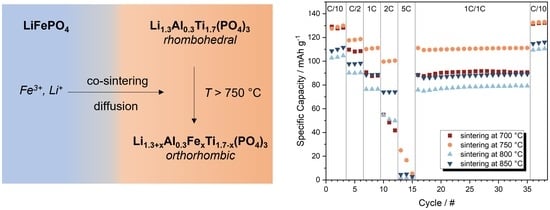
Co-Sintering of Li1.3Al0.3Ti1.7(PO4)3 and LiFePO4 in Tape-Casted Composite Cathodes for Oxide Solid-State Batteries
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of LATP-LFP Pellets
2.2. Characterization of LATP-LFP Pellets
2.3. Tape Casting of LATP-LFP Sheets
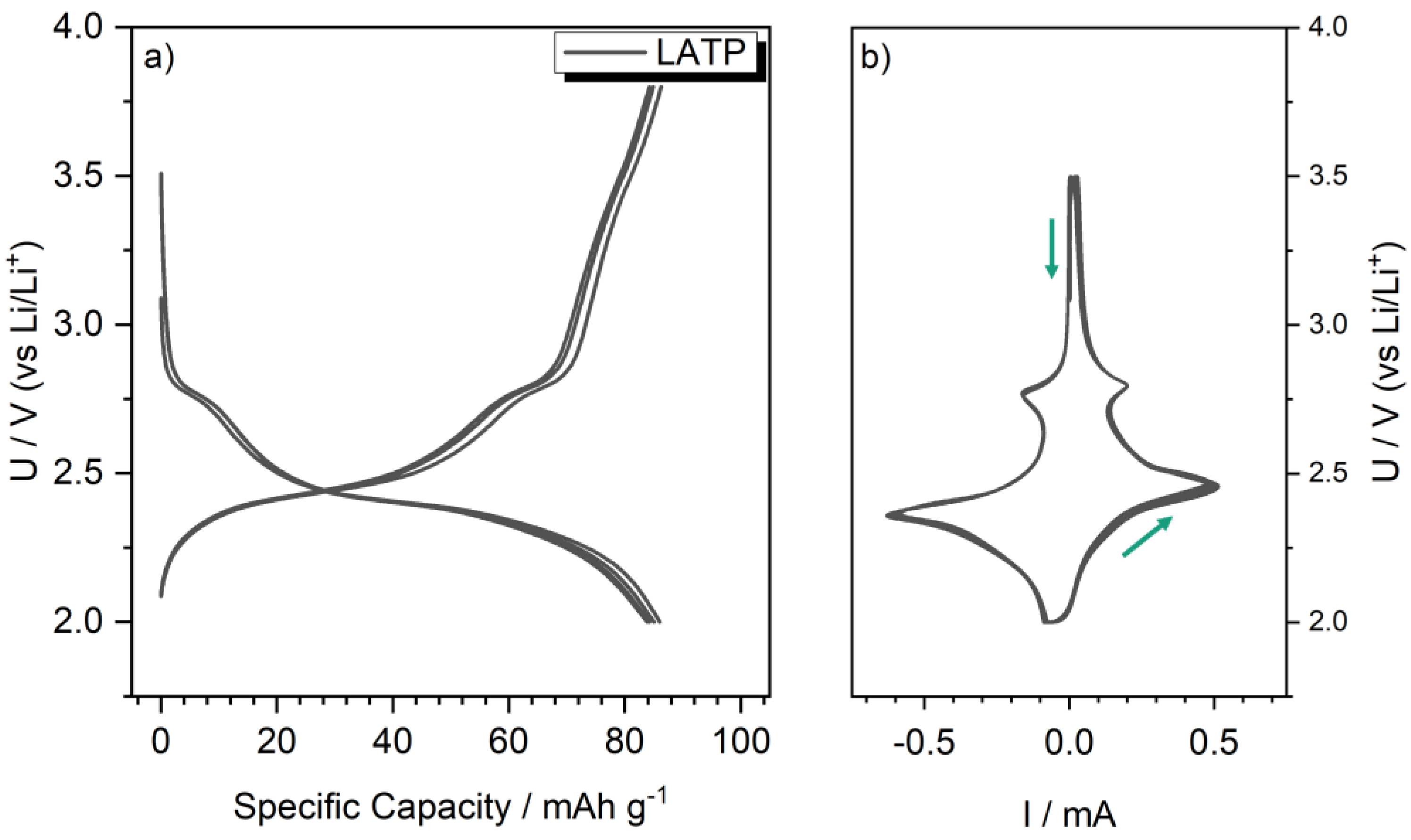
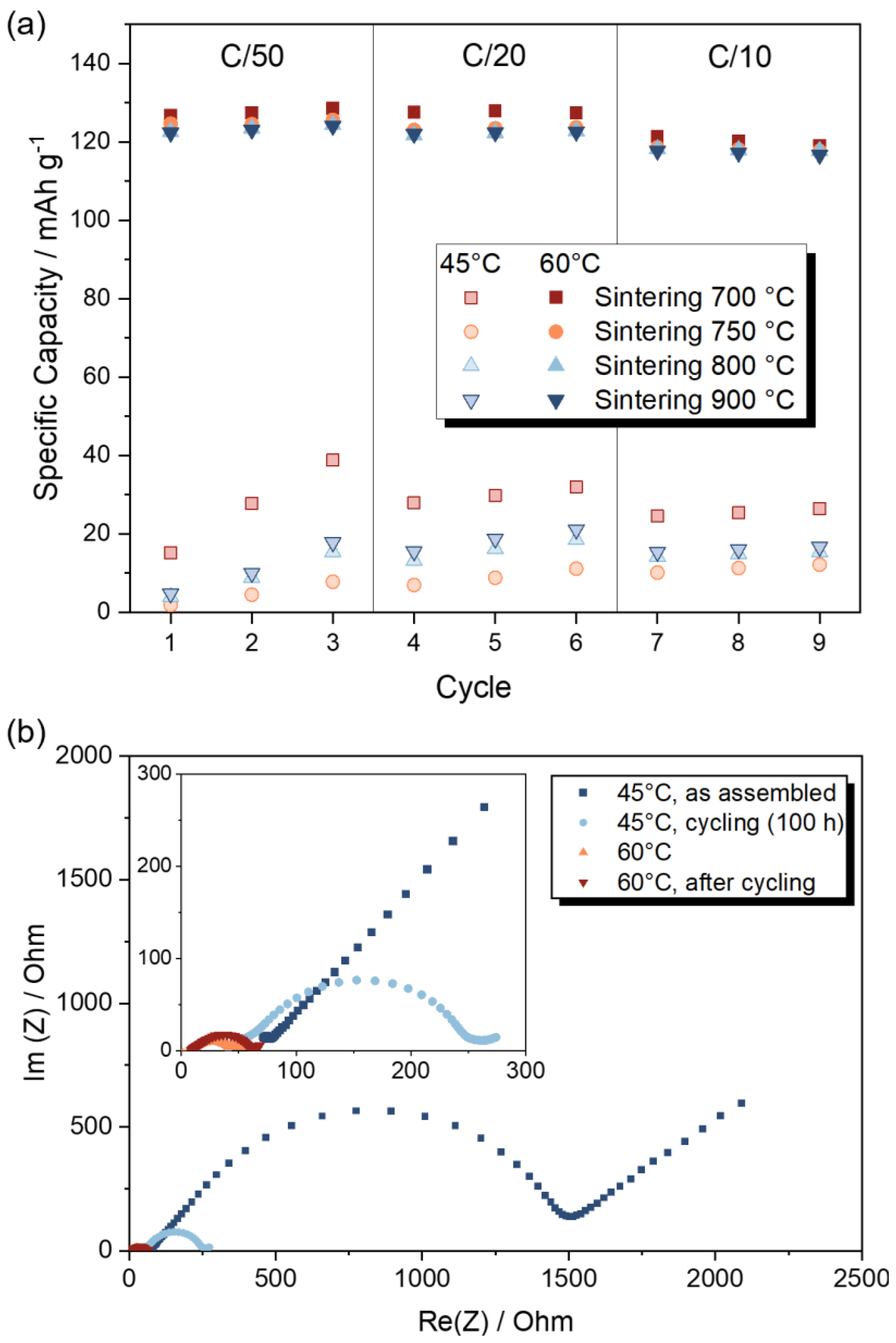
2.4. Electrochemical Characterization of LATP-LFP Sheets
3. Results
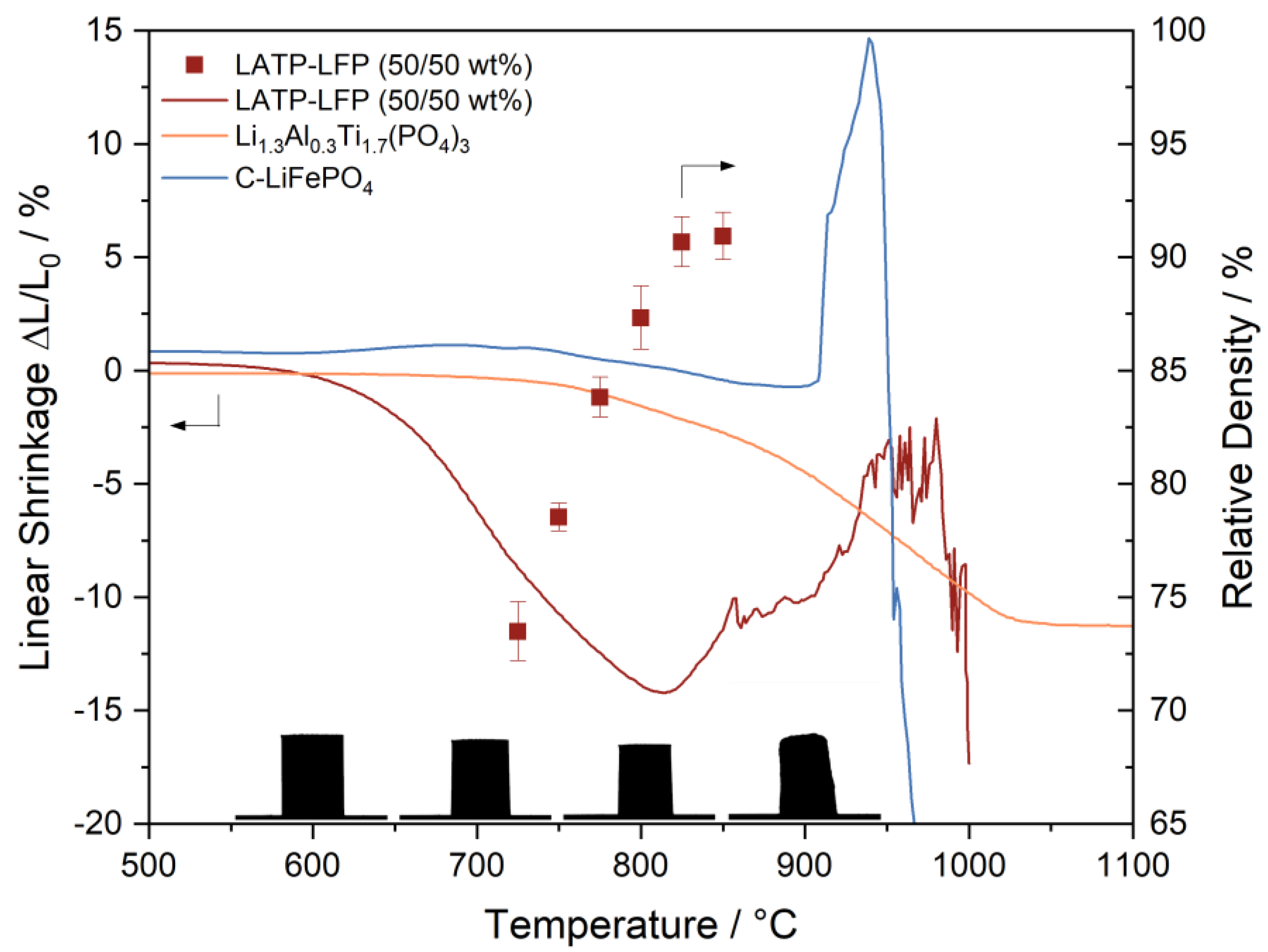
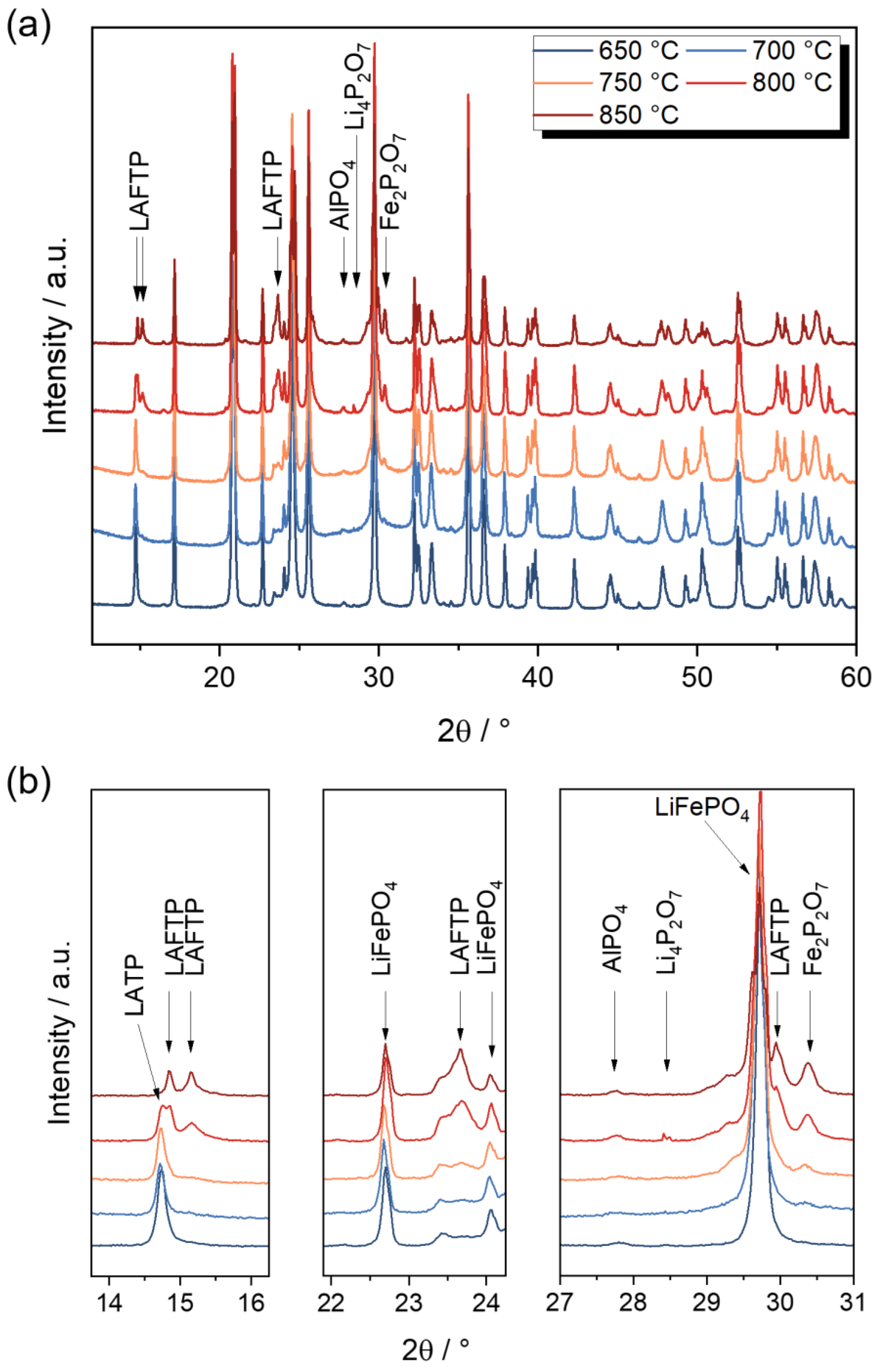
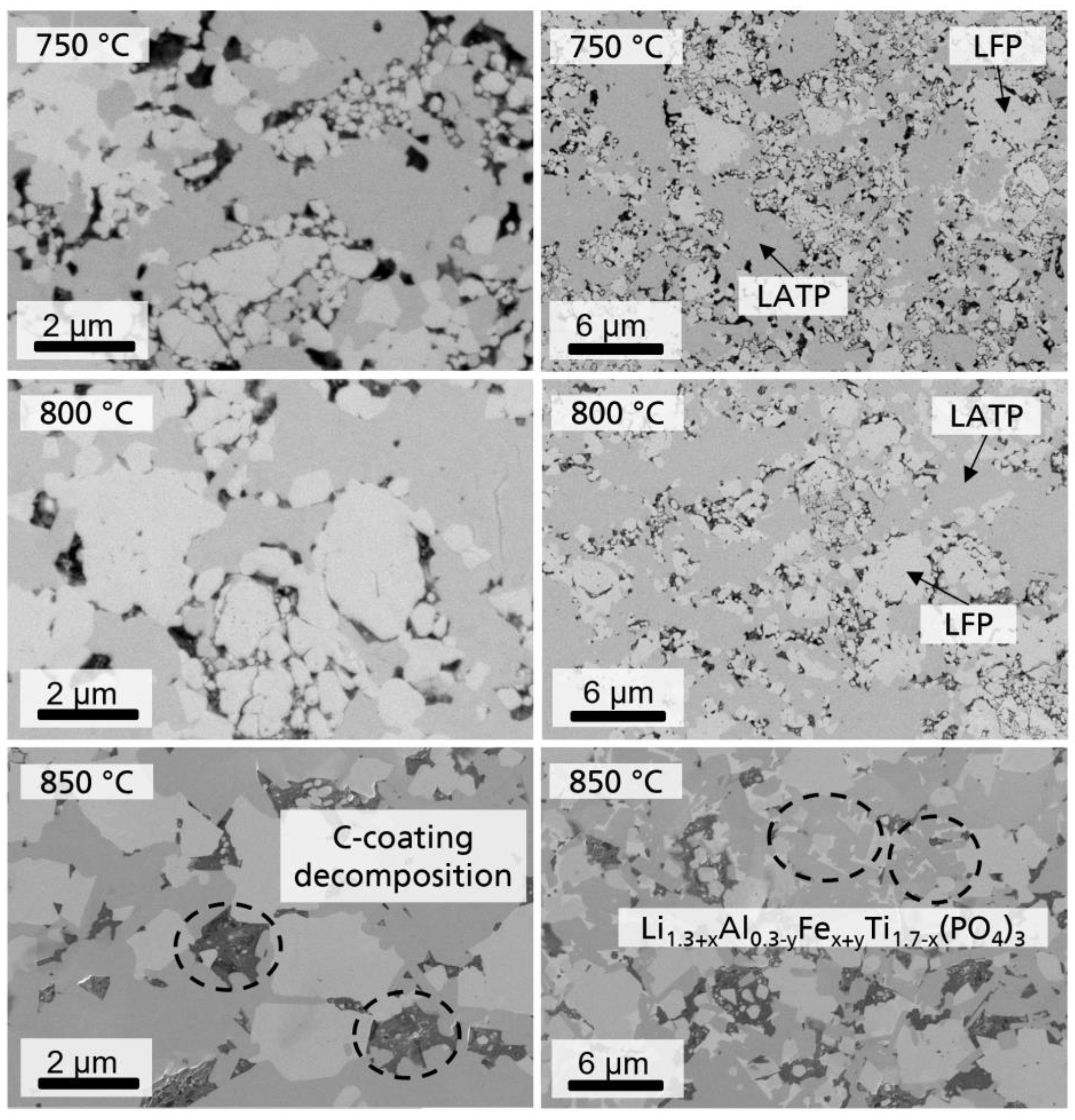
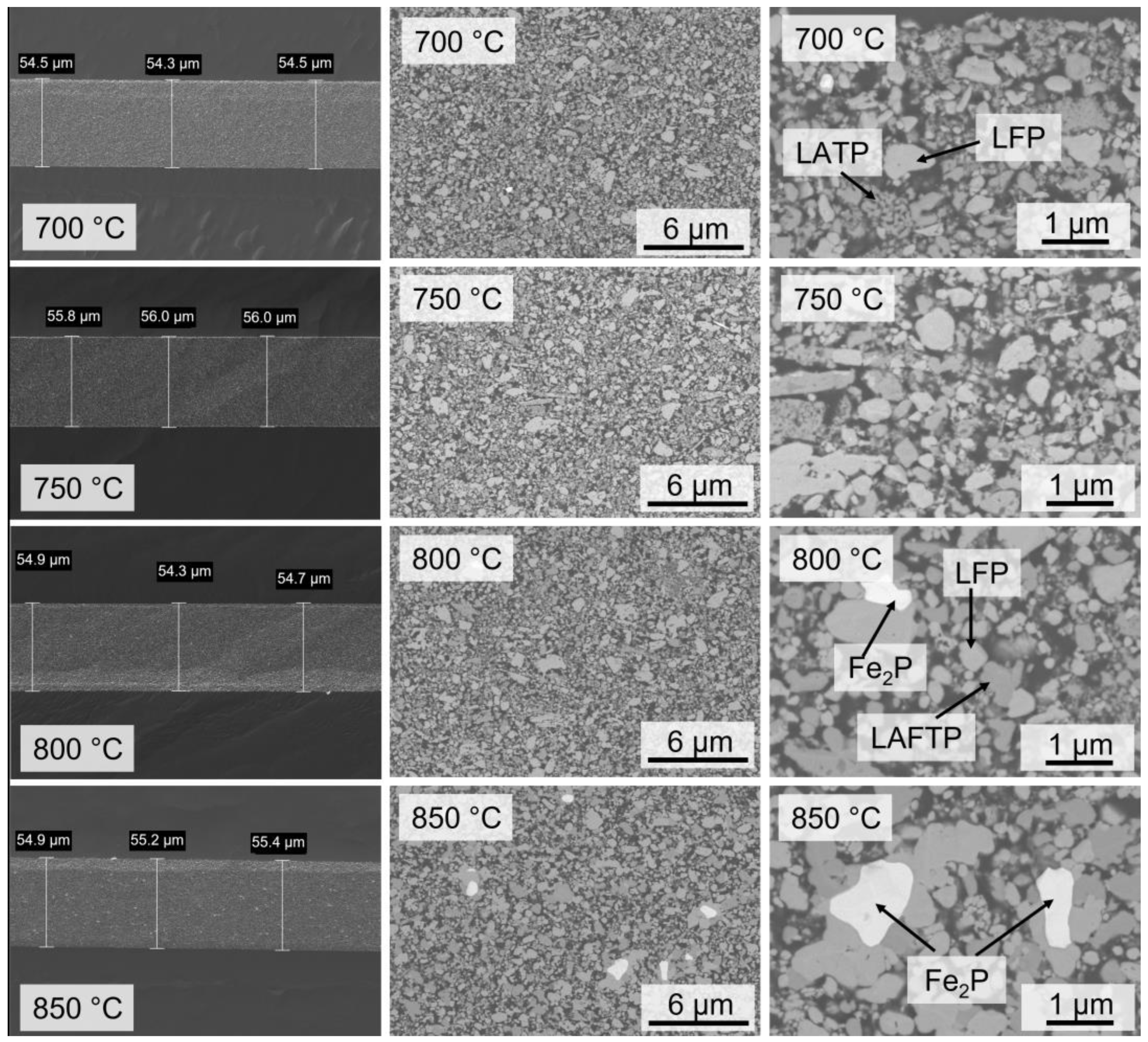
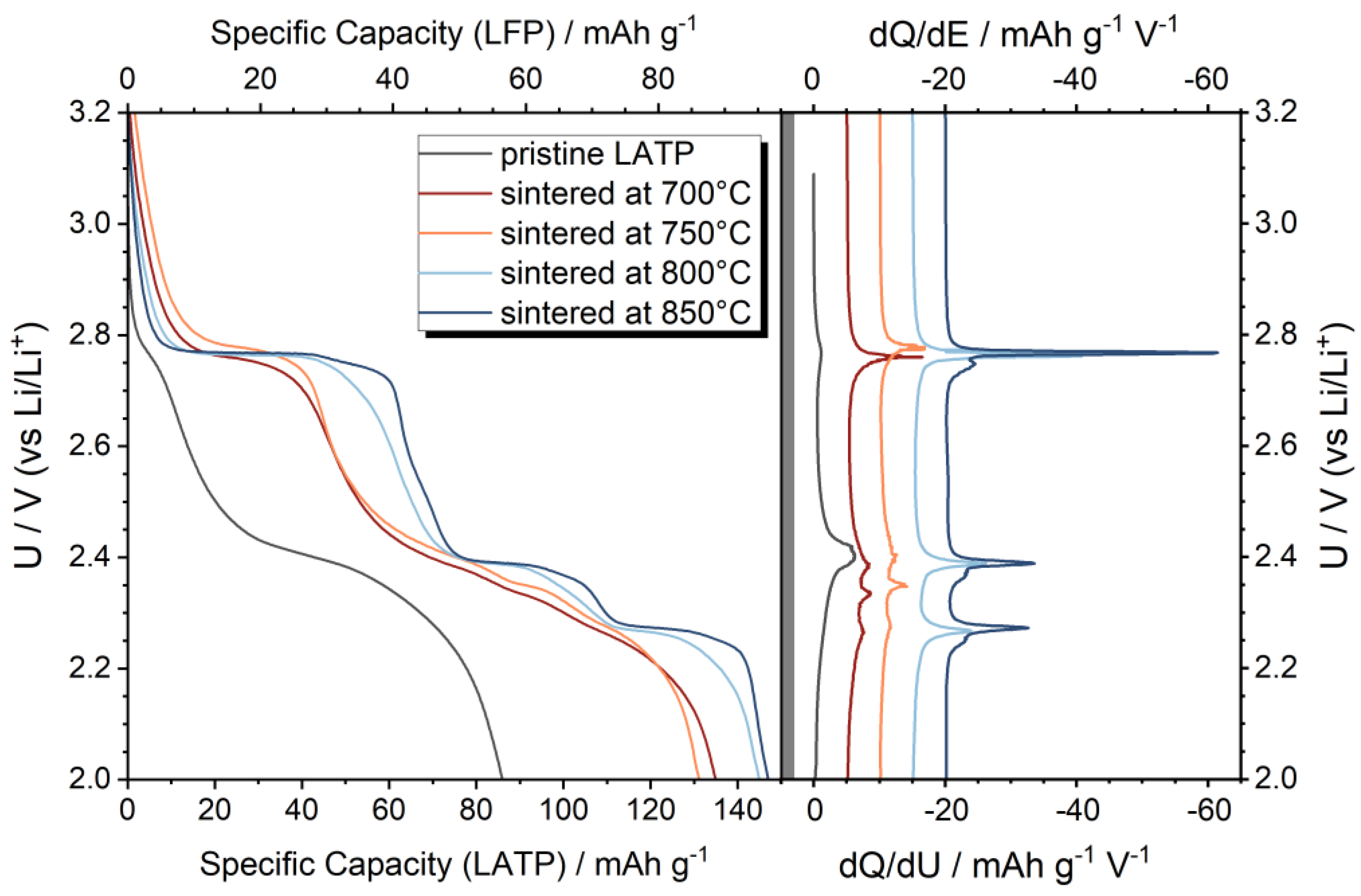
3.1. Characterization of LATP-LFP Pellets during and after Heat Treatment
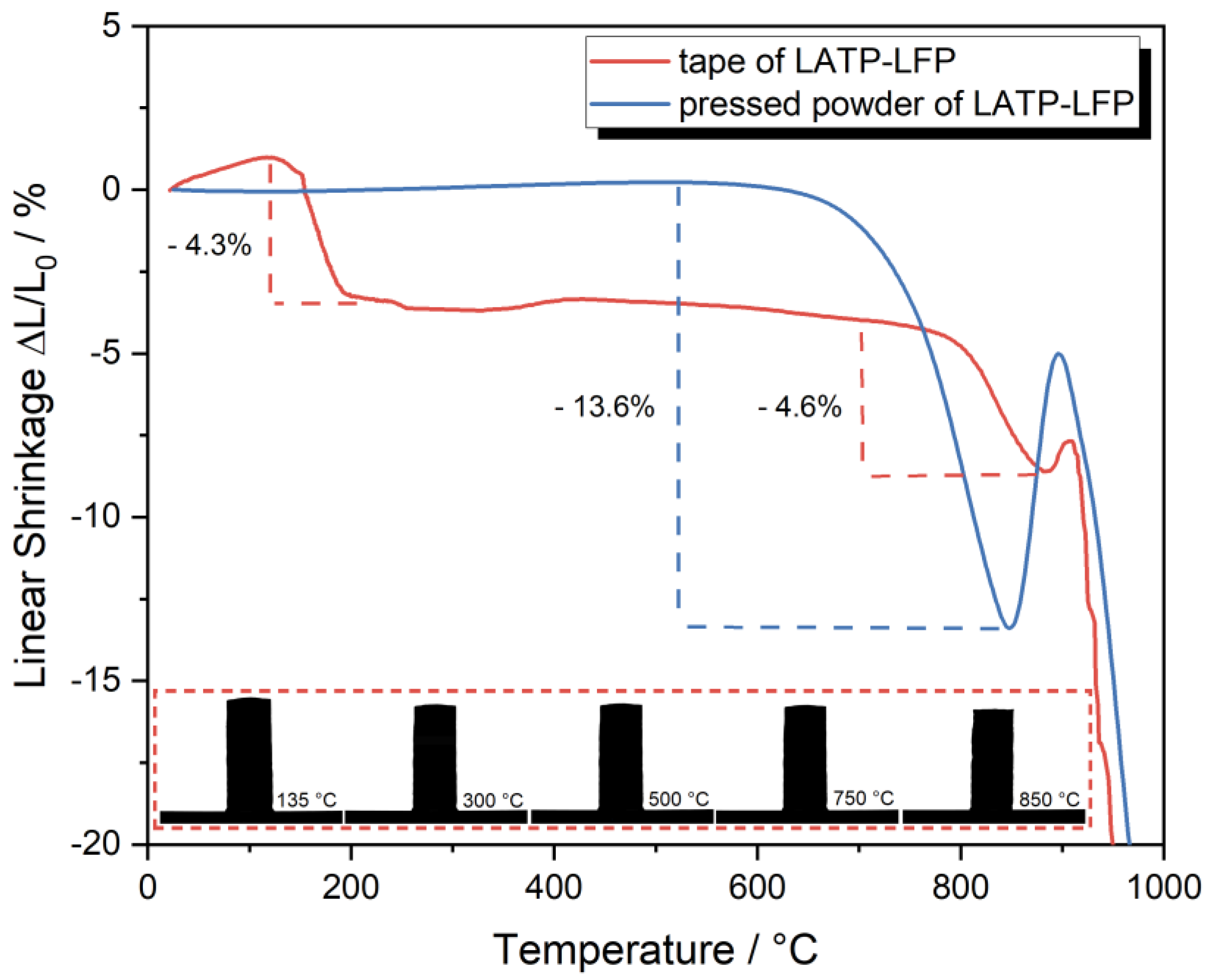
3.2. Sintering of Tape-Casted Composite Cathodes
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Heubner, C.; Voigt, K.; Marcinkowski, P.; Reuber, S.; Nikolowski, K.; Schneider, M.; Partsch, M.; Michaelis, A. From Active Materials to Battery Cells: A Straightforward Tool to Determine Performance Metrics and Support Developments at an Application-Relevant Level. Adv. Energy Mater. 2021, 11, 2102647. [Google Scholar] [CrossRef]
- Yamauchi, H.; Ikejiri, J.; Tsunoda, K.; Tanaka, A.; Sato, F.; Honma, T.; Komatsu, T. Enhanced rate capabilities in a glass-ceramic-derived sodium all-solid-state battery. Sci. Rep. 2020, 10, 9453. [Google Scholar] [CrossRef] [PubMed]
- Fertig, M.P.; Skadell, K.; Schulz, M.; Dirksen, C.; Adelhelm, P.; Stelter, M. From High- to Low-Temperature: The Revival of Sodium-Beta Alumina for Sodium Solid-State Batteries. Batter. Supercaps 2022, 5, e202100131. [Google Scholar] [CrossRef]
- TDK Electronics, AG. CeraCharge—Rechargeable Solid-State SMD Battery. Available online: https://www.tdk-electronics.tdk.com/en/ceracharge (accessed on 29 October 2023).
- Boaretto, N.; Garbayo, I.; Valiyaveettil-SobhanRaj, S.; Quintela, A.; Li, C.; Casas-Cabanas, M.; Aguesse, F. Lithium solid-state batteries: State-of-the-art and challenges for materials, interfaces and processing. J. Power Sources 2021, 502, 229919. [Google Scholar] [CrossRef]
- Lee, Y.-G.; Fujiki, S.; Jung, C.; Suzuki, N.; Yashiro, N.; Omoda, R.; Ko, D.-S.; Shiratsuchi, T.; Sugimoto, T.; Ryu, S.; et al. High-energy long-cycling all-solid-state lithium metal batteries enabled by silver–carbon composite anodes. Nat. Energy 2020, 5, 299–308. [Google Scholar] [CrossRef]
- Liu, H.; Cheng, X.-B.; Huang, J.-Q.; Yuan, H.; Lu, Y.; Yan, C.; Zhu, G.-L.; Xu, R.; Zhao, C.-Z.; Hou, L.-P.; et al. Controlling Dendrite Growth in Solid-State Electrolytes. ACS Energy Lett. 2020, 5, 833–843. [Google Scholar] [CrossRef]
- Schnell, J.; Knörzer, H.; Imbsweiler, A.J.; Reinhart, G. Solid versus Liquid—A Bottom-Up Calculation Model to Analyze the Manufacturing Cost of Future High-Energy Batteries. Energy Technol. 2020, 8, 1901237. [Google Scholar] [CrossRef]
- Mindemark, J.; Lacey, M.J.; Bowden, T.; Brandell, D. Beyond PEO—Alternative host materials for Li+ -conducting solid polymer electrolytes. Prog. Polym. Sci. 2018, 81, 114–143. [Google Scholar] [CrossRef]
- Mayer, A.; Steinle, D.; Passerini, S.; Bresser, D. Block copolymers as (single-ion conducting) lithium battery electrolytes. Nanotechnology 2021, 33, 062002. [Google Scholar] [CrossRef]
- Tong, B.; Song, Z.; Wu, H.; Wang, X.; Feng, W.; Zhou, Z.; Zhang, H. Ion transport and structural design of lithium-ion conductive solid polymer electrolytes: A perspective. Mater. Futures 2022, 1, 42103. [Google Scholar] [CrossRef]
- Waetzig, K.; Rost, A.; Heubner, C.; Coeler, M.; Nikolowski, K.; Wolter, M.; Schilm, J. Synthesis and sintering of Li1.3Al0.3Ti1.7(PO4)3 (LATP) electrolyte for ceramics with improved Li+ conductivity. J. Alloys Compd. 2020, 818, 153237. [Google Scholar] [CrossRef]
- Murugan, R.; Thangadurai, V.; Weppner, W. Fast lithium ion conduction in garnet-type Li7La3Zr2O12. Angew. Chem. Int. Ed Engl. 2007, 46, 7778–7781. [Google Scholar] [CrossRef] [PubMed]
- Reddy, M.V.; Julien, C.M.; Mauger, A.; Zaghib, K. Sulfide and Oxide Inorganic Solid Electrolytes for All-Solid-State Li Batteries: A Review. Nanomaterials 2020, 10, 1606. [Google Scholar] [CrossRef]
- Miura, A.; Rosero-Navarro, N.C.; Sakuda, A.; Tadanaga, K.; Phuc, N.H.H.; Matsuda, A.; Machida, N.; Hayashi, A.; Tatsumisago, M. Liquid-phase syntheses of sulfide electrolytes for all-solid-state lithium battery. Nat. Rev. Chem. 2019, 3, 189–198. [Google Scholar] [CrossRef]
- Li, X.; Liang, J.; Chen, N.; Luo, J.; Adair, K.R.; Wang, C.; Banis, M.N.; Sham, T.-K.; Zhang, L.; Zhao, S.; et al. Water-Mediated Synthesis of a Superionic Halide Solid Electrolyte. Angew. Chem. 2019, 131, 16579–16584. [Google Scholar] [CrossRef]
- Han, Y.; Jung, S.H.; Kwak, H.; Jun, S.; Kwak, H.H.; Lee, J.H.; Hong, S.-T.; Jung, Y.S. Single- or Poly-Crystalline Ni-Rich Layered Cathode, Sulfide or Halide Solid Electrolyte: Which Will be the Winners for All-Solid-State Batteries? Adv. Energy Mater. 2021, 11, 2100126. [Google Scholar] [CrossRef]
- Feng, J.; Wang, L.; Chen, Y.; Wang, P.; Zhang, H.; He, X. PEO based polymer-ceramic hybrid solid electrolytes: A review. Nano Converg. 2021, 8, 2. [Google Scholar] [CrossRef]
- Xue, S.; Chen, S.; Fu, Y.; Zhu, H.; Ji, Y.; Song, Y.; Pan, F.; Yang, L. Revealing the Role of Active Fillers in Li-ion Conduction of Composite Solid Electrolytes. Small 2023, 2305326. [Google Scholar] [CrossRef]
- Patel, S.V.; Banerjee, S.; Liu, H.; Wang, P.; Chien, P.-H.; Feng, X.; Liu, J.; Ong, S.P.; Hu, Y.-Y. Tunable Lithium-Ion Transport in Mixed-Halide Argyrodites Li6–xPS5–xClBrx: An Unusual Compositional Space. Chem. Mater. 2021, 33, 1435–1443. [Google Scholar] [CrossRef]
- Yu, C.; Zhao, F.; Luo, J.; Zhang, L.; Sun, X. Recent development of lithium argyrodite solid-state electrolytes for solid-state batteries: Synthesis, structure, stability and dynamics. Nano Energy 2021, 83, 105858. [Google Scholar] [CrossRef]
- Li, S.; Zhang, S.-Q.; Shen, L.; Liu, Q.; Ma, J.-B.; Lv, W.; He, Y.-B.; Yang, Q.-H. Progress and Perspective of Ceramic/Polymer Composite Solid Electrolytes for Lithium Batteries. Adv. Sci. 2020, 7, 1903088. [Google Scholar] [CrossRef] [PubMed]
- Hüttl, J.; Zapp, N.; Tanikawa, S.; Nikolowski, K.; Michaelis, A.; Auer, H. A Layered Hybrid Oxide–Sulfide All-Solid-State Battery with Lithium Metal Anode. Batteries 2023, 9, 507. [Google Scholar] [CrossRef]
- Schiffmann, N.; Bucharsky, E.C.; Schell, K.G.; Fritsch, C.A.; Knapp, M.; Hoffmann, M.J. Upscaling of LATP synthesis: Stoichiometric screening of phase purity and microstructure to ionic conductivity maps. Ionics 2021, 27, 2017–2025. [Google Scholar] [CrossRef]
- Mann, M.; Küpers, M.; Häuschen, G.; Finsterbusch, M.; Fattakhova-Rohlfing, D.; Guillon, O. Evaluation of Scalable Synthesis Methods for Aluminum-Substituted Li7La3Zr2O12 Solid Electrolytes. Materials 2021, 14, 6809. [Google Scholar] [CrossRef] [PubMed]
- Wolfenstine, J.; Allen, J.L.; Sakamoto, J.; Siegel, D.J.; Choe, H. Mechanical behavior of Li-ion-conducting crystalline oxide-based solid electrolytes: A brief review. Ionics 2018, 24, 1271–1276. [Google Scholar] [CrossRef]
- Hou, M.; Liang, F.; Chen, K.; Dai, Y.; Xue, D. Challenges and perspectives of NASICON-type solid electrolytes for all-solid-state lithium batteries. Nanotechnology 2020, 31, 132003. [Google Scholar] [CrossRef]
- Zhu, Y.; He, X.; Mo, Y. Origin of Outstanding Stability in the Lithium Solid Electrolyte Materials: Insights from Thermodynamic Analyses Based on First-Principles Calculations. ACS Appl. Mater. Interfaces 2015, 7, 23685–23693. [Google Scholar] [CrossRef]
- Uhlmann, C.; Braun, P.; Illig, J.; Weber, A.; Ivers-Tiffée, E. Interface and grain boundary resistance of a lithium lanthanum titanate (Li3xLa2/3−xTiO3, LLTO) solid electrolyte. J. Power Sources 2016, 307, 578–586. [Google Scholar] [CrossRef]
- Connell, J.G.; Fuchs, T.; Hartmann, H.; Krauskopf, T.; Zhu, Y.; Sann, J.; Garcia-Mendez, R.; Sakamoto, J.; Tepavcevic, S.; Janek, J. Kinetic versus Thermodynamic Stability of LLZO in Contact with Lithium Metal. Chem. Mater. 2020, 32, 10207–10215. [Google Scholar] [CrossRef]
- Milan, E.; Pasta, M. The role of grain boundaries in solid-state Li-metal batteries. Mater. Futures 2023, 2, 13501. [Google Scholar] [CrossRef]
- Gellert, M.; Dashjav, E.; Grüner, D.; Ma, Q.; Tietz, F. Compatibility study of oxide and olivine cathode materials with lithium aluminum titanium phosphate. Ionics 2018, 24, 1001–1006. [Google Scholar] [CrossRef]
- Miara, L.; Windmüller, A.; Tsai, C.-L.; Richards, W.D.; Ma, Q.; Uhlenbruck, S.; Guillon, O.; Ceder, G. About the Compatibility between High Voltage Spinel Cathode Materials and Solid Oxide Electrolytes as a Function of Temperature. ACS Appl. Mater. Interfaces 2016, 8, 26842–26850. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.-Y.; Choi, J.; Anandan, V.; Kim, J.-H. High-Temperature Chemical Stability of Li1.4Al0.4Ti1.6(PO4)3 Solid Electrolyte with Various Cathode Materials for Solid-State Batteries. J. Phys. Chem. C 2020, 124, 14963–14971. [Google Scholar] [CrossRef]
- Xu, Q.; Liu, Z.; Windmüller, A.; Basak, S.; Park, J.; Dzieciol, K.; Tsai, C.-L.; Yu, S.; Tempel, H.; Kungl, H.; et al. Active Interphase Enables Stable Performance for an All-Phosphate-Based Composite Cathode in an All-Solid-State Battery. Small 2022, 18, e2200266. [Google Scholar] [CrossRef] [PubMed]
- Waetzig, K.; Heubner, C.; Kusnezoff, M. Reduced Sintering Temperatures of Li+ Conductive Li1.3Al0.3Ti1.7(PO4)3 Ceramics. Crystals 2020, 10, 408. [Google Scholar] [CrossRef]
- Waetzig, K.; Rost, A.; Langklotz, U.; Matthey, B.; Schilm, J. An explanation of the microcrack formation in Li1.3Al0.3Ti1.7(PO4)3 ceramics. J. Eur. Ceram. Soc. 2016, 36, 1995–2001. [Google Scholar] [CrossRef]
- Beaupain, J.P.; Waetzig, K.; Otto, S.-K.; Henss, A.; Janek, J.; Malaki, M.; Pokle, A.; Müller, J.; Butz, B.; Volz, K.; et al. Reaction of Li1.3Al0.3Ti1.7(PO4)3 and LiNi0.6Co0.2Mn0.2O2 in Co-Sintered Composite Cathodes for Solid-State Batteries. ACS Appl. Mater. Interfaces 2021, 13, 47488–47498. [Google Scholar] [CrossRef]
- Malaki, M.; Pokle, A.; Otto, S.-K.; Henss, A.; Beaupain, J.P.; Beyer, A.; Müller, J.; Butz, B.; Wätzig, K.; Kusnezoff, M.; et al. Advanced Analytical Characterization of Interface Degradation in Ni-Rich NCM Cathode Co-Sintered with LATP Solid Electrolyte. ACS Appl. Energy Mater. 2022, 5, 4651–4663. [Google Scholar] [CrossRef]
- Li, S.-C.; Yu, J.-G. A well-designed CoTiO3 coating for uncovering and manipulating interfacial compatibility between LiCoO2 and Li1.3Al0.3Ti1.7(PO4)3 in high temperature zone. Appl. Surf. Sci. 2020, 526, 146601. [Google Scholar] [CrossRef]
- Han, X.; Wang, S.; Xu, Y.; Zhong, G.; Zhou, Y.; Liu, B.; Jiang, X.; Wang, X.; Li, Y.; Zhang, Z.; et al. All solid thick oxide cathodes based on low temperature sintering for high energy solid batteries. Energy Environ. Sci. 2021, 14, 5044–5056. [Google Scholar] [CrossRef]
- Tsai, C.-L.; Ma, Q.; Dellen, C.; Lobe, S.; Vondahlen, F.; Windmüller, A.; Grüner, D.; Zheng, H.; Uhlenbruck, S.; Finsterbusch, M.; et al. A garnet structure-based all-solid-state Li battery without interface modification: Resolving incompatibility issues on positive electrodes. Sustain. Energy Fuels 2019, 3, 280–291. [Google Scholar] [CrossRef]
- Rosen, M.; Finsterbusch, M.; Guillon, O.; Fattakhova-Rohlfing, D. Free standing dual phase cathode tapes—Scalable fabrication and microstructure optimization of garnet-based ceramic cathodes. J. Mater. Chem. A 2022, 10, 2320–2326. [Google Scholar] [CrossRef]
- Kim, K.J.; Rupp, J.L.M. All ceramic cathode composite design and manufacturing towards low interfacial resistance for garnet-based solid-state lithium batteries. Energy Environ. Sci. 2020, 13, 4930–4945. [Google Scholar] [CrossRef]
- Han, F.; Yue, J.; Chen, C.; Zhao, N.; Fan, X.; Ma, Z.; Gao, T.; Wang, F.; Guo, X.; Wang, C. Interphase Engineering Enabled All-Ceramic Lithium Battery. Joule 2018, 2, 497–508. [Google Scholar] [CrossRef]
- Zheng, C.; Tang, S.; Wen, F.; Peng, J.; Yang, W.; Lv, Z.; Wu, Y.; Tang, W.; Gong, Z.; Yang, Y. Reinforced cathode-garnet interface for high-capacity all-solid-state batteries. Mater. Futures 2022, 1, 45103. [Google Scholar] [CrossRef]
- Roitzheim, C.; Sohn, Y.J.; Kuo, L.-Y.; Häuschen, G.; Mann, M.; Sebold, D.; Finsterbusch, M.; Kaghazchi, P.; Guillon, O.; Fattakhova-Rohlfing, D. All-Solid-State Li Batteries with NCM–Garnet-Based Composite Cathodes: The Impact of NCM Composition on Material Compatibility. ACS Appl. Energy Mater. 2022, 5, 6913–6926. [Google Scholar] [CrossRef]
- Paolella, A.; Zhu, W.; Bertoni, G.; Perea, A.; Demers, H.; Savoie, S.; Girard, G.; Delaporte, N.; Guerfi, A.; Rumpel, M.; et al. Toward an All-Ceramic Cathode–Electrolyte Interface with Low-Temperature Pressed NASICON Li1.5Al0.5Ge1.5(PO4)3 Electrolyte. Adv. Mater. Interfaces 2020, 7, 2000164. [Google Scholar] [CrossRef]
- Paolella, A.; Liu, X.; Daali, A.; Xu, W.; Hwang, I.; Savoie, S.; Girard, G.; Nita, A.G.; Perea, A.; Demers, H.; et al. Enabling High-Performance NASICON-Based Solid-State Lithium Metal Batteries Towards Practical Conditions. Adv. Funct. Mater. 2021, 31, 2102765. [Google Scholar] [CrossRef]
- Nie, K.; Hong, Y.; Qiu, J.; Li, Q.; Yu, X.; Li, H.; Chen, L. Interfaces Between Cathode and Electrolyte in Solid State Lithium Batteries: Challenges and Perspectives. Front. Chem. 2018, 6, 616. [Google Scholar] [CrossRef]
- Sauriol, P.; Li, D.; Hadidi, L.; Villazon, H.; Jin, L.; Yari, B.; Gauthier, M.; Dollé, M.; Chartrand, P.; Kasprzak, W.; et al. Fe3+ reduction during melt-synthesis of LiFePO4. Can. J. Chem. Eng. 2019, 97, 2196–2210. [Google Scholar] [CrossRef]
- Ong, S.P.; Wang, L.; Kang, B.; Ceder, G. Li−Fe−P−O2 Phase Diagram from First Principles Calculations. Chem. Mater. 2008, 20, 1798–1807. [Google Scholar] [CrossRef]
- Ellis, B.; Subramanya Herle, P.; Rho, Y.H.; Nazar, L.F.; Dunlap, R.; Perry, L.K.; Ryan, D.H. Nanostructured materials for lithium-ion batteries: Surface conductivity vs. bulk ion/electron transport. Faraday Discuss. 2007, 134, 119–141; discussion 215–233, 415–4159. [Google Scholar] [CrossRef] [PubMed]
- Osterheld, R.K. Liquidus diagram for the system lithium orthophosphate-lithium metaphosphate. J. Inorg. Nucl. Chem. 1968, 30, 3173–3175. [Google Scholar] [CrossRef]
- Miyoshi, S.; Nishihara, Y.; Takada, K. Influence of Cobalt Introduction on the Phase Equilibrium and Sintering Behavior of Lithium-Ion Conductor Li1.3Al0.3Ti1.7(PO4)3. ACS Appl. Energy Mater. 2022, 5, 7515–7522. [Google Scholar] [CrossRef]
- Shannon, R.D. Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Cryst. A 1976, 32, 751–767. [Google Scholar] [CrossRef]
- Catti, M.; Comotti, A.; Di Blas, S.; Ibberson, R.M. Extensive lithium disorder in Li1.5Fe0.5Ti1.5(PO4)3 Nasicon by neutron diffraction, and the Li1+xFexTi2−x(PO4)3 phase diagram. J. Mater. Chem. 2004, 14, 835–839. [Google Scholar] [CrossRef]
- Wang, S.; Hwu, S.J. A new series of mixed-valence lithium titanium (III/IV) phosphates, Li1+xTi2(PO4)3 (0 < x < 2), with NASICON-related structures. Chem. Mater. 1992, 4, 589–595. [Google Scholar] [CrossRef]
- Shi-Chun, L.; Zu-Xiang, L. Phase relationship and ionic conductivity of Li1+xTi2−xInxP3O12 system. Solid State Ion. 1983, 9–10, 835–837. [Google Scholar] [CrossRef]
- Rettenwander, D.; Welzl, A.; Pristat, S.; Tietz, F.; Taibl, S.; Redhammer, G.J.; Fleig, J. A microcontact impedance study on NASICON-type Li1+xAlxTi2−x(PO4)3 (0 ≤ x ≤ 0.5) single crystals. J. Mater. Chem. A 2016, 4, 1506–1513. [Google Scholar] [CrossRef]
- Svitan’ko, A.I.; Novikova, S.A.; Stenina, I.A.; Skopets, V.A.; Yaroslavtsev, A.B. Microstructure and ion transport in Li1+xTi2−xMx(PO4)3 (M = Cr, Fe, Al) NASICON-type materials. Inorg. Mater. 2014, 50, 273–279. [Google Scholar] [CrossRef]
- Wang, B.; Greenblatt, M.; Wang, S.; Hwu, S.J. Ionic conductivity of lithium titanium phosphate Li1+xTi2(PO4)3 (0.2 < x < 1.72) with NASICON-related structures. Chem. Mater. 1993, 5, 23–26. [Google Scholar] [CrossRef]
- Hamdoune, S.; Tranqui, D.; Schouler, E. Ionic conductivity and crystal structure of Li1+xTi2−xInxP3O12. Solid State Ion. 1986, 18–19, 587–591. [Google Scholar] [CrossRef]
- Hartmann, P.; Leichtweiss, T.; Busche, M.R.; Schneider, M.; Reich, M.; Sann, J.; Adelhelm, P.; Janek, J. Degradation of NASICON-Type Materials in Contact with Lithium Metal: Formation of Mixed Conducting Interphases (MCI) on Solid Electrolytes. J. Phys. Chem. C 2013, 117, 21064–21074. [Google Scholar] [CrossRef]
- Jimenez, R.; Sobrados, I.; Martinez, S.; Criado, M.; Perea, B.; Sanz, J. Li conductivity in Li1+xTi2-xAlx(PO4)3 (0.3≤ x≤ 0.7) ceramics prepared from sol-gel precursors. J. Alloys Compd. 2020, 844, 156051. [Google Scholar] [CrossRef]
- He, L.; Xu, S.; Zhao, Z. Suppressing the formation of Fe2P: Thermodynamic study on the phase diagram and phase transformation for LiFePO4 synthesis. Energy 2017, 134, 962–967. [Google Scholar] [CrossRef]
- Zhu, J.; Zhao, J.; Xiang, Y.; Lin, M.; Wang, H.; Zheng, B.; He, H.; Wu, Q.; Huang, J.Y.; Yang, Y. Chemomechanical Failure Mechanism Study in NASICON-Type Li1.3Al0.3Ti1.7(PO4)3 Solid-State Lithium Batteries. Chem. Mater. 2020, 32, 4998–5008. [Google Scholar] [CrossRef]
- Bhanja, P.; Senthil, C.; Patra, A.K.; Sasidharan, M.; Bhaumik, A. NASICON type ordered mesoporous lithium-aluminum-titanium-phosphate as electrode materials for lithium-ion batteries. Microporous Mesoporous Mater. 2017, 240, 57–64. [Google Scholar] [CrossRef]
- Andersson, A.; Kalska, B.; Eyob, P.; Aernout, D.; Häggström, L.; Thomas, J.O. Lithium insertion into rhombohedral Li3Fe2(PO4)3. Solid State Ion. 2001, 140, 63–70. [Google Scholar] [CrossRef]
- Malaki, M.; Haust, J.; Beaupain, J.P.; Auer, H.; Beyer, A.; Wätzig, K.; Kusnezoff, M.; Volz, K. Probing the Interface Evolution in Co-sintered All-Phosphate Cathode-Solid Electrolyte Composites. Adv. Mater. Interfaces 2023, 2300513. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Beaupain, J.P.; Waetzig, K.; Auer, H.; Zapp, N.; Nikolowski, K.; Partsch, M.; Kusnezoff, M.; Michaelis, A. Co-Sintering of Li1.3Al0.3Ti1.7(PO4)3 and LiFePO4 in Tape-Casted Composite Cathodes for Oxide Solid-State Batteries. Batteries 2023, 9, 543. https://doi.org/10.3390/batteries9110543
Beaupain JP, Waetzig K, Auer H, Zapp N, Nikolowski K, Partsch M, Kusnezoff M, Michaelis A. Co-Sintering of Li1.3Al0.3Ti1.7(PO4)3 and LiFePO4 in Tape-Casted Composite Cathodes for Oxide Solid-State Batteries. Batteries. 2023; 9(11):543. https://doi.org/10.3390/batteries9110543
Chicago/Turabian StyleBeaupain, Jean Philippe, Katja Waetzig, Henry Auer, Nicolas Zapp, Kristian Nikolowski, Mareike Partsch, Mihails Kusnezoff, and Alexander Michaelis. 2023. "Co-Sintering of Li1.3Al0.3Ti1.7(PO4)3 and LiFePO4 in Tape-Casted Composite Cathodes for Oxide Solid-State Batteries" Batteries 9, no. 11: 543. https://doi.org/10.3390/batteries9110543