Lithium Niobate for Fast Cycling in Li-ion Batteries: Review and New Experimental Results
Abstract
1. Introduction
2. Review on Lithium Niobate for Fast Cycling in LIBs
2.1. Some Basics of Lithium Niobate
2.2. Lithium Niobate for Fast Cycling in LIBs
2.3. Lithium Niobate-Based Insertion Layers at the Electrolyte/Electrode Interface for Improved LIB Operation
2.4. Li Diffusivity in Li-Based Metal Oxides
| Oxide a | Damorphous a | Dsingle-crystal b | Damorphous/Dcrystal c | Da-LiNbO3/Da-oxide d |
|---|---|---|---|---|
| LiNbO3 | ≈1 × 10−18 | ≈1 × 10−30 | ≈1 × 1012 | 1 |
| LiTaO3 | ≈8 × 10−19 | ≈1 × 10−30 | ≈8 × 1011 | 1.25 |
| LiAlO2 | ≈4 × 10−21 | ≈1 × 10−26 | ≈4 × 105 | 2500 |
| LiGaO2 | ≈1 × 10−21 | ≈1 × 10−28 | ≈1 × 107 | 10,000 |
3. New Results Attained from Neutron Scattering and Electrochemical Experiments
3.1. Experimental Procedure
3.2. Determination of Mass Density, Free Volume, and Their Impact on Li Diffusivity
3.2.1. Mass Density and Free Volume
3.2.2. The Impact of Mass Density and Free Volume on Li Diffusivity
3.3. Electrochemical Investigations
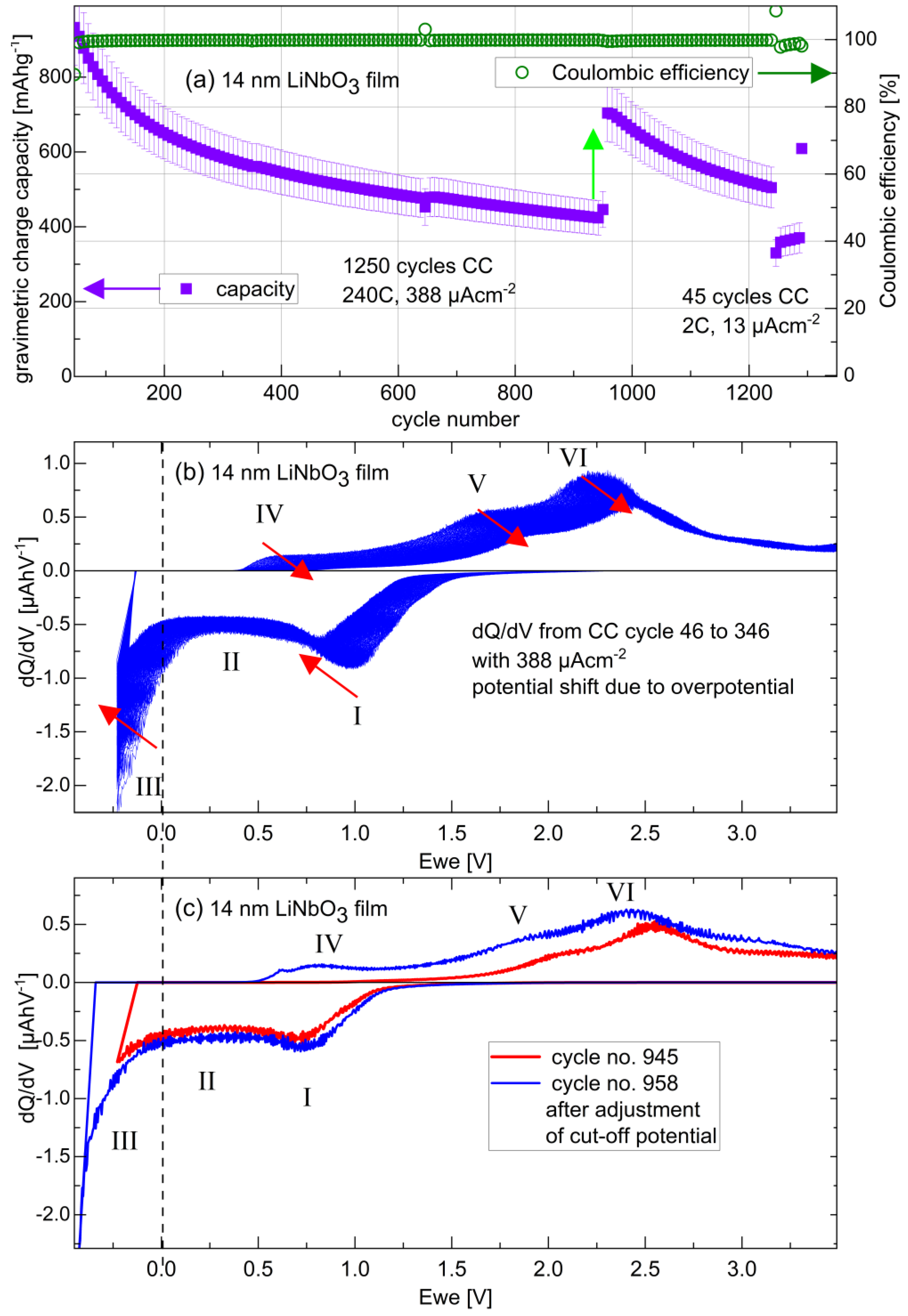
3.3.1. Potential Resolved Li+ Uptake and Release during Voltammetry and Constant Current Cycling
3.3.2. Pseudo-Capacitive Determination
3.3.3. Electrochemical Impedance Spectroscopy Investigations
3.3.4. Long-Term Cycling and Rate Capability Experiments
3.4. Remarks, Open Questions, and Outlook
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Xu, L.; Tang, S.; Cheng, Y.; Wang, K.; Liang, J.; Liu, C.; Cao, Y.-C.; Wei, F.; Mai, L. Interfaces in Solid-State Lithium Batteries. Joule 2018, 2, 1991–2015. [Google Scholar] [CrossRef]
- Jin, X.; Li, L.; Song, D.; Zhang, H.; Shi, X.; Wang, Z.; Zhang, L.; Zhu, L. LiNbO3-coated LiNi0.8Co0.1Mn0.1O2 cathode with high discharge capacity and rate performance for all-solid-state lithium battery. J. Energy Chem. 2020, 40, 39–45. [Google Scholar] [CrossRef]
- Mereacre, V.; Stüble, P.; Ghamlouce, A.; Binder, J.R. Enhancing the Stability of LiNi0.5Mn1.5O4 by Coating with LiNbO3 Solid-State Electrolyte: Novel Chemically Activated Coating Process versus Sol-Gel Method. Nanomaterials 2021, 11, 548. [Google Scholar] [CrossRef]
- Liu, X.; Shi, J.; Zheng, B.; Chen, Z.; Zhang, Y.S.M.; Xie, C.; Su, M.; Yang, Y. Constructing a High-Energy and Durable Single-Crystal NCM811 Cathode for All-Solid-State Batteries by a Surface Engineering Strategy. ACS Appl. Mater. Interfaces 2021, 13, 41669–41679. [Google Scholar] [CrossRef]
- Jiang, M.; Wu, X.; Zhang, Q.; Danilov, D.L.; Eichel, R.-A.; Notten, P.H.L. Fabrication and interfacial characterization of Ni-rich thin-film cathodes for stable Li-ion batteries. Electrochim. Acta 2021, 398, 139316. [Google Scholar] [CrossRef]
- Oudenhoven, J.F.M.; Baggetto, L.; Notten, P. All-Solid-State Lithium-Ion Microbatteries: A Review of Various Three-Dimensional Concepts. Adv. Energy Mater. 2011, 1, 10–33. [Google Scholar] [CrossRef]
- Kamaya, N.; Homma, K.; Yamakawa, Y.; Hirayama, M.; Kanno, R.; Yonemura, M.; Kamiyama, T.; Kato, Y.; Hama, S.; Kawamoto, K.; et al. A lithium superionic conductor. Nat. Mater. 2011, 10, 682–686. [Google Scholar] [CrossRef]
- Ogawa, M.; Kanda, R.; Yoshida, K.; Uemura, T.; Harada, K. High-capacity thin film lithium batteries with sulfide solid electrolytes. J. Power Sources 2012, 205, 487–490. [Google Scholar] [CrossRef]
- Ohta, N.; Takada, K.; Sakaguchi, I.; Zhang, L.; Ma, R.; Fukuda, K.; Osada, M.; Sasaki, T. LiNbO3-coated LiCoO2 as cathode material for all solid-state lithium secondary batteries. Electrochem. Commun. 2007, 9, 1486–1490. [Google Scholar] [CrossRef]
- Kato, M.; Hayashi, H.; Hasegawa, G.; Lu, X.; Miyazaki, T.; Matsuda, Y.; Kuwata, N.; Kurihara, K.; Kawamura, T. Electrochemical properties of LiCoO2 thin film surface modified by lithium tantalate and lithium niobate coatings. Solid State Ion. 2017, 308, 54–60. [Google Scholar] [CrossRef]
- Haruyama, J.; Sodeyama, K.; Han, L.; Takada, K.; Tateyama, Y. Space–Charge Layer Effect at Interface between Oxide Cathode and Sulfide Electrolyte in All-Solid-State Lithium-Ion Battery. Chem. Mater. 2014, 26, 4248–4255. [Google Scholar] [CrossRef]
- Teranishi, T.; Inohara, M.; Kano, J.; Hayashi, H.; Kishimoto, A.; Yoda, K.; Motobayashi, H.; Tasaki, Y. Synthesis of nano-crystalline LiNbO3-decorated LiCoO2 and resulting high-rate capabilities. Solid State Ion. 2018, 314, 57–60. [Google Scholar] [CrossRef]
- Xin, S.; You, Y.; Wang, S.; Gao, H.; Yin, Y.-G.; Guo, Y.-G. Solid-State Lithium Metal Batteries Promoted by Nanotechnology: Progress and Prospects. ACS Energy Lett. 2017, 2, 1385–1394. [Google Scholar] [CrossRef]
- Gao, Z.; Sun, H.; Fu, L.; Ye, F.; Zhang, Y.; Luo, W.; Huang, Y. Promises, Challenges, and Recent Progress of Inorganic Solid-State Electrolytes for All-Solid-State Lithium Batteries. Adv. Mater. 2018, 30, 1705702. [Google Scholar] [CrossRef]
- Kim, H.; Byun, D.; Chang, W.; Jung, H.-G.; Choi, W. A nano-LiNbO3 coating layer and diffusion-induced surface control towards high-performance 5 V spinel cathodes for rechargeable batteries. J. Mater. Chem. A 2017, 5, 25077–25089. [Google Scholar] [CrossRef]
- Asfaw, H.D.; Tai, C.-W.; Nyholm, L.; Edström, K. Over-Stoichiometric NbO2 Nanoparticles for a High Energy and Power Density Lithium Microbattery. ChemNanoMat 2017, 3, 646–655. [Google Scholar] [CrossRef]
- Kim, A.-Y.; Strauss, F.; Bartsch, T.; Teo, J.H.; Janek, J.; Brezesinski, T. Effect of surface carbonates on the cyclability of LiNbO3-coated NCM622 in all-solid-state batteries with lithium thiophosphate electrolytes. Sci. Rep. 2021, 11, 5367. [Google Scholar] [CrossRef] [PubMed]
- Walther, F.; Strauss, F.; Wu, X.; Mogwitz, B.; Hertle, J.; Sann, J.; Rohnke, M.; Brezesinski, T.; Janek, J. The Working Principle of a Li2CO3/LiNbO3 Coating on NCM for Thiophosphate-Based All-Solid-State Batteries. Chem. Mater. 2021, 33, 2110–2125. [Google Scholar] [CrossRef]
- Kitsche, D.; Strauss, F.; Tang, Y.; Bartnick, N.; Kim, A.-Y.; Ma, Y.; Kübel, C.; Janek, J.; Brezesinski, T. A Quasi-Multinary Composite Coating on a Nickel-Rich NCM Cathode Material for All-Solid-State Batteries. Batter. Supercaps 2022, 5, e202100397. [Google Scholar] [CrossRef]
- Mann, M.; Schwab, C.; Ihrig, M.; Finsterbusch, M.; Martin, M.; Guillon, O.; Fattakhova-Rohlfing, D. Anhydrous LiNbO3 Synthesis and Its Application for Surface Modification of Garnet Type Li-Ion Conductors. J. Electrochem. Soc. 2022, 169, 040564. [Google Scholar] [CrossRef]
- Reddy, M.V.; Subba Rao, G.V.; Chowdari, B.V.R. Metal Oxides and Oxysalts as Anode Materials for Li Ion Batteries. Chem. Rev. 2013, 113, 5364–5457. [Google Scholar] [CrossRef] [PubMed]
- Oh, G.; Hirayama, M.; Kwon, O.; Suzuki, K.; Kanno, R. Bulk-Type All Solid-State Batteries with 5 V Class LiNi0.5Mn1.5O4 Cathode and Li10GeP2S12 Solid Electrolyte. Chem. Mater. 2016, 28, 2634–2640. [Google Scholar] [CrossRef]
- Yu, H.; Wang, S.; Hu, Y.; He, G.; Bao, L.Q.; Parkin, I.P.; Jiang, H. Lithium-conductive LiNbO3 coated high-voltage LiNi0.5Co0.2Mn0.3O2 cathode with enhanced rate and cyclability. Green Energy Environ. 2022, 7, 266–274. [Google Scholar] [CrossRef]
- Jiang, M.; Zhang, Q.; Danilov, D.L.; Eichel, R.-A.; Notten, P.H.L. Formation of a Stable Solid-Electrolyte Interphase at Metallic Lithium Anodes Induced by LiNbO3 Protective Layers. ACS Appl. Energy Mater. 2021, 4, 10333–10343. [Google Scholar] [CrossRef]
- Xin, F.; Goel, A.; Chen, X.; Zhou, H.; Bai, J.; Liu, S.; Wang, F.; Zhou, G.; Whittingham, M.S. Electrochemical Characterization and Microstructure Evolution of Ni-Rich Layered Cathode Materials by Niobium Coating/Substitution. Chem. Mater. 2022, 34, 7858–7866. [Google Scholar] [CrossRef]
- Xin, F.; Whittingham, M.S. Challenges and Development of Tin-Based Anode with High Volumetric Capacity for Li-Ion Batteries. Electrochem. Energy Rev. 2020, 3, 643–655. [Google Scholar] [CrossRef]
- Hüger, E.; Jin, C.; Schmidt, H. Electrochemical investigation of ion-beam sputter-deposited carbon thin films for Li-ion batteries. J. Appl. Electrochem. 2022, 52, 1715–1732. [Google Scholar] [CrossRef]
- Hüger, E.; Jin, C.; Meyer, K.; Uxa, D.; Yang, F. Invited: Investigation of Carbon/Copper Multilayer to Examine the Influence of Copper Coating on the Li-Storage Performance of Carbon. Energies 2023, 16, 2740. [Google Scholar] [CrossRef]
- Hüger, E.; Uxa, D.; Yang, F.; Schmidt, H. The Lithiation Onset of Amorphous Silicon Thin-Film Electrodes. APL Special Topic New technologies and applications of advanced batteries. Appl. Phys. Lett. 2022, 221, 133901. [Google Scholar] [CrossRef]
- Zhang, K.; Hüger, E.; Li, Y.; Schmidt, H.; Yang, F. Review and stress analysis on the lithiation onset of amorphous silicon films. Section: Battery Materials and Interfaces: Anode, Cathode, Separators and Electrolytes or Others, Special Issue: Electrode Materials for Rechargeable Lithium Batteries. Batteries 2023, 9, 105. [Google Scholar] [CrossRef]
- Wong, K.K. (Ed.) Properties of Lithium Niobate; INSPEC Institution of Electrical Engineers: London, UK, 2002. [Google Scholar]
- Gupta, M.C.; Ballato, J. (Eds.) The Handbook of Photonics; CRC Press Taylor & Francis Group: Boca Raton, FL, USA, 2007. [Google Scholar]
- Volk, T.; Wöhleke, M. Lithium Niobate: Defects, Photorefraction and Ferroelectric Switching; Springer: Berlin/Heidelberg, Germany, 2008. [Google Scholar]
- Sánchez-Dena, O.; Fierro-Ruiz, C.S.; Villalobos-Mendoza, S.D.; Carrillo Flores, D.M.; Elizalde-Galindo, J.T.; Farías, R. Lithium Niobate Single Crystals and Powders Reviewed—Part I. Crystals 2020, 10, 973. [Google Scholar] [CrossRef]
- Indris, S.; Bork, D.; Heitjans, P. Nanocrystalline Oxide Ceramics Prepared by High-Energy Ball Milling. J. Mater. Synth. Process. 2000, 8, 245–250. [Google Scholar] [CrossRef]
- Masoud, M. Diffusivity and Ionic Conductivity in Lithium Niobate and Related Glasses and Glass Composites. Ph.D. Thesis, Leibniz University Hannover, Hannover, Germany, 2005. [Google Scholar]
- Rahn, J.; Hüger, E.; Dörrer, L.; Ruprecht, B.; Heitjans, P.; Schmidt, H. Self-Diffusion of Lithium in Amorphous Lithium Niobate Layers. Z. Phys. Chem. 2012, 226, 439–448. [Google Scholar] [CrossRef]
- Hüger, E.; Wormeester, H.; Osuch, K. Subsurface miscibility of metal overlayers with V, Nb and Ta substrates. Surf. Sci. 2005, 580, 173–194. [Google Scholar] [CrossRef]
- Kuz’minov, Y. Lithium Niobate Crystals; Cambridge International Science Publishing: Cambridge, UK, 1999. [Google Scholar]
- Peng, Q.; Cohen, R.E. Origin of pyroelectricity in LiNbO3. Phys. Rev. B 2011, 83, 220103. [Google Scholar] [CrossRef]
- Lüdtke, F.; Buse, K.; Sturman, B. Hidden Reservoir of Photoactive Electrons in LiNbO3 Crystals. Phys. Rev. Lett. 2012, 109, 026603. [Google Scholar] [CrossRef]
- Wang, C.; Zhang, M.; Chen, X.; Bertrand, M.; Shams-Ansari, A.; Chandrasekhar, S.; Winzer, P.; Lončar, M. Integrated lithium niobate electro-optic modulators operating at CMOS-compatible voltages. Nature 2018, 562, 101–104. [Google Scholar] [CrossRef]
- Sturman, B.; Kösters, M.; Haertle, D.; Becher, C.; Buse, K. Optical cleaning owing to the bulk photovoltaic effect. Phys. Rev. B 2009, 80, 245319. [Google Scholar] [CrossRef]
- Kösters, M.; Sturman, B.; Werheit, P.; Haertle, D.; Buse, K. Optical cleaning of congruent lithium niobate crystals. Nat. Photonics 2009, 3, 510–513. [Google Scholar] [CrossRef]
- Chen, K.; Chen, G.; Ruan, Z.; Fan, X.; Zhang, J.; Gan, R.; Liu, J.; Dai, D.; Guo, C.; Liu, L. Four-channel CWDM transmitter chip based on thin-film lithium niobate platform. J. Semicond. 2022, 43, 112301. [Google Scholar] [CrossRef]
- Masoud, M.; Heitjans, P. Impedance spectroscopy study of Li ion dynamics in single crystal, microcrystalline, nanocrystalline, and amorphous LiNbO3. Defect and Diffusion Forum 2005, 237–240, 1016–1021. [Google Scholar] [CrossRef]
- Heitjans, P.; Masoud, M.; Feldhoff, A.; Wilkening, M. NMR and impedance studies of nanocrystalline and amorphous ion conductors: Lithium niobate as a model system. Faraday Discuss. 2007, 134, 67–82. [Google Scholar] [CrossRef] [PubMed]
- Wolff, D.; Weber, S.; Graumann, T.; Zebrowski, S.; Mainusch, N.; Dilger, N.; Cerdas, F.; Zelnner, S. An Environmental and Technical Evaluation of Vacuum-Based Thin Film Technologies: Lithium Niobate Coated Cathode Active Material for Use in All-Solid-State Battery Cells. Energies 2023, 16, 1278. [Google Scholar] [CrossRef]
- Shi, J.; Fritze, H.; Borchardt, G.; Becker, K.-D. Defect chemistry, redox kinetics, and chemical diffusion of lithium deficient lithium niobate. Phys. Chem. Chem. Phys. 2011, 13, 6925–6930. [Google Scholar] [CrossRef] [PubMed]
- Fan, Q.; Lei, L.; Yin, G.; Sun, Y. Self-weaving CNT–LiNbO3 nanoplate–polypyrrole hybrid as a flexible anode for Li-ion batteries. Chem. Commun. 2014, 50, 2370–2373. [Google Scholar] [CrossRef]
- Reddy, M.V.; Varadaraju, U.V. Lithium Insertion into Niobates with Columbite-Type Structure: Interplay between Structure-Composition and Crystallite Size. J. Phys. Chem. C 2011, 115, 25121–25124. [Google Scholar] [CrossRef]
- Xu, H.; Shu, J.; Hu, X.; Sun, Y.; Luo, W.; Huang, Y. Electrospun porous LiNb3O8 nanofibers with enhanced lithium-storage properties. J. Mater. Chem. A 2013, 1, 15053–15059. [Google Scholar] [CrossRef]
- Fan, Q.; Lei, L.; Sun, Y. Facile synthesis of a 3D-porous LiNbO3 nanocomposite as a novel electrode material for lithium ion batteries. Nanoscale 2014, 6, 7188–7192. [Google Scholar] [CrossRef] [PubMed]
- Griffith, K.; Wiaderek, K.; Cibin, G.; Marbella, L.; Grey, C. Niobium tungsten oxides for high-rate lithium-ion energy storage. Nature 2018, 559, 556–563. [Google Scholar] [CrossRef]
- Griffith, K.; Forse, A.; Griffin, J.; Grey, C. High-rate intercalation without nanostructuring in metastable Nb2O5 bronze phases. J. Am. Chem. Soc. 2016, 138, 8888–8899. [Google Scholar] [CrossRef]
- Griffith, K.; Senyshyn, A.; Grey, C. Structural stability from crystallographic shear in TiO2–Nb2O5 phases: Cation ordering and lithiation behavior of TiNb24O62. Inorg. Chem. 2017, 56, 4002–4010. [Google Scholar] [CrossRef]
- Sun, H.; Mei, L.; Liang, J.; Zhao, Z.; Lee, C.; Fei, H.; Ding, M.; Lau, J.; Li, M.; Wang, C.; et al. Three-dimensional holey-graphene/niobia composite architectures for ultrahigh-rate energy storage. Science 2017, 356, 599–604. [Google Scholar] [CrossRef]
- Augustyn, V.; Come, J.; Lowe, M.; Kim, J.; Taberna, P.-L.; Tolbert, S.; Abruna, H.; Simon, P.; Dunn, B. High-rate electrochemical energy storage through Li+ intercalation pseudocapacitance. Nat. Mater. 2013, 12, 518–522. [Google Scholar] [CrossRef]
- Augustyn, V.; Simon, P.; Dunn, B. Pseudocapacitive oxide materials for high-rate electrochemical energy storage. Energy Environ. Sci. 2014, 7, 1597–1614. [Google Scholar] [CrossRef]
- Simon, P.; Gogotski, Y.; Dunn, B. Where do batteries end and supercapacitors begin? Science 2014, 343, 1210–1211. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Wang, K.; Zhang, G.; Li, S.; Xu, Y.; Zhang, X.; Zhang, X.; Zheng, S.; Sun, X.; Ma, Y. Fast Charging Anode Materials for Lithium-Ion Batteries: Current Status and Perspectives. Adv. Funct. Mater. 2022, 32, 2200796. [Google Scholar] [CrossRef]
- Shen, P.; Zhang, B.; Wang, Y.; Liu, X.; Yu, C.; Xu, T.; Mofarah, S.S.; Yu, Y.; Liu, Y.; Sun, H.; et al. Nanoscale niobium oxides anode for electrochemical lithium and sodium storage: A review of recent improvements. J. Nanostructure Chem. 2021, 11, 33–68. [Google Scholar] [CrossRef]
- Shen, F.; Sun, Z.; He, Q.; Sun, J.; Kaner, R.B.; Shao, Y. Niobium pentoxide based materials for high rate rechargeable electrochemical energy storage. Mater. Horiz. 2021, 8, 1130–1152. [Google Scholar] [CrossRef]
- Yang, Y.; Zhao, J. Wadsley–Roth Crystallographic Shear Structure Niobium-Based Oxides: Promising Anode Materials for High-Safety Lithium-Ion Batteries. Adv. Sci. 2021, 8, 2004855. [Google Scholar] [CrossRef]
- Ding, H.; Song, Z.; Zhang, H.; Li, X. Niobium-based oxide anodes toward fast and safe energy storage: A review. Mater. Today Nano 2020, 11, 100082. [Google Scholar] [CrossRef]
- Lovett, A.J.; Kursumovic, A.; Dutton, S.; Qi, Z.; He, Z.; Wang, H.; MacManus-Driscoll, J.L. Lithium-based vertically aligned nanocomposite films incorporating LixLa0.32(Nb0.7Ti0.32)O3 electrolyte with high Li+ ion conductivity. APL Mater. 2022, 10, 051102. [Google Scholar] [CrossRef]
- Gellert, M.; Gries, K.I.; San, J.; Pfeifer, E.; Volz, K.; Roling, B. Impedance spectroscopic study of the charge transfer resistance at the interface between a LiNi0.5Mn1.5O4 high-voltage cathode film and a LiNbO3 coating film. Solid State Ion. 2016, 287, 8–12. [Google Scholar] [CrossRef]
- Wang, B.; Zhao, Y.; Banis, M.N.; Sun, Q.; Adair, K.R.; Li, R.; Sham, T.-K.; Sun, X. Atomic layer deposition of lithium niobium oxides as potential solid-state electrolytes for lithium-ion batteries. ACS Appl. Mater. Interfaces 2018, 10, 1654–1661. [Google Scholar] [CrossRef] [PubMed]
- Heitjans, P.; Wilkening, M. Ion dynamics at interfaces: Nuclear magnetic resonance studies. MRS Bull. 2009, 34, 915–922. [Google Scholar] [CrossRef]
- Jian, Z.; Lu, X.; Fang, Z.; Hu, Y.-S.; Zhou, J.; Chen, W.; Chen, L. LiNb3O8 as a novel anode material for lithium-ion batteries. Electrochem. Commun. 2011, 13, 1127–1130. [Google Scholar] [CrossRef]
- Goldner, R.B.; Liu, T.-Y.; Goldner, M.A.; Gerouki, A.; Haas, T.E. Rechargeable Thin Film Battery and Method for Making the Same. U.S. Patent 6,982,132 B1, 3 January 2006. Application number: 09/638,444; Filed: 14 August 2000. Available online: https://www.osti.gov/biblio/1175601 (accessed on 23 April 2023).
- Glass, A.M.; Lines, M.E.; Nassau, K. Elektrische Geräte mit einem mit Elektroden Versehenen Festen Amorphen Material. European Patent EP0000785 B1, 5 August 1981. Application number: 78100638.2; Date of filing: 10 August 1978. Available online: https://patents.google.com/patent/EP0000785B1/de (accessed on 23 April 2023).
- Takada, K.; Ohta, N.; Zhang, L.; Fukuda, K.; Skaguchi, I.; Ma, R.; Osada, M.; Sasaki, T. Interfacial modification for high-power solid-state lithium batteries. Solid State Ion. 2008, 179, 1333–1337. [Google Scholar] [CrossRef]
- Pralong, V.; Reddy, M.A.; Caignaert, V.; Malo, S.; Lebedev, O.I.; Varadaraju, U.V.; Raveau, B. A New Form of LiNbO3 with a Lamellar Structure Showing Reversible Lithium Intercalation. Chem. Mater. 2011, 23, 1915–1922. [Google Scholar] [CrossRef]
- Li, J.; Liu, W.-W.; Zhou, H.-M.; Liu, Z.-Z.; Chen, B.-R.; Sun, W.-J. Anode material NbO for Li-ion battery and its electrochemical properties. Rare Met. 2018, 37, 118–122. [Google Scholar] [CrossRef]
- Reichmann, B.; Bard, A.J. The Application of Nb2O5 as a Cathode in Nonaqueous Lithium Cells. J. Electrochem. Soc. 1981, 128, 344. [Google Scholar] [CrossRef]
- Han, J.-T.; Liu, D.-Q.; Song, S.-H.; Kim, Y.; Goodenough, J.B. Lithium Ion Intercalation Performance of Niobium Oxides: KNb5O13 and K6Nb10.8O30. Chem. Mater. 2009, 21, 4753–4755. [Google Scholar] [CrossRef]
- Lakhdar, A.; Geary, H.; Houk, M.; Gastol, D.; Groombridge, A.S.; Slater, P.R.; Kendrick, E. Optimization of Electrode and Cell Design for Ultrafast-Charging Lithium-Ion Batteries Based on Molybdenum Niobium Oxide Anodes. ACS Appl. Energy Mater. 2022, 5, 11229–11240. [Google Scholar] [CrossRef]
- Glass, A.M.; Nassau, K.; Negran, T.J. Ionic conductivity of quenched alkali niobate and tantalate glasses. J. Appl. Phys. 1978, 49, 4808–4811. [Google Scholar] [CrossRef]
- Goldner, R.B.; Haas, T.; Wong, K.-K.; Seward, G. Thin Film Ion Conducting Coating. U.S. Patent 4,832,463, 23 May 1989. Application number 93,782; Date of Filing: 8 September 1987. Available online: https://patents.google.com/patent/US4832463A/en (accessed on 23 April 2023).
- Takada, K.; Ohta, N.; Zhang, L.; Xu, X.; Hang, B.T.; Ohnishi, T.; Osada, M.; Sasaki, T. Interfacial phenomena in solid-state lithium battery with sulfide solid electrolyte. Solid State Ion. 2012, 225, 594–597. [Google Scholar] [CrossRef]
- Knauth, P. Inorganic Solid Li Ion Conductors: An Overview. Solid State Ion. 2009, 180, 911–916. [Google Scholar] [CrossRef]
- Horopanitis, E.E.; Perentzis, G.; Karagiannidis, P.G.; Papadimitriou, L. Electrical properties of LiNbO3 (electrolyte)/Cu (anode) bi-layers. Mat. Sci. Engin. B 2011, 176, 512–514. [Google Scholar] [CrossRef]
- Chadwick, A.V.; Savin, S.L.P. Structure and dynamics in nanoionic materials. Solid State Ion. 2006, 177, 3001–3008. [Google Scholar] [CrossRef]
- Li, W.; Hub, C.; Zhou, M.; Wang, K.; Lia, H.; Cheng, S.; Jiang, K. The Electrochemical Synthesis of LiNbO2 in Molten Salts and its Application for Lithium Ion Batteries with High Rate Capability. Electrochim. Acta 2016, 189, 231–236. [Google Scholar] [CrossRef]
- Ye, R.; Ohta, K.; Baba, M. Electrochemical Properties of Amorphous Nb2O5 Thin Film and Its Application to Rechargeable Thin Film Lithium Ion Batteries. ECS Trans. 2016, 73, 49–55. [Google Scholar] [CrossRef]
- Lv, Z.; Meng, W.; Yang, Y.; Zhang, Y.; Ye, M.; Li, C.C. Nb-based compounds for rapid lithium-ion storage and diffusion. J. Power Sources 2021, 496, 229840. [Google Scholar] [CrossRef]
- Lee, D.-C.; Shank, J.C.; Tellekamp, M.B.; Doolittle, W.A.; Alamgir, F.M. Thin-Film Lithium Niobites and Their Chemical Properties for Lithium-Ion Storage and Diffusion. ChemElectroChem 2019, 6, 5109–5115. [Google Scholar] [CrossRef]
- Goldner, R.B.; Haas, T.E.; Seward, G.; Wong, K.K.; Norton, P.; Foley, G.; Berera, G.; Wei, G.; Schulz, S.; Chapman, R. Thin film solid state ionic materials for electrochromic smart window glass. Solid State Ion. 1988, 28–30, 1715–1721. [Google Scholar] [CrossRef]
- Goldner, R.B.; Arntz, F.O.; Morel, B.; Haas, T.E.; Wong, K.-K. Ion-Beam Based Deposition of Coatings for Electrochromic Devices. U.S. Patent 5,051,274, 24 September 1991. Application number 405,271; Date of Filing: 11 September 1989. Available online: https://patents.google.com/patent/US5051274 (accessed on 23 April 2023).
- Schmitt, M.; Heusing, S.; Aegerter, M.A.; Pawlicke, A.; Avellaneda, C. Electrochromic properties of Nb2O5 sol–gel coatings. Sol. Energy Mater. Sol. Cells 1998, 54, 9–17. [Google Scholar] [CrossRef]
- Zhu, Z.; Kushima, A.; Yin, Z.; Qi, L.; Amine, K.; Lu, J.; Li, J. Anion-redox nanolithia cathodes for Li-ion batteries. Nat. Energy 2016, 1, 16111. [Google Scholar] [CrossRef]
- Wang, Z.; Li, Z.; Fu, Y.Q. Composites of piezoelectric materials and silicon as anodes for lithium-ion batteries. ChemElectroChem 2017, 4, 1523–1527. [Google Scholar] [CrossRef]
- Wang, Z. Modelling and simulation of piezoelectrically driven self-charging lithium ion batteries. ACS Appl. Mater. Interfaces 2017, 9, 15893–15897. [Google Scholar] [CrossRef]
- Wilkening, M.; Bork, D.; Indris, S.; Heitjans, P. Diffusion in amorphous LiNbO3 studied by 7Li NMR—Comparison with the nano- and microcrystalline material. Phys. Chem. Chem. Phys. 2002, 4, 3246–3251. [Google Scholar] [CrossRef]
- Chadwick, A.V.; Pooley, M.J.; Savin, S.L.P. Lithium ion transport and microstructure in nanocrystalline lithium niobate. Phys. Stat. Sol. C 2005, 2, 302–305. [Google Scholar] [CrossRef]
- Bork, D.; Heitjans, P. NMR investigations on ion dynamics and structure in nanocrystalline and polycrystalline LiNbO3. J. Phys. Chem. B 2001, 105, 9162–9170. [Google Scholar] [CrossRef]
- Bork, D.; Heitjans, P. NMR relaxation study of ion dynamics in nanocrystalline and polycrystalline LiNbO3. J. Phys. Chem. B 1998, 102, 7303–7306. [Google Scholar] [CrossRef]
- Sumets, M.; Ievlev, V.; Dybov, V.; Kostyuchenko, A.; Serikov, D.; Kannykin, S.; Belonogov, E. Synthesis and properties of multifunctional Si–LiNbO3 heterostructures for non-volatile memory units. J. Mater. Sci. Mater. Electron. 2019, 30, 16562–16570. [Google Scholar] [CrossRef]
- Sumets, M.; Ievlev, V.; Dybov, V.; Serikov, D.; Belonogov, E.; Grebennikov, A. Transport properties and crystallization of Li–Nb–O system on silicon. Mater. Sci. Semicond. Process 2022, 142, 106519. [Google Scholar] [CrossRef]
- Guo, C.; Liu, Z.; Han, K.; Zhang, L.; Ding, X.; Wang, X.; Mai, L. Nano-Sized Niobium Tungsten Oxide Anode for Advanced Fast-Charge Lithium-Ion Batteries. Small 2022, 18, 2107365. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.; Kulkarni, P.; Balaji, S.; Senthil-Kumar, P. Exploration of electrochemical and lithium transport properties of BaNb3.6O10 as an anode material for lithium-ion batteries. J. Alloys Compd. 2020, 830, 154306. [Google Scholar] [CrossRef]
- Reddy, M.V.; Varadaraju, U.V. Facile Insertion of Lithium into Nanocrystalline AlNbO4 at Room Temperature. Chem. Mater. 2008, 20, 4557–4559. [Google Scholar] [CrossRef]
- Kobayashi, S.; Watanabe, H.; Kato, T.; Mizuno, F. Atomic-Scale Observations of Oxygen Release Degradation in Sulfide-Based All-Solid-State Batteries with Layered Oxide Cathodes. ACS Appl. Mater. Interfaces 2022, 14, 39459–39466. [Google Scholar] [CrossRef]
- Li, Z.; Yuan, M.; Liu, H.; Liu, J.; Xie, S.; Wang, T.; Yan, J. Improving the electrochemical properties of the Li-rich cathode material 0.5Li2MnO3·0.5LiNi1/3Co1/3Mn1/3O2 by coating the bi-functional amorphous LiNbO3. J. Mater. Res. 2020, 37, 3831–3841. [Google Scholar] [CrossRef]
- Kaur, G.; Gates, B.D. Review: Surface Coatings for Cathodes in Lithium Ion Batteries: From Crystal Structures to Electrochemical Performance. J. Electrochem. Soc. 2022, 169, 043504. [Google Scholar] [CrossRef]
- Morino, Y. Degradation rate at the Solid–Solid interface of sulfide-based solid Electrolyte–Cathode active material. J. Power Sources 2022, 541, 231672. [Google Scholar] [CrossRef]
- Morino, Y.; Kanada, S. Degradation Analysis by X-ray Absorption Spectroscopy for LiNbO3 Coating of Sulfide-Based All-Solid-State Battery Cathode. ACS Appl. Mater. Interfaces 2023, 15, 2979–2984. [Google Scholar] [CrossRef]
- Uhlendorf, J.; Ruprecht, B.; Witt, E.; Chandran, C.V.; Dörrer, L.; Hüger, E.; Strauß, F.; Heitjans, P.; Schmidt, H. Slow Lithium Transport in Metal Oxides on the Nanoscale. Z. Phys. Chem. 2017, 231, 1423–1442. [Google Scholar] [CrossRef]
- Uxa, D.; Hüger, E.; Meyer, K.; Dörrer, L.; Schmidt, H. Lithium-ion Diffusion in Near-stoichiometric Polycrystalline and Monocrystalline LiCoO2. Chem. Mater. 2023; 35, 3307–3315. [Google Scholar] [CrossRef]
- Braun, C. Parratt32 or the Reflectometry Tool, HMI, Berlin. Available online: http://www.helmholtz-berlin.de (accessed on 17 November 2007).
- Available online: http://www.ncnrs.nist.gov/resources/sldcalc.html (accessed on 22 December 2022).
- Hüger, E.; Rothe, H.; Frant, M.; Grohmann, S.; Hildebrand, G.; Liefeith, K. Atomic force microscopy and thermodynamics on taro, a self-cleaning plant leaf. Appl. Phys. Lett. 2009, 95, 033702. [Google Scholar] [CrossRef]
- Strafela, M.; Fischer, J.; Music, D.; Chang, K.; Schneider, J.; Leiste, H.; Rinke, M.; Bergfeldt, T.; Seifert, H.J.; Ulrich, S. Dependence of the constitution, microstructure and electrochemical behaviour of magnetron sputtered Li–Ni–Mn–Co–O thin film cathodes for lithium-ion batteries on the working gas pressure and annealing conditions. Int. J. Mater. Res. 2017, 108, 879–886. [Google Scholar] [CrossRef]
- Tan, G.; Wu, F.; Lu, J.; Chen, R.; Li, L.; Amine, K. Controllable crystalline preferred orientation in Li–Co–Ni–Mn oxide cathode thin films for all-solid-state lithium batteries. Nanoscale 2014, 6, 10611–10622. [Google Scholar] [CrossRef]
- Venkatachalam, P.; Kesavan, T.; Maduraiveeran, G.; Kundu, M.; Sasidharan, M. Self-assembled mesoporous Nb2O5 as a high performance anode material for rechargeable lithium ion batteries. Mater. Res. Express 2019, 6, 035502. [Google Scholar] [CrossRef]
- Ouendi, S.; Arico, C.; Blanchard, F.; Codron, J.-L.; Wallart, X.; Taberna, P.-L.; Roussel, P.; Clavier, L.; Simon, P.; Lethien, C. Synthesis of T-Nb2O5 thin-films deposited by Atomic Layer Deposition for miniaturized electrochemical energy storage devices. Energy Storage Mater. 2019, 16, 581–588. [Google Scholar] [CrossRef]
- Ji, Q.; Gao, X.; Zhang, Q.; Jin, L.; Wang, D.; Xia, Y.; Yin, S.; Xia, S.; Hohn, N.; Zuo, X.; et al. Dental Resin Monomer Enables Unique NbO2/Carbon Lithium-Ion Battery Negative Electrode with Exceptional Performance. Adv. Funct. Mater. 2019, 29, 1904961. [Google Scholar] [CrossRef]
- Xia, R.; Zhao, K.; Kuo, L.-Y.; Zhang, L.; Cunha, D.M.; Wang, Y.; Huang, S.; Zheng, J.; Boukamp, B.; Kaghazchi, P.; et al. Nickel Niobate Anodes for High Rate Lithium-Ion Batteries. Adv. Energy Mater. 2022, 12, 2102972. [Google Scholar] [CrossRef]
- Jiang, Y.; Zhang, D.; Li, Y.; Yuan, T.; Bahlawane, N.; Liang, C.; Sun, W.; Lu, Y.; Yan, M. Amorphous Fe2O3 as a high-capacity, high-rate and long-life anode material for lithium ion batteries. Nano Energy 2014, 4, 23–30. [Google Scholar] [CrossRef]
- Cabana, J.; Monconduit, L.; Larcher, D.; Rosa Palacin, M. Beyond intercalation-based Li-ion batteries: The state of the art and challenges of electrode materials reacting through conversion reactions. Adv. Mater. 2010, 22, E170–E192. [Google Scholar] [CrossRef]
- Grugeon, S.; Laruelle, S.; Dupont, L.; Tarascon, J.-M. An update on the reactivity of nanoparticles Co-based compounds toward Li. Solid State Sci. 2003, 5, 895–904. [Google Scholar] [CrossRef]
- Wang, J.; Polleux, J.; Lim, J.; Dunn, B. Pseudocapacitive Contributions to Electrochemical Energy Storage in TiO2 (Anatase) Nanoparticles. J. Phys. Chem. C 2007, 111, 14925–14931. [Google Scholar] [CrossRef]
- Dhara, A.; Sarkar, S.K.; Mitra, S. Controlled 3D Carbon Nanotube Architecture Coated with MoOx Material by ALD Technique: A High Energy Density Lithium-Ion Battery Electrode. Adv. Mater. Interfaces 2017, 4, 1700332. [Google Scholar] [CrossRef]
- Yan, S.; Abhilash, K.P.; Tang, L.; Yang, M.; Ma, Y.; Xia, Q.; Guo, Q.; Xia, H. Research Advances of Amorphous Metal Oxides in Electrochemical Energy Storage and Conversion. Small 2019, 15, 1804371. [Google Scholar] [CrossRef]
- Maier, J. Nanoionics: Ion transport and electrochemical storage in confined systems. Nat. Mater. 2005, 4, 805–815. [Google Scholar] [CrossRef] [PubMed]
- Haverkate, L.A.; Chan, W.K.; Mulder, F.W. Large Space-Charge Effects in a Nanostructured Proton Conductor. Adv. Funct. Mater. 2010, 20, 4107–4116. [Google Scholar] [CrossRef]
- Fu, L.; Chen, C.-C.; Samuelis, D.; Maier, J. Thermodynamics of Lithium Storage at Abrupt Junctions: Modeling and Experimental Evidence. PRL 2014, 112, 208301. [Google Scholar] [CrossRef]
- Hüger, E.; Osuch, K. Quantum well states in thin (110)-oriented Au films and k-space symmetry. Eur. Phys. J. B 2004, 37, 149–162. [Google Scholar] [CrossRef]
- Brousse, T.; Belanger, D.; Long, J.W. To Be or Not To Be Pseudocapacitive? J. Electrochem. Soc. 2015, 162, A5185–A5189. [Google Scholar] [CrossRef]
- Fleischmann, S.; Mitchell, J.B.; Wang, R.; Zhan, C.; Jiang, D.; Presser, V.; Augustyn, V. Pseudocapacitance: From Fundamental Understanding to High Power Energy Storage Materials. Chem. Rev. 2020, 120, 6738–6782. [Google Scholar] [CrossRef]
- Bisquert, J.; Garcia-Belmonte, G.; Bueno, P.; Longo, E.; Bulhoes, L.O.S. Impedance of constant phase element (CPE)-blocked diffusion in film electrodes. J. Electroanal. Chem. 1998, 452, 229–234. [Google Scholar] [CrossRef]
- Diard, J.-P.; Montella, C. Diffusion-trapping impedance under restricted linear diffusion conditions. J. Electroanal. Chem. 2003, 557, 19–36. [Google Scholar] [CrossRef]
- Hatchard, T.D.; Dahn, J.R. In Situ XRD and Electrochemical Study of the Reaction of Lithium with Amorphous Silicon. J. Electrochem. Soc. 2004, 151, A838. [Google Scholar] [CrossRef]
- Obravac, M.N.; Christensen, L. Structural Changes in Silicon Anode during Lithium Insertion/Extraction. Electrochem. Solid-State Lett. 2004, 7, A93. [Google Scholar] [CrossRef]
- Key, B.; Morcrette, M.; Tarascon, J.-M.; Grey, C.P. Pair Distribution Function Analysis and Solid State NMR Studies of Silicon Electrodes for Lithium Ion Batteries: Understanding the (De)lithiation Mechanisms. J. Am. Chem. Soc. 2011, 133, 503–512. [Google Scholar] [CrossRef]
- Ogata, K.; Salager, E.; Kerr, C.J.; Fraser, A.E.; Ducati, C.; Morris, A.J.; Hofmann, S.; Grey, C.P. Revealing lithium–silicide phase transformations in nano-structured silicon-based lithium ion batteries via in situ NMR spectroscopy. Nat. Commun. 2014, 5, 3217. [Google Scholar] [CrossRef]
- Bao, W.; Fang, C.; Cheng, D.; Zhang, Y.; Lu, B.; Tan, D.H.S.; Shimizu, R.; Sreenarayanan, B.; Bai, S.; Li, W.; et al. Quantifying lithium loss in amorphous silicon thin-film anodes via titration-gas chromatography. Cell Rep. Phys. Sci. 2021, 2, 100597. [Google Scholar] [CrossRef]
- Baggetto, L.; Notten, P.H.L. Lithium-Ion (De)Insertion Reaction of Germanium Thin-Film Electrodes: An Electrochemical and In Situ XRD Study. J. Electrochem. Soc. 2009, 156, A169–A175. [Google Scholar] [CrossRef]
- Kim, H.; Choi, W.; Yoon, J.; Um, J.H.; Lee, W.; Kim, J.; Cabana, J.; Yoon, W.-S. Exploring Anomalous Charge Storage in Anode Materials for Next-Generation Li Rechargeable Batteries. Chem. Rev. 2020, 120, 6934–6976. [Google Scholar] [CrossRef]
- Uxa, D.; Jerliu, B.; Hüger, E.; Dörrer, L.; Horisberger, M.; Stahn, J.; Schmidt, H. On the Lithiation Mechanism of Amorphous Silicon Electrodes in Li-Ion Batteries. J. Phys. Chem. C 2019, 123, 22027–22039. [Google Scholar] [CrossRef]
- Dörrer, L.; Heller, R.; Schmidt, H. Tracer Diffusion in Proton-Exchanged Congruent LiNbO3 Crystals as a Function of Hydrogen Content. Phys. Chem. Chem. Phys. 2022, 24, 16139–16147. [Google Scholar] [CrossRef]
- Kuhn, A.; Pérez-Flores, J.C.; Prado-Gonjal, J.; Morán, E.; Hoelzel, M.; Díez-Gómez, V.; Sobrados, I.; Sanz, J.; García-Alvarado, F. Lithium Intercalation Mechanism and Critical Role of Structural Water in Layered H2V3O8 High-Capacity Cathode Material for Lithium-Ion Batteries. Chem. Mater. 2022, 34, 694–705. [Google Scholar] [CrossRef]


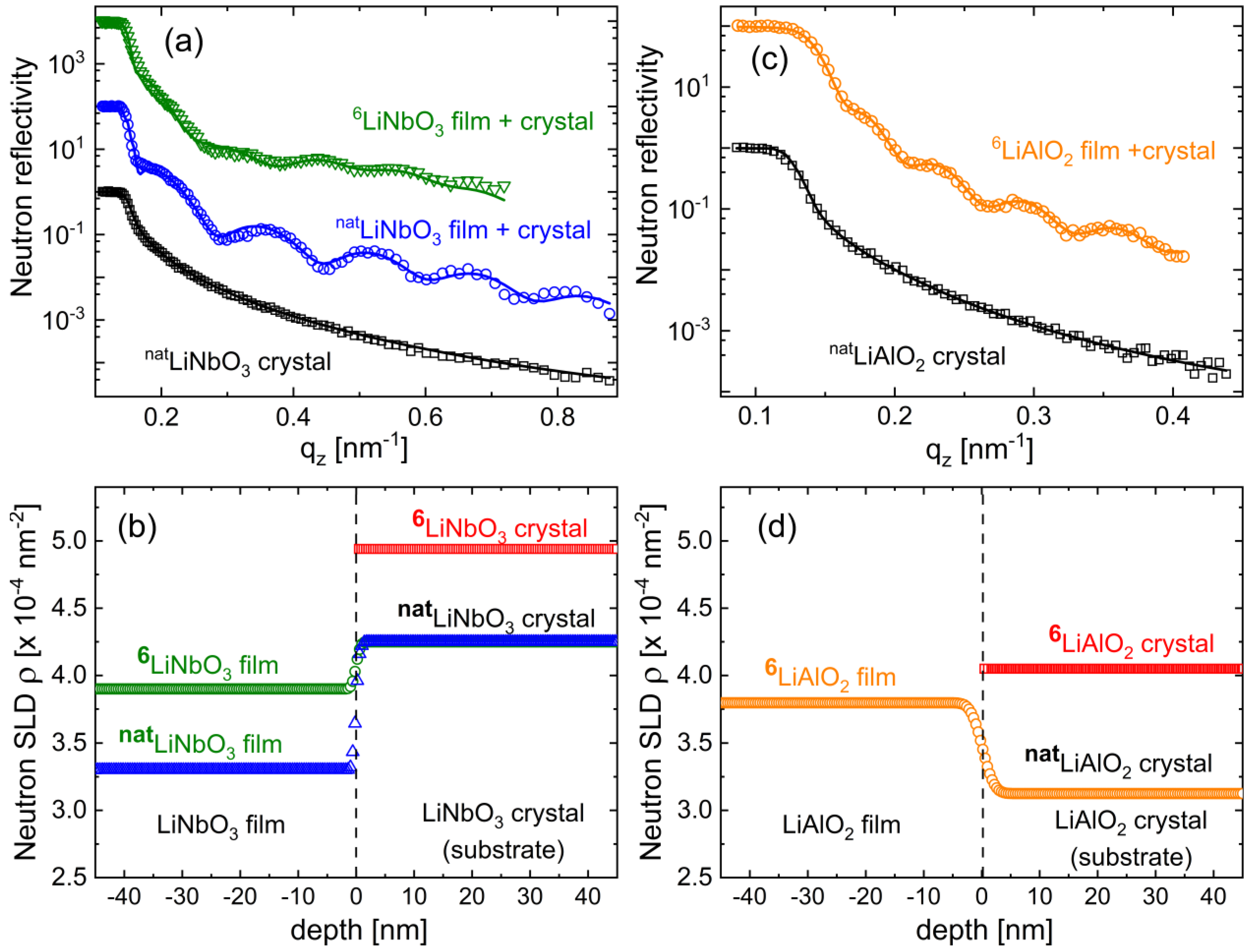

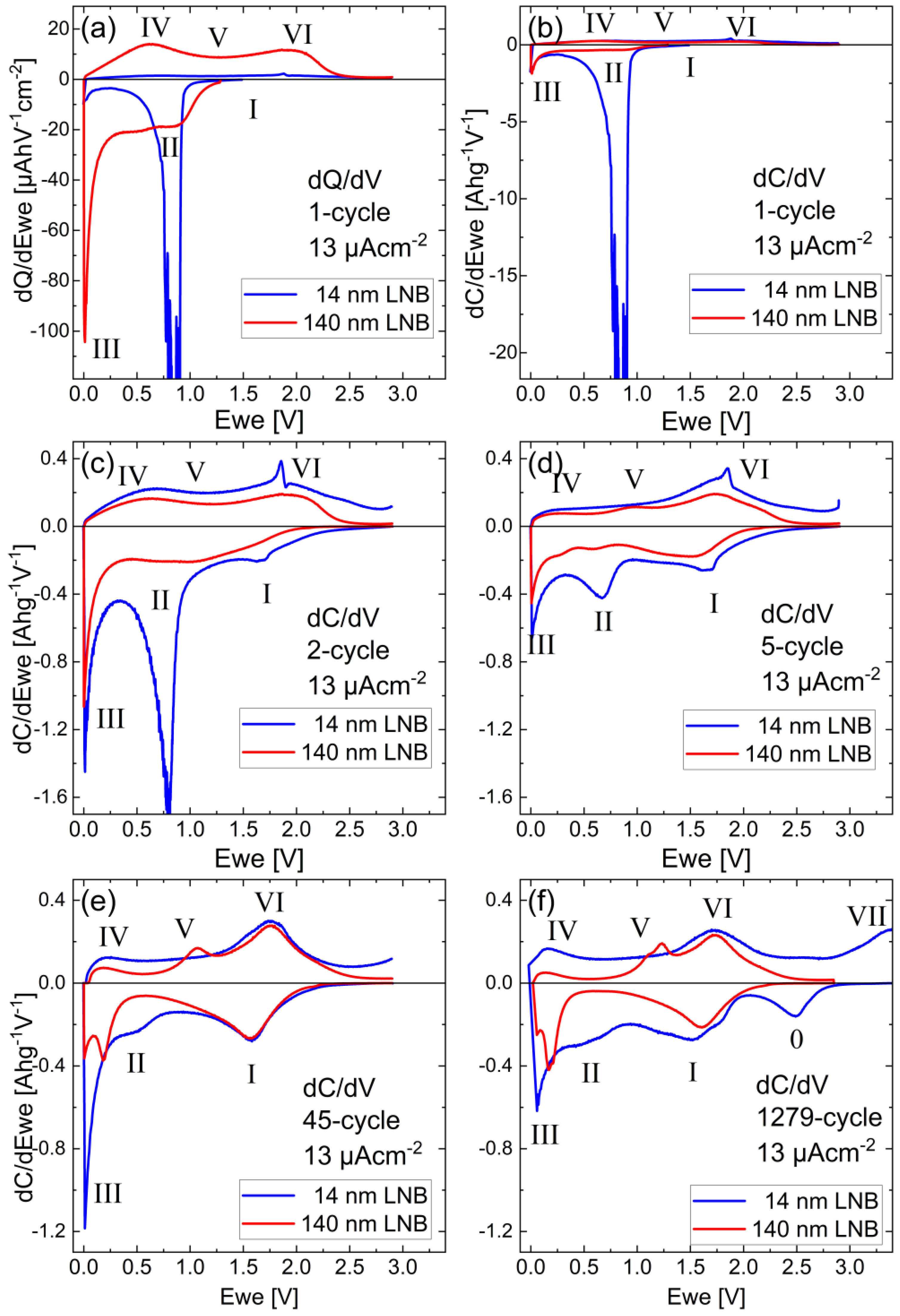

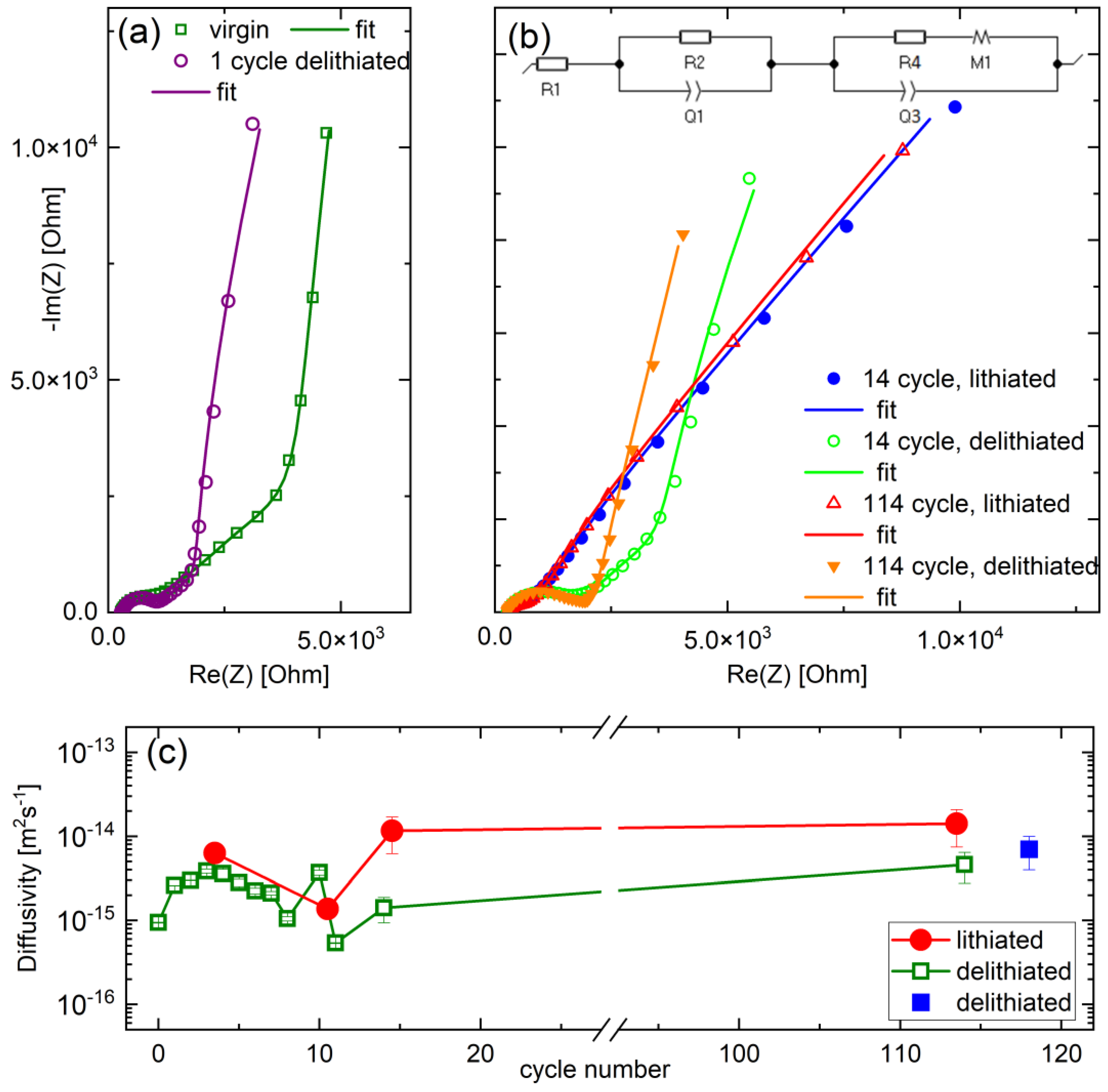
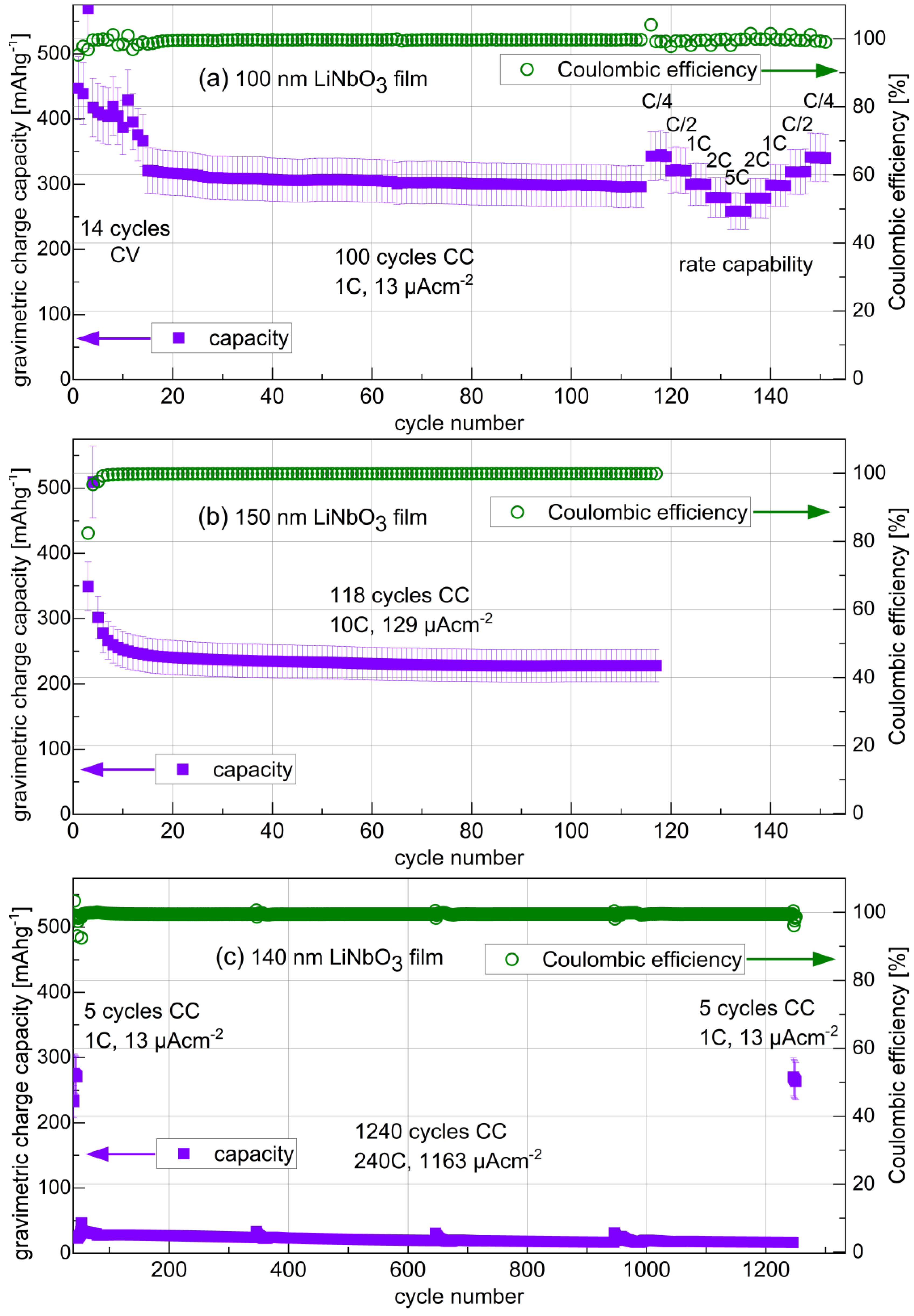
| Sample a | SLD (10−4 nm−2) | Mass Density (gcm−3) | ρamorphous-film/ρcrystal |
|---|---|---|---|
| natLiNbO3-wafer | 4.25 ± 0.20 | 4.60 ± 0.22 | -- |
| natLiNbO3-film | 3.30 ± 0.07 | 3.60 ± 0.08 | 0.78 |
| 6LiNbO3-film | 3.90 ± 0.15 | 3.61 ± 0.14 | 0.78 |
| natLiAlO2-wafer | 3.13 ± 0.17 | 2.61 ± 0.14 | -- |
| 6LiAlO2-film | 3.80 ± 0.12 | 2.43 ± 0.08 | 0.93 |
| Amorphous Film Electrode | Cycle Number | Current Density (µAcm−2) | Film Gravimetric Capacity (mAhg−1) | Maximal Gravimetric Capacity (mAhg−1) [Refs.] | Film Mass Density (gcm−3) | Film volumetric Capacity (mAhcm−3) | Maximal Volumetric capacity (mAhcm−3) | Theoretical Volumetric capacity (mAhcm−3) [Refs.] |
|---|---|---|---|---|---|---|---|---|
| 14 nm LiNbO3 | 600 | 388 | 500 | 402 [62,63,64,87] | 3.6 | 1800 | 1447 | 1769 [64] |
| 14 nm LiNbO3 | 970 | 388 | 700 | 402 [62,63,64,87] | 3.6 | 2520 | 1447 | 1769 [64] |
| 14 nm silicon | 600 | 1163 | 300 | 3579 [29,30,134,135,136,137,138] | 2 | 600 | 7158 | 8322 [134,135,136] |
| 16 nm carbon | 600 | 1163 | 50 | 372 [27] | 2 | 100 | 744 | 833 [136] |
| 16 nm germanium | 260 | 1163 | 20 | 1385 [139] | 4 | 80 | 5540 | 7366 [139] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hüger, E.; Riedel, L.; Zhu, J.; Stahn, J.; Heitjans, P.; Schmidt, H. Lithium Niobate for Fast Cycling in Li-ion Batteries: Review and New Experimental Results. Batteries 2023, 9, 244. https://doi.org/10.3390/batteries9050244
Hüger E, Riedel L, Zhu J, Stahn J, Heitjans P, Schmidt H. Lithium Niobate for Fast Cycling in Li-ion Batteries: Review and New Experimental Results. Batteries. 2023; 9(5):244. https://doi.org/10.3390/batteries9050244
Chicago/Turabian StyleHüger, Erwin, Lukas Riedel, Jing Zhu, Jochen Stahn, Paul Heitjans, and Harald Schmidt. 2023. "Lithium Niobate for Fast Cycling in Li-ion Batteries: Review and New Experimental Results" Batteries 9, no. 5: 244. https://doi.org/10.3390/batteries9050244
APA StyleHüger, E., Riedel, L., Zhu, J., Stahn, J., Heitjans, P., & Schmidt, H. (2023). Lithium Niobate for Fast Cycling in Li-ion Batteries: Review and New Experimental Results. Batteries, 9(5), 244. https://doi.org/10.3390/batteries9050244






