New Type of Alkaline Rechargeable Battery—Ni-Ni Battery
Abstract
:1. Introduction
2. Experimental Setup
3. Results
3.1. Electrochemical Measurements
3.2. X-ray Diffraction Analysis
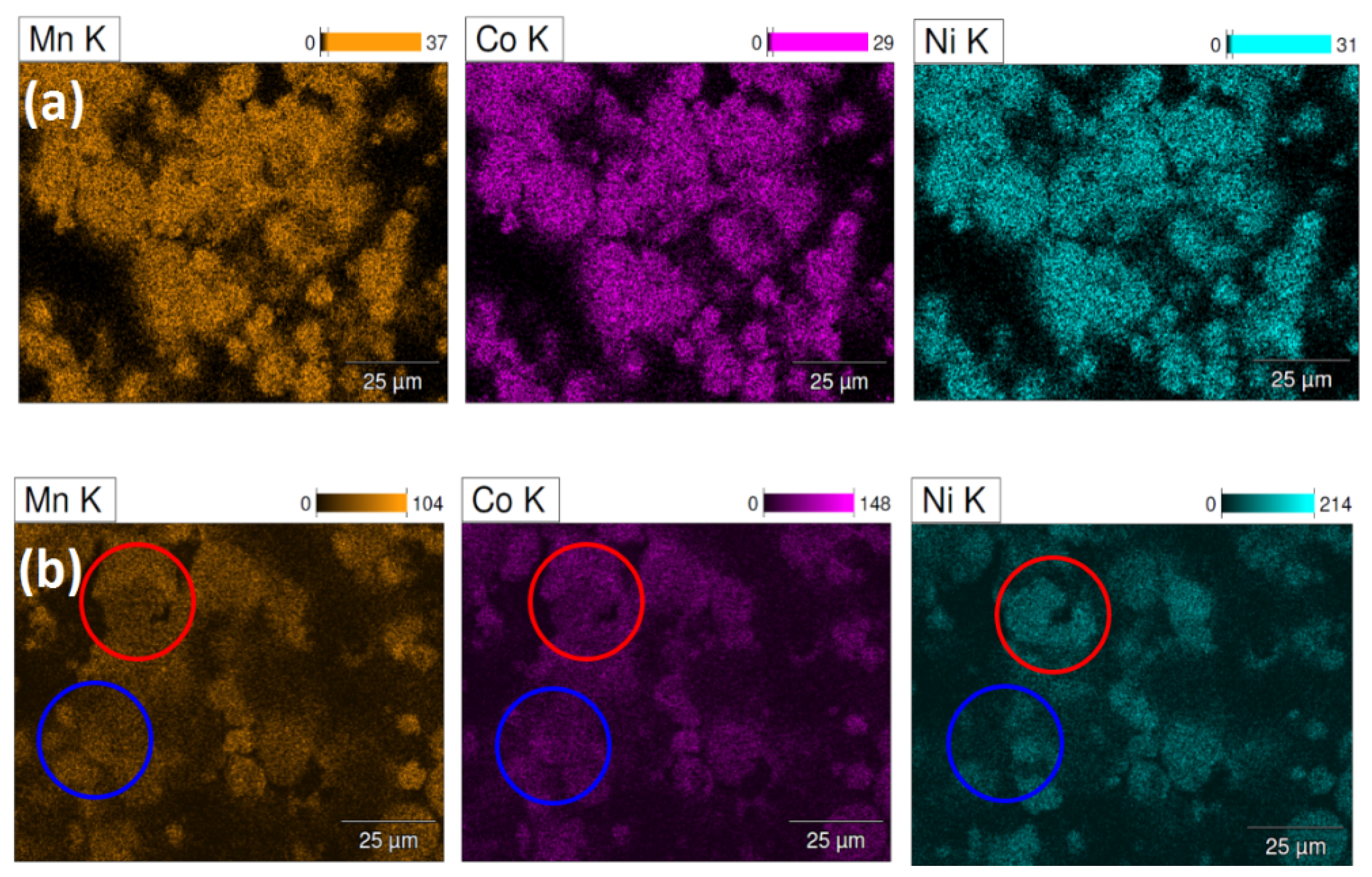
3.3. Scanning Electron Microscopy/Energy Dispersive Spectroscopy Characterizations
3.4. Transmission Electron Microscopy Characterizations
3.5. Discussion
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| TM | Transition metal |
| Ni/MH | Nickel/metal hydride |
| MH | Metal hydride |
| EV | Electric vehicle |
| DOD | Degree of disorder |
| M–O | Metal–oxygen |
| RANGE | Robust Affordable Next Generation EV |
| CSTR | Continuous stirring tank reactor |
| rpm | Revolution per minute |
| PVDF | Polyvinylidene fluoride |
| CV | Cyclic voltammetry |
| XRD | X-Ray diffraction |
| SEM | Scanning electron microscopy |
| EDS | X-Ray energy dispersive spectroscopy |
| DP | Discharge peak |
| CP | Charge peak |
| TEM | Transmission electron microscopy |
| Powder diffraction file | |
| EELS | Electron energy loss spectroscope |
References
- Edison, T.A. Reversible Galvanic Battery. U.S. Patent 678,722, 16 July 1901. [Google Scholar]
- Edison, T.A. Reversible Galvanic Battery. U.S. Patent 692,507, 4 February 1902. [Google Scholar]
- Shukla, A.K.; Venugopalan, S.; Hariprakash, B. Nickel-based rechargeable batteries. J. Power Sources 2001, 100, 125–148. [Google Scholar] [CrossRef]
- Morioka, Y.; Narukawa, S.; Itou, T. State-of-the-art of alkaline rechargeable batteries. J. Power Sources 2001, 100, 107–116. [Google Scholar] [CrossRef]
- Tarascon, J.M. Key challenges in future Li-battery research. Philos. Trans. R. Soc. A 2010, 368, 3227–3241. [Google Scholar] [CrossRef] [PubMed]
- Carmichael, C. Making Economical, Green, High-Energy Nickel-Manganese (NiMn) Batteries. Available online: http://www.saers.com/recorder/craig/TurquoiseEnergy/BatteryMaking/BatteryMaking.html (accessed on 1 April 2016).
- Chang, S.; Young, K.; Nei, J.; Fierro, C. Reviews on the U.S. patents regarding nickel/metal hydride batteries. Batteries 2016, 2. [Google Scholar] [CrossRef]
- Ouchi, T.; Young, K.; Moghe, D. Reviews on the Japanese patent applications regarding nickel/metal hydride batteries. Batteries 2016, 2. [Google Scholar] [CrossRef]
- Jiang, W.; Wu, Y.; Cheng, Y.; Wang, L. Industrial application of nickel-iron battery and its recent research progress. Chin. J. Appl. Chem. 2014, 31, 749–756. [Google Scholar]
- Gao, X.P.; Yao, S.M.; Yan, T.Y.; Zhou, Z. Alkaline rechargeable Ni/Co batteries: Cobalt hydroxides as negative electrode materials. Energy Environ. Sci. 2009, 2, 502–505. [Google Scholar] [CrossRef]
- Gao, X.P.; Yang, H.X. Multi-electron reaction materials for high energy density batteries. Energy Environ. Sci. 2010, 3, 174–189. [Google Scholar] [CrossRef]
- PowerGenix Batteries. The Nickel-Zinc Battery. Available online: http://www.powergenix.com/the-nickel-zinc-battery/ (accessed on 6 April 2016).
- Wikipedia Webpage. Nickel-Cadmium Battery. Available online: https://en.wikipedia.org/wiki/Nickel%E2%80%93cadmium_battery/ (accessed on 6 April 2016).
- Lide, D.R. CRC Handbook of Chemistry and Physics, 74th ed.; CRC Press Inc.: Boca Raton, FA, USA, 1993. [Google Scholar]
- Pourbaix, M. Atlas of Electrochemical Equilibrium in Aqueous Solutions; National Association of Corrosion Engineers: Houston, TX, USA, 1974. [Google Scholar]
- Nimmermark, A.; Ohrstrom, L.; Reedijk, J. Metal-ligand bond lengths and strengths: Are they correlated? A detailed CSD analysis. Z. Krist. Cryst. Mater. 2013, 228, 311–317. [Google Scholar] [CrossRef]
- Wikipedia Webpage. General Motors EV1. Available online: https://en.wikipedia.org/wiki/General_Motors_EV1 (accessed on 17 May 2016).
- Matthé, R.; Eberle, U. The Voltec System: Energy Storage and Electric Propulsion. Available online: https://www.selidori.com/tech/scarica.php?id_doc=1090 (accessed on 17 May 2016).
- Tanoue, K.; Yanagihara, H.; Kusumi, H. Hybrid Is A Key Technology for Future Automobiles. In Hydrogen Technology; Léon, A., Ed.; Springer: Berlin, Germany, 2008; pp. 235–272. [Google Scholar]
- Zelinsky, M.; Koch, J.; Fetcenko, M. Heat Tolerant NiMH Batteries for Stationary Power. Available online: https://www.battcon.com/PapersFinal2010/ZelinskyPaper2010Final_12.pdf (accessed on 28 March 2016).
- Zelinsky, M.; Koch, J. Batteries and Heat—A Recipe for Success? Available online: https://www.battcon.com/PapersFinal2013/16-Mike%20Zelinsky%20-%20Batteries%20and%20Heat.pdf (accessed on 28 March 2016).
- Zhao, X.; Ma, L.; Shen, X. Co-based anode materials for alkaline rechargeable Ni/Co batteries: A review. J. Mater. Chem. 2012, 22, 277–285. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, Y.; Xiao, L.; Song, D.; Wang, Y.; Jiao, L.; Yuan, H. Structure and electrochemical behaviors of a series of Co-B alloys. Electrochim. Acta 2008, 53, 2265–2271. [Google Scholar] [CrossRef]
- Wang, Y.; Lee, J.M.; Wang, X. An investigation of the origin of the electrochemical hydrogen storage capacities of the ball-milled Co–Si composites. Int. J. Hydrog. Energy 2010, 35, 1669–1673. [Google Scholar] [CrossRef]
- Cao, Y.; Zhou, W.; Li, X.; Ai, X.; Gao, X.; Yang, H. Electrochemical hydrogen storage behaviors of ultrafine Co-P particles prepared by direct ball-milling method. Electrochim. Acta 2006, 51, 4285–4290. [Google Scholar] [CrossRef]
- Wang, Q.; Jiao, L.; Du, H.; Peng, W.; Liu, S.; Wang, Y.; Yuan, H. Electrochemical hydrogen storage property of Co-S alloy prepared by ball-milling method. Int. J. Hydrog. Energy 2010, 35, 8357–8362. [Google Scholar] [CrossRef]
- Sapru, K.; Reichman, B.; Reger, A.; Ovshinsky, S.R. Rechargeable Battery and Electrode Used Therein. U.S. Patent 4,623,597, 18 November 1986. [Google Scholar]
- Han, Y.; Wang, Y.; Wang, Y.; Jiao, L.; Yuan, H. Characterization of CoB-silica nanochains hydrogen storage composite prepared by in-situ reduction. Int. J. Hydrog. Energy 2010, 35, 8177–8181. [Google Scholar] [CrossRef]
- Yao, S.M.; Xi, K.; Li, G.R.; Gao, X. Preparation and electrochemical properties of Co-Si3N4 nanocomposites. J. Power Sources 2008, 184, 657–662. [Google Scholar] [CrossRef]
- Du, H.; Jiao, L.; Wang, Q.; Peng, W.; Song, D.; Wang, Y.; Yuan, H. Structure and electrochemical properties of ball-milled Co-carbon nanotube composites as negative electrode material of alkaline rechargeable batteries. J. Power Sources 2011, 196, 5751–5755. [Google Scholar] [CrossRef]
- Li, L.; Xu, Y.; An, C.; Wang, Y.; Jiao, L.; Yuan, H. Enhanced electrochemical properties of Co/CMK-3 composite as negative material for alkaline secondary battery. J. Power Sources 2013, 238, 117–122. [Google Scholar] [CrossRef]
- Xu, Y.; Wang, X.; An, C.; Wang, Y.; Jiao, L.; Yuan, H. Effect of the length and surface area on electrochemical performance of cobalt oxide nanowires for alkaline secondary battery application. J. Power Sources 2014, 272, 703–710. [Google Scholar] [CrossRef]
- Fierro, C.; Fetcenko, M.A.; Young, K.; Ovshinsky, S.R.; Sommers, B.; Harrison, C. Nickel Hydroxide Positive Electrode Material Exhibiting Improved Conductivity and Engineered Activation Energy. U.S. Patent 6,228,535, 8 May 2001. [Google Scholar]
- Fierro, C.; Fetcenko, M.A.; Young, K.; Ovshinsky, S.R.; Sommers, B.; Harrison, C. Nickel Hydroxide Positive Electrode Material Exhibiting Improved Conductivity and Engineered Activation Energy. U.S. Patent 6,447,953, 10 September 2002. [Google Scholar]
- Ovshinsky, S.R.; Corrigan, D.; Venkatesan, S.; Young, R.; Fierro, C.; Fetcenko, M.A. Chemically and Compositionally Modified Solid Solution Disordered Multiphase Nickel Hydroxide Positive Electrode for Alkaline Rechargeable Electrochemical Cells. U.S. Patent 5,348,822, 20 September 1994. [Google Scholar]
- Wong, D.F.; Young, K.; Wang, L.; Nei, J.; Ng, K.Y.S. Evolution of stacking faults in substituted nickel hydroxide spherical powders. J. Alloys Compd. 2016. submitted. [Google Scholar]
- Young, K.; Ng, K.Y.S.; Bendersky, L.A. A technical report of the robust affordable next generation energy storage system-BASF program. Batteries 2016, 2. [Google Scholar] [CrossRef]
- Fierro, C.; Zallen, A.; Koch, J.; Fetcenko, M.A. The influence of nickel-hydroxide composition and microstructure on the high-temperature performance of nickel metal hydride batteries. J. Electrochem. Soc. 2006, 153, A492–A496. [Google Scholar] [CrossRef]
- Powder Diffraction File (PDF) Database; International Centre for Diffraction Data: Newtown Square, PA, USA, 2011.
- Siozios, A.; Zoubos, H.; Pliatsikas, N.; Koutsogeorgis, D.C.; Vourlias, G.; Pavlidou, E.; Cranton, W.; Patsalas, P. Growth an annealing strategies to control the microstructure of AlN:Ag nanocomposite films for plasmonic applications. Surf. Coat. Technol. 2014, 255, 28–36. [Google Scholar] [CrossRef]
- Ishijima, Y.; Kannari, S.; Kurishita, H.; Hasegawa, M.; Hiraoka, Y.; Takida, T.; Takebe, K. Processing of fine-grained W materials without detrimental phases and their mechanical properties ta 200–432 K. Mater. Sci. Eng. A 2008, 473, 7–15. [Google Scholar] [CrossRef]
- Wang, M.; Du, J.; Deng, Q.; Tian, Z.; Zhu, J. The effect of phosphorus on the microstructure and mechanical properties of ATI 718Plus alloy. Mater. Sci. Eng. A 2015, 626, 382–389. [Google Scholar] [CrossRef]
- Fu, H.; Xiao, Q.; Li, Y. A study of the microstructures and properties of Fe-V-W-Mo alloy modified by rare earth. Mater. Sci. Eng. A 2005, 395, 281–287. [Google Scholar] [CrossRef]
- Nei, J.; Young, K.; Rotarov, D. Studies in Mg-Ni based metal hydride electrode with electrolytes composed of various hydroxides. Batteries 2016. submitted. [Google Scholar]
- Yan, S.; Young, K.; Ng, K.Y.S. Effects of salt additives to the KOH electrolyte used in Ni/MH batteries. Batteries 2015, 1, 54–73. [Google Scholar] [CrossRef]
- BASF Catalysts. NCM Cathode Materials. Available online: http://www.catalysts.basf.com/p02/USWeb-Internet/catalysts/en/content/microsites/catalysts/prods-inds/batt-mats/NCM (accessed on 17 May 2016).
- Fetcenko, M.A. BASF-ANL Collaboration on NCM Cathode Materials. Available online: http://www.energy.gov/sites/prod/files/2014/11/f19/Fetcenko%20-%20Industry%20Partners%20Panel_0.pdf (accessed on 17 May 2016).








| Battery | Anode (Charge/Discharge) | Pros | Cons | Commercial Product | Energy Density |
|---|---|---|---|---|---|
| Ni-Mn | Mn/Mn(OH)2 | • Low cost | Only theoretical | No | 130–190 Wh·kg−1 [6] |
| • High voltage (2.2 V) | |||||
| Ni-Fe | Fe/Fe(OH)2 | • Low cost | • Low energy density | Yes | 30 Wh·kg−1 [9] |
| • Long cycle life | • Low power | ||||
| Ni-Co | Co/Co(OH)2 | • High capacity | High cost | No | Projected to be 165 Wh·kg−1 [10,11] |
| Ni-Zn | Zn/Zn(OH)2 | • Low cost | Cycle life still has room to improve | Yes | 65‒120 Wh·kg−1 [12] |
| • Higher voltage (1.5 V) | |||||
| Ni-Cd | Cd/Cd(OH)2 | • Low cost | Yes | 40‒60 Wh·kg−1 [13] | |
| • Long cycle life | • Toxic | ||||
| • High power at low temperature | • Low energy density |
| Hydroxide | Formula Weight | Theoretical Capacity (mAh·g−1) | Density (g·cm−3) | Solubility in Cold Water (g·100·cm−3) | E0(Mt) in Equation (7) [15] (V) | M–O Bond Strength (kJ·mol−1·M) |
|---|---|---|---|---|---|---|
| Mn(OH)2 | 88.94 | 603 | 3.258 | 0.0002 | −0.163 | 402.9 |
| Fe(OH)2 | 89.55 | 597 | 3.4 | 0.00015 | 0.493 | 390.4 |
| Co(OH)2 | 92.93 | 577 | 3.597 | 0.00032 | 0.659 | 384.5 |
| Ni(OH)2 | 92.69 | 578 | 4.15 | 0.013 | 0.648 | 382.0 |
| Zn(OH)2 | 99.39 | 539 | 3.258 | 0.0002 | 0.034 | 180 [16] |
| Cd(OH)2 | 146.41 | 366 | 4.79 | 0.00025 | 0.583 | 235.6 |
| M in M(OH)2 | Ni | Mn | Ni0.91Co0.09 | Ni0.91Zn0.09 | Ni0.91Mn0.09 | Ni0.33Co0.33Mn0.33 | Ni0.4Co0.2Mn0.4 |
|---|---|---|---|---|---|---|---|
| First cycle | 1.0 | 0.0 | 0.0 | 0.0 | 0.0 | 17.2 | 14.5 |
| Fifth cycle | 0.7 | 0.0 | 0.0 | 0.0 | 0.0 | 20.7 | 20.1 |
| Lattice Constant (Å) | Pristine | At the First Discharge Voltage Plateau | After First Charge | After Discharge |
|---|---|---|---|---|
| a | 2.92 | 3.09 | 3.11 | 3.02 |
| c | 13.44 | 13.82 | 13.87 | 13.85 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, L.; Young, K.-H.; Shen, H.-T. New Type of Alkaline Rechargeable Battery—Ni-Ni Battery. Batteries 2016, 2, 16. https://doi.org/10.3390/batteries2020016
Wang L, Young K-H, Shen H-T. New Type of Alkaline Rechargeable Battery—Ni-Ni Battery. Batteries. 2016; 2(2):16. https://doi.org/10.3390/batteries2020016
Chicago/Turabian StyleWang, Lixin, Kwo-Hsiung Young, and Hao-Ting Shen. 2016. "New Type of Alkaline Rechargeable Battery—Ni-Ni Battery" Batteries 2, no. 2: 16. https://doi.org/10.3390/batteries2020016







