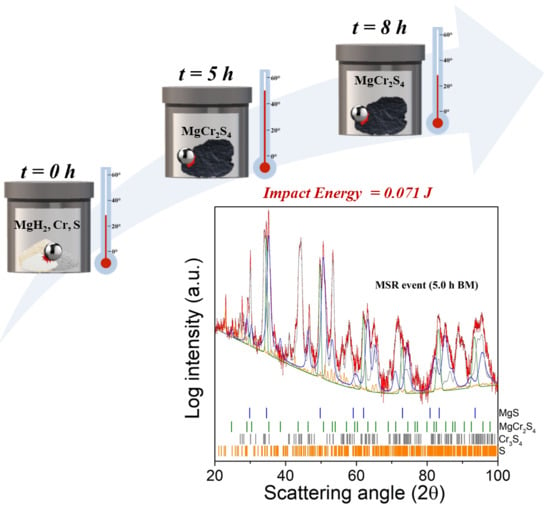
Solvent-Free Mechanochemical Approach towards Thiospinel MgCr2S4 as a Potential Electrode for Post-Lithium Ion Batteries
Abstract
:1. Introduction
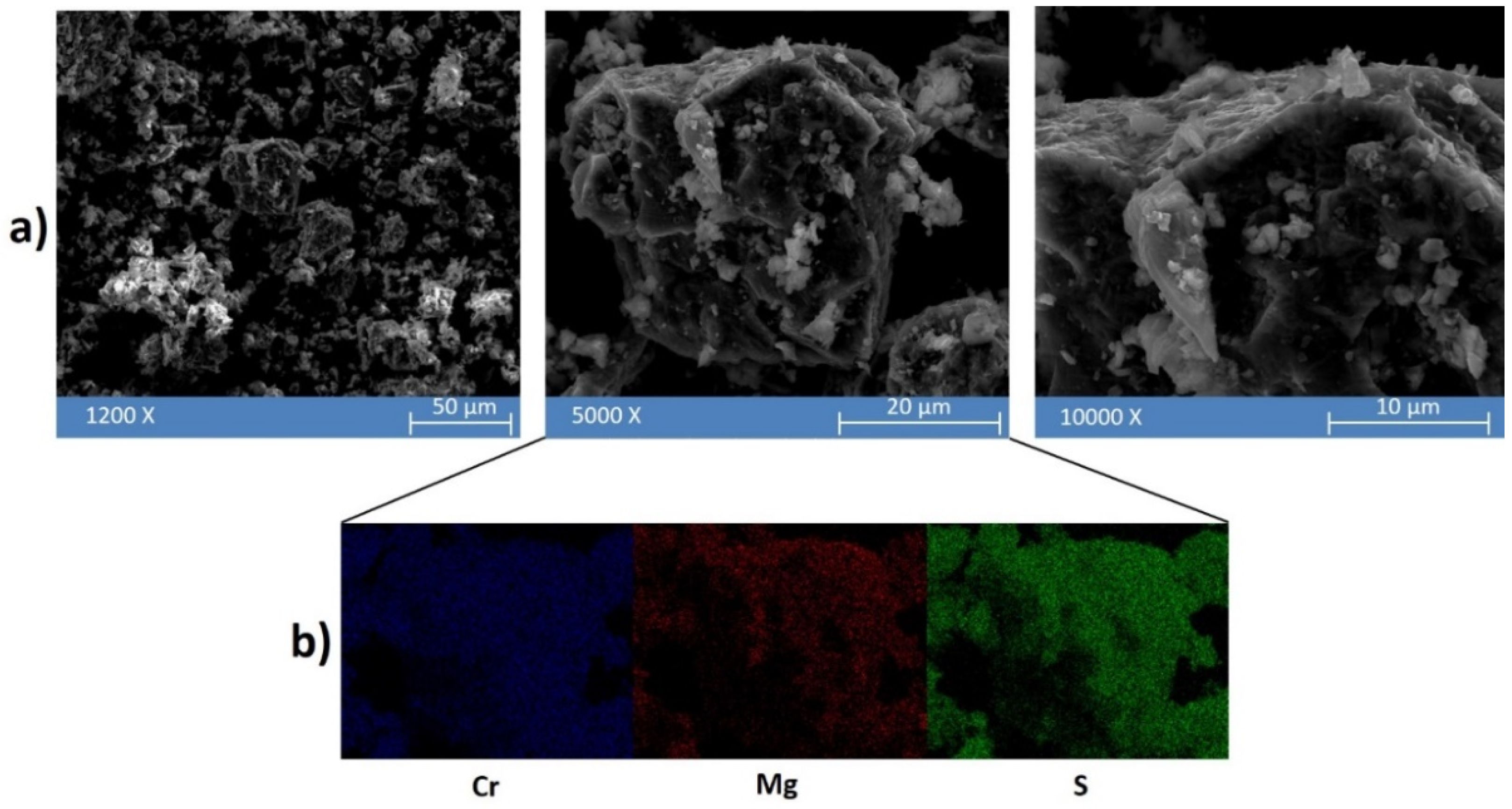
2. Results and Discussion
3. Materials and Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Dunn, B.; Kamath, H.; Tarascon, J.-M. Electrical Energy Storage for the Grid: A Battery of Choices. Science 2011, 334, 928–935. [Google Scholar] [CrossRef] [Green Version]
- Schipper, F.; Aurbach, D. A brief review: Past, present and future of lithium ion batteries. Russ. J. Electrochem. 2016, 52, 1095–1121. [Google Scholar] [CrossRef]
- Islam, M.S.; Fisher, C.A.J. Lithium and sodium battery cathode materials: Computational insights into voltage, diffusion and nanostructural properties. Chem. Soc. Rev. 2014, 43, 185–204. [Google Scholar] [CrossRef] [Green Version]
- Ding, Y.; Cano, Z.P.; Yu, A.; Lu, J.; Chen, Z. Automotive Li-Ion Batteries: Current Status and Future Perspectives. Electrochem. Energy Rev. 2019, 2, 1–28. [Google Scholar] [CrossRef]
- Fares, R.L.; Webber, M.E. The impacts of storing solar energy in the home to reduce reliance on the utility. Nat. Energy 2017, 2, 17001. [Google Scholar] [CrossRef]
- Heelan, J.; Gratz, E.; Zheng, Z.; Wang, Y.; Chen, M.; Apelian, D.; Wang, Y. Current and Prospective Li-Ion Battery Recycling and Recovery Processes. JOM 2016, 68, 2632–2638. [Google Scholar] [CrossRef] [Green Version]
- Martin, G.; Rentsch, L.; Höck, M.; Bertau, M. Lithium market research—Global supply, future demand and price development. Energy Storage Mater. 2017, 6, 171–179. [Google Scholar] [CrossRef]
- Canepa, P.; Gautam, G.S.; Hannah, D.C.; Malik, R.; Liu, M.; Gallagher, K.G.; Persson, K.A.; Ceder, G. Odyssey of Multivalent Cathode Materials: Open Questions and Future Challenges. Chem. Rev. 2017, 117, 4287–4341. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Zhang, Q. Recent Progress in Multivalent Metal (Mg, Zn, Ca, and Al) and Metal-Ion Rechargeable Batteries with Organic Materials as Promising Electrodes. Small 2019, 15, e1805061. [Google Scholar] [CrossRef]
- Aurbach, D.; Lu, Z.; Schechter, A.; Gofer, Y.; Gizbar, H.; Turgeman, R.; Cohen, Y.; Moshkovich, M.; Levi, E. Prototype systems for rechargeable magnesium batteries. Nature 2000, 407, 724–727. [Google Scholar] [CrossRef]
- Robba, A.; Vizintin, A.; Bitenc, J.; Mali, G.; Arčon, I.; Kavčič, M.; Žitnik, M.; Bučar, K.; Aquilanti, G.; Martineau-Corcos, C.; et al. Mechanistic Study of Magnesium–Sulfur Batteries. Chem. Mater. 2017, 29, 9555–9564. [Google Scholar] [CrossRef]
- Ma, Z.; Macfarlane, D.R.; Kar, M. Mg Cathode Materials and Electrolytes for Rechargeable Mg Batteries: A Review. Batter. Supercaps 2019, 2, 115–127. [Google Scholar] [CrossRef]
- Bertasi, F.; Hettige, C.; Sepehr, F.; Bogle, X.; Pagot, G.; Vezzù, K.; Negro, E.; Paddison, S.J.; Greenbaum, S.G.; Vittadello, M.; et al. A Key concept in Magnesium Secondary Battery Electrolytes. ChemSusChem 2015, 8, 3069–3076. [Google Scholar] [CrossRef] [PubMed]
- Ponrouch, A.; Palacín, M.R. Post-Li batteries: Promises and challenges. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2019, 377, 20180297. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Han, L.; Wang, Y.; Li, X.; Lu, J.-L.; Hu, X. Microstructure Characteristics of Cathode Materials for Rechargeable Magnesium Batteries. Small 2019, 15, e1900105. [Google Scholar] [CrossRef]
- Mohtadi, R.; Mizuno, F. Magnesium batteries: Current state of the art, issues and future perspectives. Beilstein J. Nanotechnol. 2014, 5, 1291–1311. [Google Scholar] [CrossRef]
- Bodenez, V.; Dupont, L.; Laffont, L.; Armstrong, A.R.; Shaju, K.M.; Bruce, P.G.; Tarascon, J.-M. The reaction of lithium with CuCr2S4—Lithium intercalation and copper displacement/extrusion. J. Mater. Chem. 2007, 17, 3238. [Google Scholar] [CrossRef]
- Liu, M.; Rong, Z.; Malik, R.; Canepa, P.; Jain, A.; Ceder, G.; Persson, K.A. Spinel compounds as multivalent battery cathodes: A systematic evaluation based on ab initio calculations. Energy Environ. Sci. 2015, 8, 964–974. [Google Scholar] [CrossRef] [Green Version]
- Rong, Z.; Malik, R.; Canepa, P.; Gautam, G.S.; Liu, M.; Jain, A.; Persson, K.; Ceder, G. Materials Design Rules for Multivalent Ion Mobility in Intercalation Structures. Chem. Mater. 2015, 27, 6016–6021. [Google Scholar] [CrossRef]
- Kim, C.; Phillips, P.J.; Key, B.; Yi, T.; Nordlund, D.; Yu, Y.-S.; Bayliss, R.D.; Han, S.-D.; He, M.; Zhang, Z.; et al. Direct Observation of Reversible Magnesium Ion Intercalation into a Spinel Oxide Host. Adv. Mater. 2015, 27, 3377–3384. [Google Scholar] [CrossRef]
- Van Stapele, R. Chapter 8 Sulphospinels. In Handbook of Ferromagnetic Materials; North-Holland Publishing Company: Amsterdam, The Netherlands, 1982; Volume 3, pp. 603–745. [Google Scholar] [CrossRef]
- Liu, M.; Jain, A.; Rong, Z.; Qu, X.; Canepa, P.; Malik, R.; Ceder, G.; Persson, K.A. Evaluation of sulfur spinel compounds for multivalent battery cathode applications. Energy Environ. Sci. 2016, 9, 3201–3209. [Google Scholar] [CrossRef] [Green Version]
- Wustrow, A.; Key, B.; Phillips, P.J.; Sa, N.; Lipton, A.S.; Klie, R.F.; Vaughey, J.T.; Poeppelmeier, K.R. Synthesis and Characterization of MgCr2S4 Thiospinel as a Potential Magnesium Cathode. Inorg. Chem. 2018, 57, 8634–8638. [Google Scholar] [CrossRef] [PubMed]
- Miura, A.; Ito, H.; Bartel, C.; Sun, W.; Rosero-Navarro, N.C.; Tadanaga, K.; Nakata, H.; Maeda, K.; Ceder, G. Selective metathesis synthesis of MgCr2S4 by control of thermodynamic driving forces. Mater. Horiz. 2020, 7, 1310–1316. [Google Scholar] [CrossRef] [Green Version]
- Shaikh, M.; Iqbal, M.; Akhter, J.I.; Ahmad, M.; Zaman, Q.; Akhtar, M.; Moughal, M.; Ahmed, Z.; Farooque, M. Alloying of immiscible Ge with Al by ball milling. Mater. Lett. 2003, 57, 3681–3685. [Google Scholar] [CrossRef]
- Delogu, F.; Cocco, G. Kinetics of structural evolution in immiscible Ag–Cu and Co–Cu systems under mechanical processing conditions. Mater. Sci. Eng. A 2005, 402, 208–214. [Google Scholar] [CrossRef]
- Goo, N.; Hirscher, M. Synthesis of the nanocrystalline MgS and its interaction with hydrogen. J. Alloys Compd. 2005, 404, 503–506. [Google Scholar] [CrossRef]
- Kyoi, D.; Rönnebro, E.; Kitamura, N.; Ueda, A.; Ito, M.; Katsuyama, S.; Sakai, T. The first magnesium–chromium hydride synthesized by the gigapascal high-pressure technique. J. Alloys Compd. 2003, 361, 252–256. [Google Scholar] [CrossRef]
- Takacs, L. Self-sustaining reactions induced by ball milling. Prog. Mater. Sci. 2002, 47, 355–414. [Google Scholar] [CrossRef]
- Baláž, P.; Baláž, M.; Achimovičová, M.; Bujňáková, Z.; Dutková, E. Chalcogenide mechanochemistry in materials science: Insight into synthesis and applications (a review). J. Mater. Sci. 2017, 52, 11851–11890. [Google Scholar] [CrossRef]
- Liang, B.Y.; Wang, M.Z. Synthesis of Ti3SiC2 by mechanically induced self-sustaining reaction: Some mechanistic aspects. Int. J. Self-Propagating High-Temp. Synth. 2012, 21, 172–177. [Google Scholar] [CrossRef]
- International Tables for Crystallography. J. Appl. Crystallogr. 1983, 16, 284. [CrossRef] [Green Version]
- Lide, D.R. Crc Handbook of Chemistry and Physics: A Ready-Reference Book of Chemical and Physical Data; CRC Press: Boca Raton, FL, USA, 2004. [Google Scholar]
- Wheast, R.C. Crc Standard thermodynamic properties of chemical substances. In CRC Handbook of Chemistry and Physics; CRC PRESS: Boca Raton, FL, USA, 2012; Volume 13, pp. 4–41. [Google Scholar] [CrossRef] [Green Version]
- Waldner, P.; Sitte, W. Thermodynamic modeling of the Cr—S system. Int. J. Mater. Res. 2011, 102, 1216–1225. [Google Scholar] [CrossRef]
- Abilov, C.; Kuliyev, A.; Hasanova, M. Phase Equilibra and Some Electrophycal Properties in the CuCr2S4—In System. JMEST 2015, 2, 267–270. [Google Scholar]
- Delogu, F.; Mulas, G.; Schiffini, L.; Cocco, G. Mechanical work and conversion degree in mechanically induced processes. Mater. Sci. Eng. A 2004, 382, 280–287. [Google Scholar] [CrossRef]
- Deidda, C.; Delogu, F.; Maglia, F.; Anselmi-Tamburini, U.; Cocco, G. Mechanical processing and self-sustaining high-temperature synthesis of TiC powders. Mater. Sci. Eng. A 2004, 800–803. [Google Scholar] [CrossRef]
- Takacs, L. Ball Milling-Induced Combustion in Powder Mixtures Containing Titanium, Zirconium, or Hafnium. J. Solid State Chem. 1996, 125, 75–84. [Google Scholar] [CrossRef]
- Lutterotti, L. Total pattern fitting for the combined size-strain-stress-texture determination in thin film diffraction. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. Atoms 2010, 268, 334–340. [Google Scholar] [CrossRef]





| Reagents | ΔfH° (KJ mol−1 at 298 K) | Products | ΔfH° (KJ mol−1 at 298 K) |
|---|---|---|---|
| MgH2 | −75.30 [34] | Cr2S3, Cr3S4 | −467.14 and −629.67 [35] |
| Cr | 0.00 [34] | MgS | −346.00 [34] |
| S | 0.00 [34] | MgCr2S4 | −869.90 [36] |
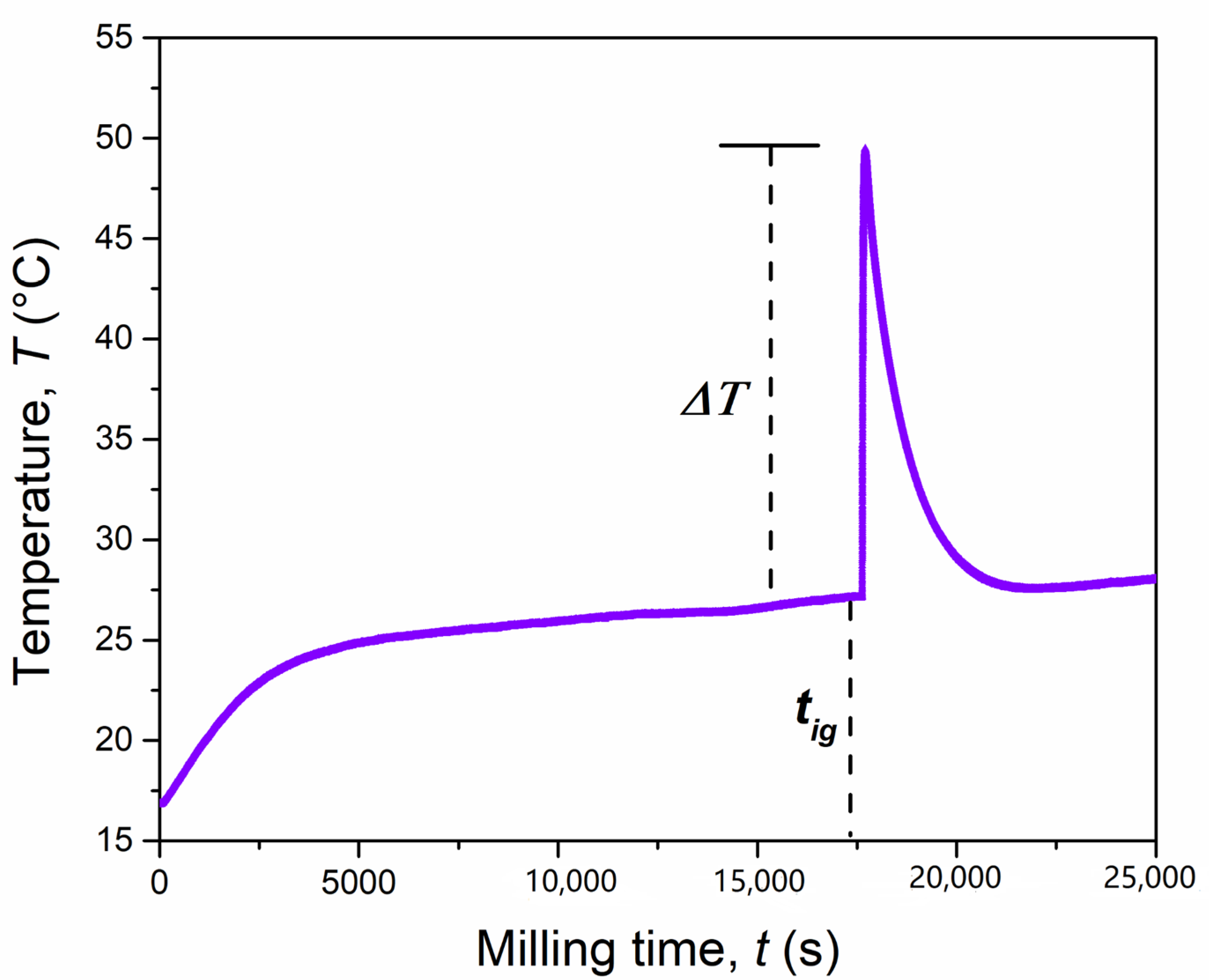
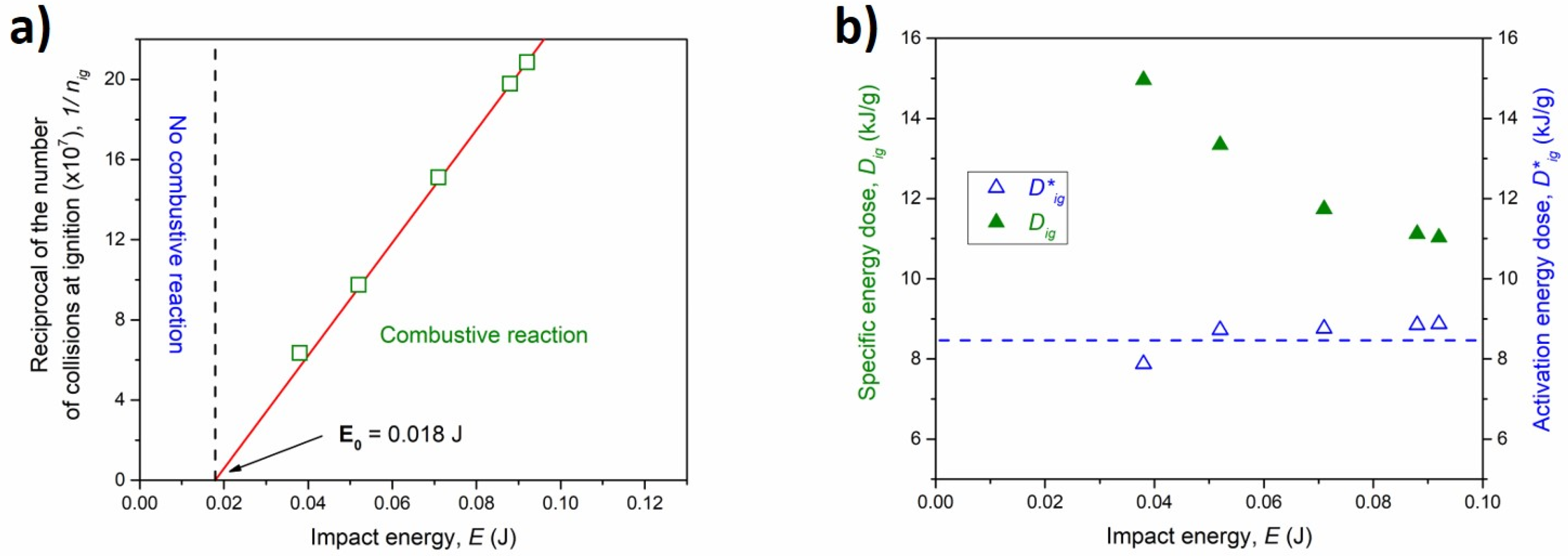
| Powder Mass, mp (g) | Ball Mass, mb (g) | Milling Speed, ν (rpm) | Collision Frequency, N (Hz) | Impact Energy, E (J) | Ignition Time, tig (h) | Specific Energy Dose, Dig (kJ g−1) |
|---|---|---|---|---|---|---|
| 4 | 8 | 640 | 21.34 | 0.038 | 20.50 | 14.96 |
| 4 | 8 | 750 | 25.00 | 0.052 | 11.40 | 13.34 |
| 4 | 8 | 875 | 29.20 | 0.071 | 6.29 | 11.74 |
| 4 | 8 | 975 | 32.50 | 0.086 | 4.32 | 11.12 |
| 4 | 8 | 1000 | 33.30 | 0.090 | 4.01 | 11.029 |
| 4 | 2 | 875 | 29.20 | 0.017 | NO MSR | - |
| 4 | 8 | 555 | 18.50 | 0.014 | NO MSR | - |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Caggiu, L.; Enzo, S.; Stievano, L.; Berthelot, R.; Gerbaldi, C.; Falco, M.; Garroni, S.; Mulas, G. Solvent-Free Mechanochemical Approach towards Thiospinel MgCr2S4 as a Potential Electrode for Post-Lithium Ion Batteries. Batteries 2020, 6, 43. https://doi.org/10.3390/batteries6030043
Caggiu L, Enzo S, Stievano L, Berthelot R, Gerbaldi C, Falco M, Garroni S, Mulas G. Solvent-Free Mechanochemical Approach towards Thiospinel MgCr2S4 as a Potential Electrode for Post-Lithium Ion Batteries. Batteries. 2020; 6(3):43. https://doi.org/10.3390/batteries6030043
Chicago/Turabian StyleCaggiu, Laura, Stefano Enzo, Lorenzo Stievano, Romain Berthelot, Claudio Gerbaldi, Marisa Falco, Sebastiano Garroni, and Gabriele Mulas. 2020. "Solvent-Free Mechanochemical Approach towards Thiospinel MgCr2S4 as a Potential Electrode for Post-Lithium Ion Batteries" Batteries 6, no. 3: 43. https://doi.org/10.3390/batteries6030043