Three-Dimensional Printing, an Emerging Advanced Technique in Electrochemical Energy Storage and Conversion
Abstract
:1. Introduction
2. Electrocatalysis
3. Secondary Battery
3.1. Three-Dimensionally Printed Cathode
3.2. Three-Dimensionally Printed Anode
3.3. Three-Dimensionally Printed Electrolyte
3.4. Three-Dimensionally Printed Membrane
4. Supercapacitors
5. Advanced Detection Techniques
6. Summary and Perspective
- Three-dimensional printing can theoretically print any relatively complex structure. The application of the 3D printing technique has experienced a surge in popularity due to its advantages in terms of design flexibility, material efficiency, and the simplified creation of intricate structures. A diverse range of materials, including polymers, metals, ceramics, thermosets, resins, and esters, may be further leveraged for 3D printing through various procedures.
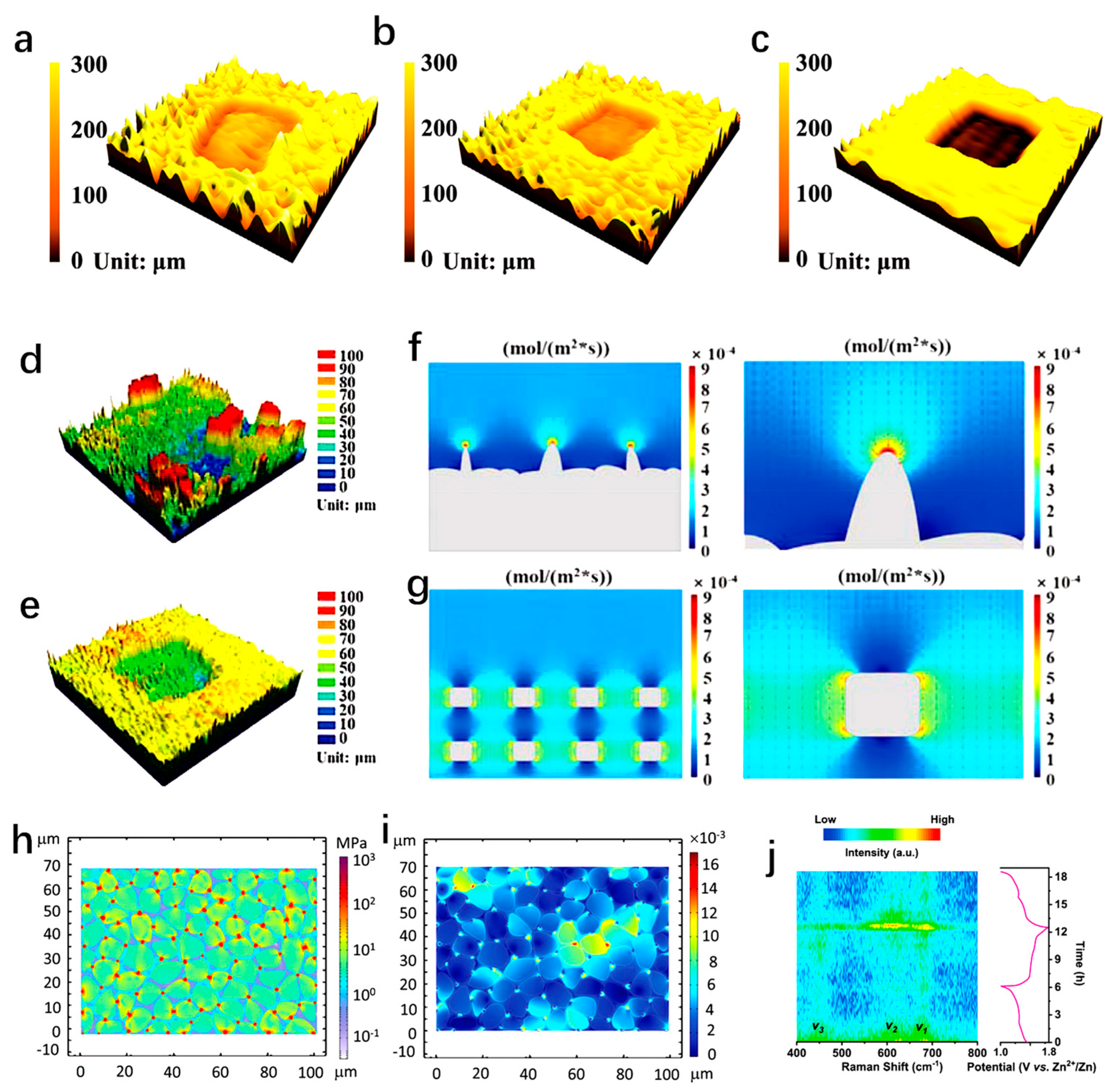
- With an increase in electrode thickness, the bulk structure experiences reduced ion diffusion rates due to the presence of an excessive amount of dead mass. This leads to a marked decrease in rate performance. Therefore, the advancement of multi-scale electrodes is particularly essential for attaining superior electrochemical performance. The application of 3D printing technology offers a potential solution for improving the performance of high-load electrodes. Versus conventional thick electrode fabrication techniques (coating and calendaring), the 3D printing process can create 3D structured electrodes with shorter ion diffusion paths and lower resistance. Thus, higher energy density can be generated by creating porous structures with larger surface areas, improved electrode reactions and ions transport, and increased utilization of space.
- Nowadays, there is a limited amount of research on applicable materials for 3D printing because this technology is still being refined and improved. Expanding the selection of materials for the 3D printing of electrochemical device components, as well as research and development on electrochemical energy-related applications, is still an important topic to be investigated.
- Three-dimensional printing has clear technical advantages, but also limitations. It still cannot meet the requirements of large-scale production. Moreover, in some cases, the quality of 3D-printed products does not surpass traditional technologies, as completely controlling the void ratio, density and uniformity during printing process is still difficult. In addition, the materials available for 3D printing are still relatively limited, and the 3D-printed objects are easily affected by the external environment, such as temperature and humidity. To some extent, they hinder the wide application of 3D printing technology. It is essential to continuously optimize the production process to improve the quality and performance of energy storage devices.
- The cost of 3D printing remains a significant challenge that needs to be addressed. Reduced 3D printing costs can drive the widespread adoption and application of this technology, allowing more people to benefit from its advantages. To reduce the cost, innovation, productivity and transformation in the manufacturing sector are highly needed. Simultaneously, adequate market competition, technological exchanges, and integration could also trigger effective cost reduction.
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Steenhuis, H.-J.; Fang, X.; Ulusemre, T. Global Diffusion of Innovation during the Fourth Industrial Revolution: The Case of Additive Manufacturing or 3D Printing. Int. J. Technol. Manag. 2019, 17, 2050005. [Google Scholar]
- Saadi, M.A.S.R.; Maguire, A.; Pottackal, N.T.; Thakur, M.S.H.; Ikram, M.M.; Hart, A.J.; Ajayan, P.M.; Rahman, M.M. Direct Ink Writing: A 3D Printing Technology for Diverse Materials. Adv. Mater. 2022, 34, 2108855. [Google Scholar] [CrossRef]
- Bao, Y.; Paunović, N.; Leroux, J.-C. Challenges and Opportunities in 3D Printing of Biodegradable Medical Devices by Emerging Photopolymerization Techniques. Adv. Funct. Mater. 2022, 32, 2109864. [Google Scholar] [CrossRef]
- Xu, L.; Thompson, C.V. Electrochemically controlled reversible formation of organized channel arrays in nanoscale-thick RuO2 films: Implications for mechanically stable thin films and microfluidic devices. ACS Appl. Nano Mater. 2021, 4, 13700–13707. [Google Scholar] [CrossRef]
- Ligon, S.C.; Liska, R.; Stampfl, J.; Gurr, M.; Mülhaupt, R. Polymers for 3D Printing and Customized Additive Manufacturing. Chem. Rev. 2017, 117, 10212–10290. [Google Scholar] [CrossRef]
- Goh, G.L.; Zhang, H.; Chong, T.H.; Yeong, W.Y. 3D Printing of Multilayered and Multimaterial Electronics: A Review. Adv. Electron. Mater. 2021, 7, 2100445. [Google Scholar] [CrossRef]
- Chen, Z.; Sun, X.; Shang, Y.; Xiong, K.; Xu, Z.; Guo, R.; Cai, S.; Zheng, C. Dense ceramics with complex shape fabricated by 3D printing: A review. J. Adv. Ceram. 2021, 10, 195–218. [Google Scholar] [CrossRef]
- Costa, C.M.; Gonçalves, R.; Lanceros-Méndez, S. Recent advances and future challenges in printed batteries. Energy Stor. Mater. 2020, 28, 216–234. [Google Scholar] [CrossRef]
- Yang, Y.; Yuan, W.; Zhang, X.; Yuan, Y.; Wang, C.; Ye, Y.; Huang, Y.; Qiu, Z.; Tang, Y. Overview on the applications of three-dimensional printing for rechargeable lithium-ion batteries. Appl. Energy 2020, 257, 114002. [Google Scholar] [CrossRef]
- Liu, H.; Zhang, H.; Han, W.; Lin, H.; Li, R.; Zhu, J.; Huang, W. 3D Printed Flexible Strain Sensors: From Printing to Devices and Signals. Adv. Mater. 2021, 33, 2004782. [Google Scholar] [CrossRef]
- Zhou, L.-Y.; Fu, J.; He, Y. A Review of 3D Printing Technologies for Soft Polymer Materials. Adv. Funct. Mater. 2020, 30, 2000187. [Google Scholar] [CrossRef]
- Zeng, L.; Li, P.; Yao, Y.; Niu, B.; Niu, S.; Xu, B. Recent progresses of 3D printing technologies for structural energy storage devices. Mater. Today Nano 2020, 12, 100094. [Google Scholar] [CrossRef]
- Gao, X.; Liu, K.; Su, C.; Zhang, W.; Dai, Y.; Parkin, I.P.; Carmalt, C.J.; He, G. From bibliometric analysis: 3D printing design strategies and battery applications with a focus on zinc-ion batteries. SmartMat 2023, e1197. [Google Scholar] [CrossRef]
- Shen, K.; Mei, H.; Li, B.; Ding, J.; Yang, S. 3D Printing Sulfur Copolymer-Graphene Architectures for Li-S Batteries. Adv. Energy Mater. 2018, 8, 1701527. [Google Scholar] [CrossRef]
- Zhang, C.J.; Kremer, M.P.; Seral-Ascaso, A.; Park, S.-H.; McEvoy, N.; Anasori, B.; Gogotsi, Y.; Nicolosi, V. Stamping of Flexible, Coplanar Micro-Supercapacitors Using MXene Inks. Adv. Funct. Mater. 2018, 28, 1705506. [Google Scholar] [CrossRef]
- Shi, H.H.; Pan, Y.; Xu, L.; Feng, X.; Wang, W.; Potluri, P.; Hu, L.; Hasan, T.; Huang, Y.Y.S. Sustainable electronic textiles towards scalable commercialization. Nat. Mater. 2023. online ahead of print. [Google Scholar] [CrossRef]
- Mubarak, S.; Dhamodharan, D.; Byun, H.-S. Recent advances in 3D printed electrode materials for electrochemical energy storage devices. J. Energy Chem. 2023, 81, 272–312. [Google Scholar] [CrossRef]
- Zong, W.; Chui, N.; Tian, Z.; Li, Y.; Yang, C.; Rao, D.; Wang, W.; Huang, J.; Wang, J.; Lai, F.; et al. Ultrafine MoP Nanoparticle Splotched Nitrogen-Doped Carbon Nanosheets Enabling High-Performance 3D-Printed Potassium-Ion Hybrid Capacitors. Adv. Sci. 2021, 8, 2004142. [Google Scholar] [CrossRef] [PubMed]
- Faria, R.; Marques, P.; Garcia, R.; Moura, P.; Freire, F.; Delgado, J.; de Almeida, A.T. Primary and secondary use of electric mobility batteries from a life cycle perspective. J. Power Sources 2014, 262, 169–177. [Google Scholar] [CrossRef]
- Chia, H.N.; Wu, B.M. Recent advances in 3D printing of biomaterials. J. Biol. Eng. 2015, 9, 1–14. [Google Scholar] [CrossRef]
- Gross, B.C.; Erkal, J.L.; Lockwood, S.Y.; Chen, C.; Spence, D.M. Evaluation of 3D printing and its potential impact on biotechnology and the chemical sciences. Anal. Chem. 2014, 86, 3240–3253. [Google Scholar] [CrossRef] [PubMed]
- Peng, M.; Shi, D.; Sun, Y.; Cheng, J.; Zhao, B.; Xie, Y.; Zhang, J.; Guo, W.; Jia, Z.; Liang, Z. 3D Printed Mechanically Robust Graphene/CNT Electrodes for Highly Efficient Overall Water Splitting. Adv. Mater. 2020, 32, 1908201. [Google Scholar] [CrossRef] [PubMed]
- Chang, S.; Huang, X.; Ong, C.Y.A.; Zhao, L.; Li, L.; Wang, X.; Ding, J. High loading accessible active sites via designable 3D-printed metal architecture towards promoting electrocatalytic performance. J. Mater. Chem. A 2019, 7, 18338–18347. [Google Scholar] [CrossRef]
- Ying, Y.; Browne, M.P.; Pumera, M. Metal–organic-frameworks on 3D-printed electrodes: In situ electrochemical transformation towards the oxygen evolution reaction. Sustain. Energy Fuels 2020, 4, 3732–3738. [Google Scholar] [CrossRef]
- Iffelsberger, C.; Ng, S.; Pumera, M. Catalyst coating of 3D printed structures via electrochemical deposition: Case of the transition metal chalcogenide MoSx for hydrogen evolution reaction. Appl. Mater. Today 2020, 20, 100654. [Google Scholar] [CrossRef]
- Santos, P.L.D.; Rowley-Neale, S.J.; Ferrari, A.G.M.; Bonacin, J.A.; Banks, C.E. Ni-Fe (Oxy) hydroxide Modified Graphene Additive Manufactured (3D-Printed) Electrochemical Platforms as an Efficient Electrocatalyst for the Oxygen Evolution Reaction. ChemElectroChem 2019, 6, 5633–5641. [Google Scholar] [CrossRef]
- Long, J.W.; Dunn, B.; Rolison, D.R.; White, H.S. Three-dimensional battery architectures. Chem. Rev. 2004, 104, 4463–4492. [Google Scholar] [CrossRef]
- Chen, C.; Jiang, J.; He, W.; Lei, W.; Hao, Q.; Zhang, X. 3D Printed High-Loading Lithium-Sulfur Battery Toward Wearable Energy Storage. Adv. Funct. Mater. 2020, 30, 1909469. [Google Scholar] [CrossRef]
- Li, J.; Leu, M.C.; Panat, R.; Park, J. A hybrid three-dimensionally structured electrode for lithium-ion batteries via 3D printing. Mater. Des. 2017, 119, 417–424. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, C.; Xie, H.; Gao, T.; Yao, Y.; Pastel, G.; Han, X.; Li, Y.; Zhao, J.; Fu, K. 3D-printed all-fiber li-ion battery toward wearable energy storage. Adv. Funct. Mater. 2017, 27, 1703140. [Google Scholar] [CrossRef]
- Cai, J.; Fan, Z.; Jin, J.; Shi, Z.; Dou, S.; Sun, J.; Liu, Z. Expediting the electrochemical kinetics of 3D-printed sulfur cathodes for Li–S batteries with high rate capability and areal capacity. Nano Energy 2020, 75, 104970. [Google Scholar] [CrossRef]
- Li, J.; Kong, Z.; Liu, X.; Zheng, B.; Fan, Q.H.; Garratt, E.; Schuelke, T.; Wang, K.; Xu, H.; Jin, H. Strategies to anode protection in lithium metal battery: A review. InfoMat 2021, 3, 1333–1363. [Google Scholar] [CrossRef]
- Yuan, H.; Ding, X.; Liu, T.; Nai, J.; Wang, Y.; Liu, Y.; Liu, C.; Tao, X. A review of concepts and contributions in lithium metal anode development. Mater. Today 2022, 53, 173–196. [Google Scholar] [CrossRef]
- Li, R.; Fan, Y.; Zhao, C.; Hu, A.; Zhou, B.; He, M.; Chen, J.; Yan, Z.; Pan, Y.; Long, J. Air-Stable Protective Layers for Lithium Anode Achieving Safe Lithium Metal Batteries. Small Methods 2023, 7, 2201177. [Google Scholar] [CrossRef] [PubMed]
- Song, L.; Ning, D.; Chai, Y.; Ma, M.; Zhang, G.; Wang, A.; Su, H.; Hao, D.; Zhu, M.; Zhang, J.; et al. Correlating Solid Electrolyte Interphase Composition with Dendrite-Free and Long Life-Span Lithium Metal Batteries via Advanced Characterizations and Simulations. Small Methods 2023, 7, 2300168. [Google Scholar] [CrossRef]
- Lyu, Z.; Lim, G.J.; Guo, R.; Pan, Z.; Zhang, X.; Zhang, H.; He, Z.; Adams, S.; Chen, W.; Ding, J. 3D-printed electrodes for lithium metal batteries with high areal capacity and high-rate capability. Energy Stor. Mater. 2020, 24, 336–342. [Google Scholar] [CrossRef]
- Yan, J.; Zhi, G.; Kong, D.; Wang, H.; Xu, T.; Zang, J.; Shen, W.; Xu, J.; Shi, Y.; Dai, S. 3D printed rGO/CNT microlattice aerogel for a dendrite-free sodium metal anode. J. Mater. Chem. A 2020, 8, 19843–19854. [Google Scholar] [CrossRef]
- McOwen, D.W.; Xu, S.; Gong, Y.; Wen, Y.; Godbey, G.L.; Gritton, J.E.; Hamann, T.R.; Dai, J.; Hitz, G.T.; Hu, L. 3D-printing electrolytes for solid-state batteries. Adv. Mater. 2018, 30, 1707132. [Google Scholar] [CrossRef] [PubMed]
- Cheng, M.; Jiang, Y.; Yao, W.; Yuan, Y.; Deivanayagam, R.; Foroozan, T.; Huang, Z.; Song, B.; Rojaee, R.; Shokuhfar, T. Elevated-Temperature 3D Printing of Hybrid Solid-State Electrolyte for Li-Ion Batteries. Adv. Mater. 2018, 30, 1800615. [Google Scholar] [CrossRef]
- Liu, Y.; Qiao, Y.; Zhang, Y.; Yang, Z.; Gao, T.; Kirsch, D.; Liu, B.; Song, J.; Yang, B.; Hu, L. 3D printed separator for the thermal management of high-performance Li metal anodes. Energy Stor. Mater. 2018, 12, 197–203. [Google Scholar] [CrossRef]
- Shi, C.; Yu, M. Flexible solid-state lithium-sulfur batteries based on structural designs. Energy Stor. Mater. 2023, 57, 429–459. [Google Scholar] [CrossRef]
- Foster, C.W.; Zou, G.Q.; Jiang, Y.; Down, M.P.; Liauw, C.M.; Ferrari, A.G.M.; Ji, X.; Smith, G.C.; Kelly, P.J.; Banks, C.E. Next-generation additive manufacturing: Tailorable graphene/polylactic (acid) filaments allow the fabrication of 3D printable porous anodes for utilisation within lithium-ion batteries. Batter. Supercaps 2019, 2, 448–453. [Google Scholar] [CrossRef]
- Zhang, C.; Shen, K.; Li, B.; Li, S.; Yang, S. Continuously 3D printed quantum dot-based electrodes for lithium storage with ultrahigh capacities. J. Mater. Chem. A 2018, 6, 19960–19966. [Google Scholar] [CrossRef]
- Saleh, M.S.; Li, J.; Park, J.; Panat, R. 3D printed hierarchically-porous microlattice electrode materials for exceptionally high specific capacity and areal capacity lithium ion batteries. Addit. Manuf. 2018, 23, 70–78. [Google Scholar] [CrossRef]
- Airoldi, L.; Anselmi-Tamburini, U.; Vigani, B.; Rossi, S.; Mustarelli, P.; Quartarone, E. Additive Manufacturing of Aqueous-Processed LiMn2O4 Thick Electrodes for High-Energy-Density Lithium-Ion Batteries. Batter. Supercaps 2020, 3, 1040–1050. [Google Scholar] [CrossRef]
- Yee, D.W.; Citrin, M.A.; Taylor, Z.W.; Saccone, M.A.; Tovmasyan, V.L.; Greer, J.R. Hydrogel-Based Additive Manufacturing of Lithium Cobalt Oxide. Adv. Mater. Technol. 2021, 6, 2000791. [Google Scholar] [CrossRef]
- Shen, K.; Ding, J.; Yang, S. 3D printing quasi-solid-state asymmetric micro-supercapacitors with ultrahigh areal energy density. Adv. Energy Mater. 2018, 8, 1800408. [Google Scholar] [CrossRef]
- Yang, W.; Yang, J.; Byun, J.J.; Moissinac, F.P.; Xu, J.; Haigh, S.J.; Domingos, M.; Bissett, M.A.; Dryfe, R.A.; Barg, S. 3D Printing of Freestanding MXene Architectures for Current-Collector-Free Supercapacitors. Adv. Mater. 2019, 31, 1902725. [Google Scholar] [CrossRef] [PubMed]
- He, H.; Zeng, L.; Luo, D.; He, J.; Li, X.; Guo, Z.; Zhang, C. 3D Printing of Electron/Ion-Flux Dual-Gradient Anodes for Dendrite-Free Zinc Batteries. Adv. Mater. 2023, 35, 2211498. [Google Scholar] [CrossRef]
- Zeng, L.; He, H.; Chen, H.; Luo, D.; He, J.; Zhang, C. 3D Printing Architecting Reservoir-Integrated Anode for Dendrite-Free, Safe, and Durable Zn Batteries. Adv. Energy Mater. 2022, 12, 2103708. [Google Scholar] [CrossRef]
- Shi, G.; Peng, X.; Zeng, J.; Zhong, L.; Sun, Y.; Yang, W.; Zhong, Y.L.; Zhu, Y.; Zou, R.; Admassie, S.; et al. A Liquid Metal Microdroplets Initialized Hemicellulose Composite for 3D Printing Anode Host in Zn-Ion Battery. Adv. Mater. 2023, 35, 2300109. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Wan, Y.; Sun, K.; Zhang, M.; Wang, C.; He, Z.; Li, Q.; Wang, N.; Zhang, Y.; Hu, H.; et al. Reconciling Mass Loading and Gravimetric Performance of MnO2 Cathodes by 3D-Printed Carbon Structures for Zinc-Ion Batteries. Adv. Funct. Mater. 2023, 33, 2215076. [Google Scholar] [CrossRef]
- Zhou, S.; Huang, S.; Yan, M.; Long, Y.; Liang, H.; Ruan, S.; Zeng, Y.; Cui, H.Z. A scalable, eco-friendly, and ultrafast solar steam generator fabricated using evolutional 3D printing. J. Mater. Chem. A 2021, 9, 9909–9917. [Google Scholar] [CrossRef]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, S.; Xue, S.; Wang, Y.; Zhang, G.; Arif, N.; Li, P.; Zeng, Y.-J. Three-Dimensional Printing, an Emerging Advanced Technique in Electrochemical Energy Storage and Conversion. Batteries 2023, 9, 546. https://doi.org/10.3390/batteries9110546
Zhang S, Xue S, Wang Y, Zhang G, Arif N, Li P, Zeng Y-J. Three-Dimensional Printing, an Emerging Advanced Technique in Electrochemical Energy Storage and Conversion. Batteries. 2023; 9(11):546. https://doi.org/10.3390/batteries9110546
Chicago/Turabian StyleZhang, Shu, Shuyue Xue, Yaohui Wang, Gufei Zhang, Nayab Arif, Peng Li, and Yu-Jia Zeng. 2023. "Three-Dimensional Printing, an Emerging Advanced Technique in Electrochemical Energy Storage and Conversion" Batteries 9, no. 11: 546. https://doi.org/10.3390/batteries9110546





