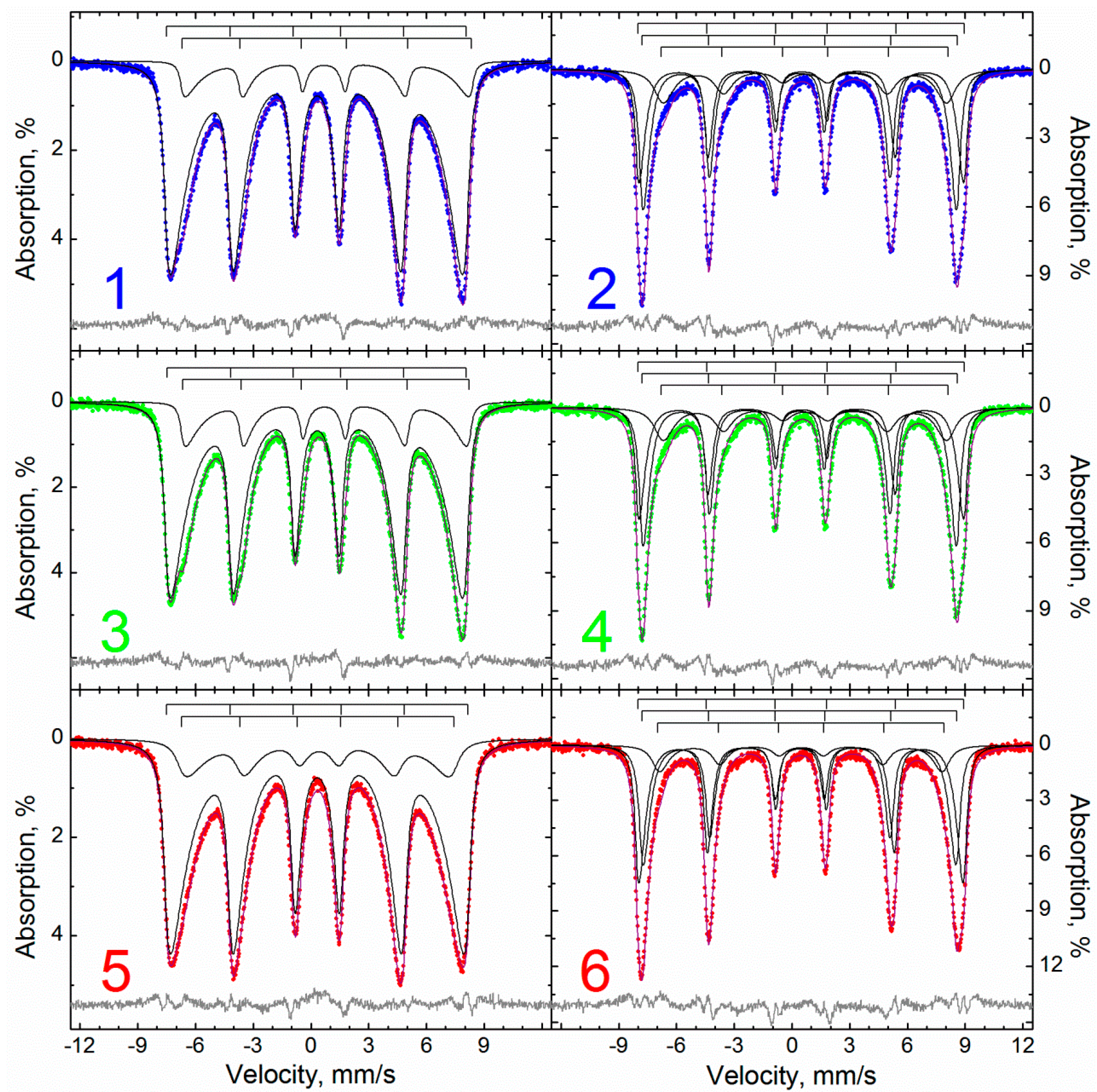
| Sample | M | MA | MAox |
|---|---|---|---|
| a1, Å | 8.3643 ± 0.0003 | 8.3531 ± 0.0003 | 8.3511 ± 0.0004 |
| χ2 | 1 | 0.8 | 0.8 |
| X | 0.22 | 0.12 | 0.1 |
| δ | 0.16 | 0.23 | 0.25 |
| , % | 51.3 | 27.3 | 24.2 |
| Fe3−δO4 compound | Fe2.84O4 | Fe2.76O4 | Fe2.75O4 |
| DXRD, nm | 21.7 ± 0.1 | 20.9 ± 0.2 | 23.3 ± 1.9 |
| Temperature, K | 295 | 78 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Sample | Subspectra | δ 1 | Δ | Gexp | Hhf | S | δ | Δ | Gexp | Hhf | S |
| mm/s | кOe | % | mm/s | кOe | % | ||||||
| M | 1 | 0.33 | 0.00 | 0.65 | 476.7 | 30 | 0.49 | −0.02 | 0.56 | 522.3 | 33 |
| 2 | 0.42 | −0.08 | 0.77 | 445.3 | 23 | 0.40 | 0.01 | 0.50 | 504.3 | 24 | |
| 3 | 0.41 | −0.05 | 1.19 | 405 | 30 | 0.47 | −0.01 | 0.74 | 481.0 | 21 | |
| 4 | 0.36 | 0.00 | 0.94 | 342 | 9 | 0.67 | −0.02 | 1.62 | 440 | 19 | |
| 5 | 0.23 | −0.28 | 0.92 | 253 | 6 | ||||||
| 6 | 0.32 | 2.28 | 0.40 | 2 | 0.44 | 2.67 | 0.38 | 2 | |||
| MA | 1 | 0.32 | 0.00 | 0.64 | 475.1 | 30 | 0.50 | −0.03 | 0.54 | 521.6 | 30 |
| 2 | 0.46 | −0.10 | 0.79 | 442.6 | 26 | 0.39 | 0.02 | 0.46 | 504.4 | 24 | |
| 3 | 0.43 | −0.04 | 1.22 | 400 | 28 | 0.48 | −0.03 | 0.73 | 483.7 | 23 | |
| 4 | 0.35 | 0.02 | 0.84 | 337.0 | 7 | 0.68 | −0.03 | 1.47 | 443 | 22 | |
| 5 | 0.25 | −0.26 | 0.92 | 251 | 7 | ||||||
| 6 | 0.34 | 2.35 | 0.42 | 2 | 0.42 | 2.64 | 0.28 | 1 | |||
| MAox | 1 | 0.33 | 0.00 | 0.66 | 479.2 | 23 | 0.47 | 0.00 | 0.56 | 521.3 | 35 |
| 2 | 0.34 | −0.03 | 0.73 | 450.0 | 18 | 0.41 | −0.01 | 0.53 | 503.4 | 28 | |
| 3 | 0.36 | −0.04 | 1.36 | 410 | 37 | 0.43 | 0.00 | 0.65 | 476.5 | 18 | |
| 4 | 0.35 | 0.01 | 0.98 | 338.7 | 10 | 0.52 | −0.05 | 1.51 | 435 | 17 | |
| 5 | 0.31 | −0.09 | 1.00 | 248.3 | 8 | ||||||
| 6 | 0.33 | 2.23 | 0.40 | 3 | 0.45 | 2.72 | 0.35 | 3 | |||
| Spectra Description Method | P(H) | MSSPR | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Sample | Temperature | Hdmax 1 | Hdw ± √D | δ | Δ | Gexp | Hhf | α | S | d |
| K | кOe | mm/s | кOe | % | nm | |||||
| M | 295 | 476.3 | 415 ± 78 | 0.31 | 0.02 | 0.46 | 482.8 | 4.78 | 88 | 15.49 |
| 0.75 | 0.16 | 0.33 | 465.6 | 12 | ||||||
| 78 | 512.6 | 482 ± 71 | 0.49 | 0.02 | 0.40 | 526.9 | 18.1 | 39 | ||
| 0.41 | 0.00 | 0.44 | 508.0 | 43 | ||||||
| 0.63 | 0.00 | 1.00 | 461.2 | 18 | ||||||
| MA | 295 | 477.3 | 414 ± 79 | 0.31 | 0.03 | 0.44 | 482.2 | 4.91 | 83 | 15.63 |
| 0.74 | 0.09 | 0.34 | 461.6 | 17 | ||||||
| 78 | 508.4 | 481 ± 69 | 0.49 | 0.01 | 0.39 | 525.8 | 18.6 | 35 | ||
| 0.41 | 0.00 | 0.43 | 507.9 | 44 | ||||||
| 0.68 | 0.01 | 1.04 | 462.4 | 21 | ||||||
| MAox | 295 | 476.6 | 409 ± 82 | 0.33 | 0.00 | 0.47 | 485.4 | 4.57 | 82 | 15.27 |
| 0.39 | 0.06 | 0.85 | 439.9 | 18 | ||||||
| 78 | 511.7 | 483 ± 67 | 0.47 | 0.00 | 0.40 | 525.8 | 17.3 | 45 | ||
| 0.41 | 0.01 | 0.45 | 507.4 | 41 | ||||||
| 0.47 | 0.01 | 0.84 | 461.1 | 14 | ||||||
| Model | CM | MSSPR | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Temperature, K | 295 | 75 | 295 | 295 | ||||||||
| Sample | Xms | δ | Fe3−δO4 | Xms | δ | Fe3−δO4 | Xms | δ | Fe3−δO4 | Xms | δ | Fe3−δO4 |
| M | 0.048 | 0.292 | Fe2.708O4 | 0.051 | 0.289 | Fe2.711O4 | 0.067 | 0.277 | Fe2.723O4 | 0.038 | 0.300 | Fe2.700O4 |
| MA | 0.078 | 0.268 | Fe2.732O4 | 0.062 | 0.281 | Fe2.719O4 | 0.095 | 0.254 | Fe2.746O4 | 0.058 | 0.284 | Fe2.716O4 |
| MAox | 0.009 | 0.325 | Fe2.675O4 | 0.015 | 0.320 | Fe2.680O4 | 0.013 | 0.322 | Fe2.678O4 | 0.004 | 0.329 | Fe2.671O4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dzeranov, A.; Bondarenko, L.; Pankratov, D.; Dzhardimalieva, G.; Jorobekova, S.; Saman, D.; Kydralieva, K. Impact of Silica-Modification and Oxidation on the Crystal Structure of Magnetite Nanoparticles. Magnetochemistry 2023, 9, 18. https://doi.org/10.3390/magnetochemistry9010018
Dzeranov A, Bondarenko L, Pankratov D, Dzhardimalieva G, Jorobekova S, Saman D, Kydralieva K. Impact of Silica-Modification and Oxidation on the Crystal Structure of Magnetite Nanoparticles. Magnetochemistry. 2023; 9(1):18. https://doi.org/10.3390/magnetochemistry9010018
Chicago/Turabian StyleDzeranov, Artur, Lyubov Bondarenko, Denis Pankratov, Gulzhian Dzhardimalieva, Sharipa Jorobekova, Daniel Saman, and Kamila Kydralieva. 2023. "Impact of Silica-Modification and Oxidation on the Crystal Structure of Magnetite Nanoparticles" Magnetochemistry 9, no. 1: 18. https://doi.org/10.3390/magnetochemistry9010018
APA StyleDzeranov, A., Bondarenko, L., Pankratov, D., Dzhardimalieva, G., Jorobekova, S., Saman, D., & Kydralieva, K. (2023). Impact of Silica-Modification and Oxidation on the Crystal Structure of Magnetite Nanoparticles. Magnetochemistry, 9(1), 18. https://doi.org/10.3390/magnetochemistry9010018









