Design of Sodium Titanate Nanowires as Anodes for Dual Li,Na Ion Batteries
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
3. Results and Discussions
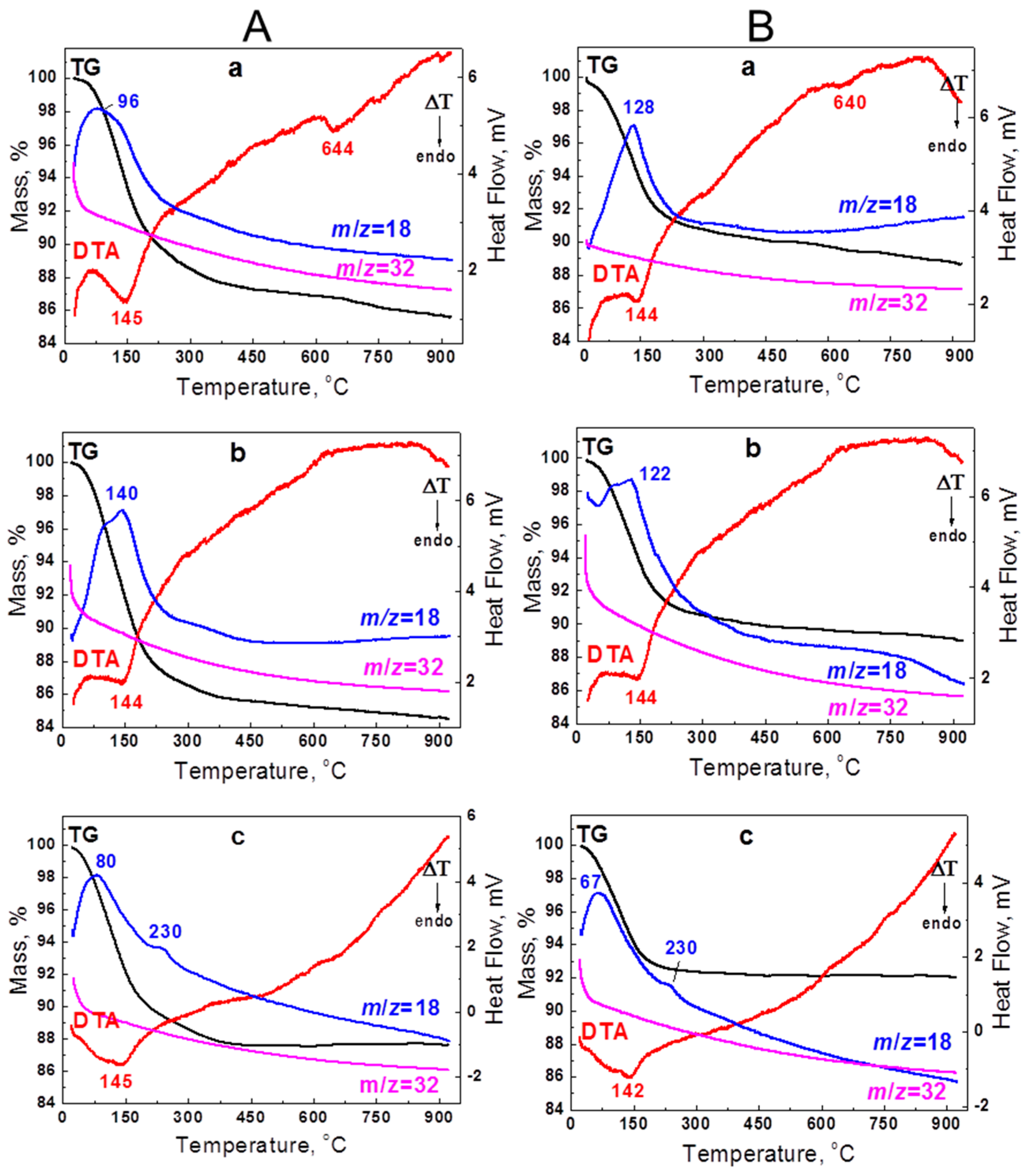
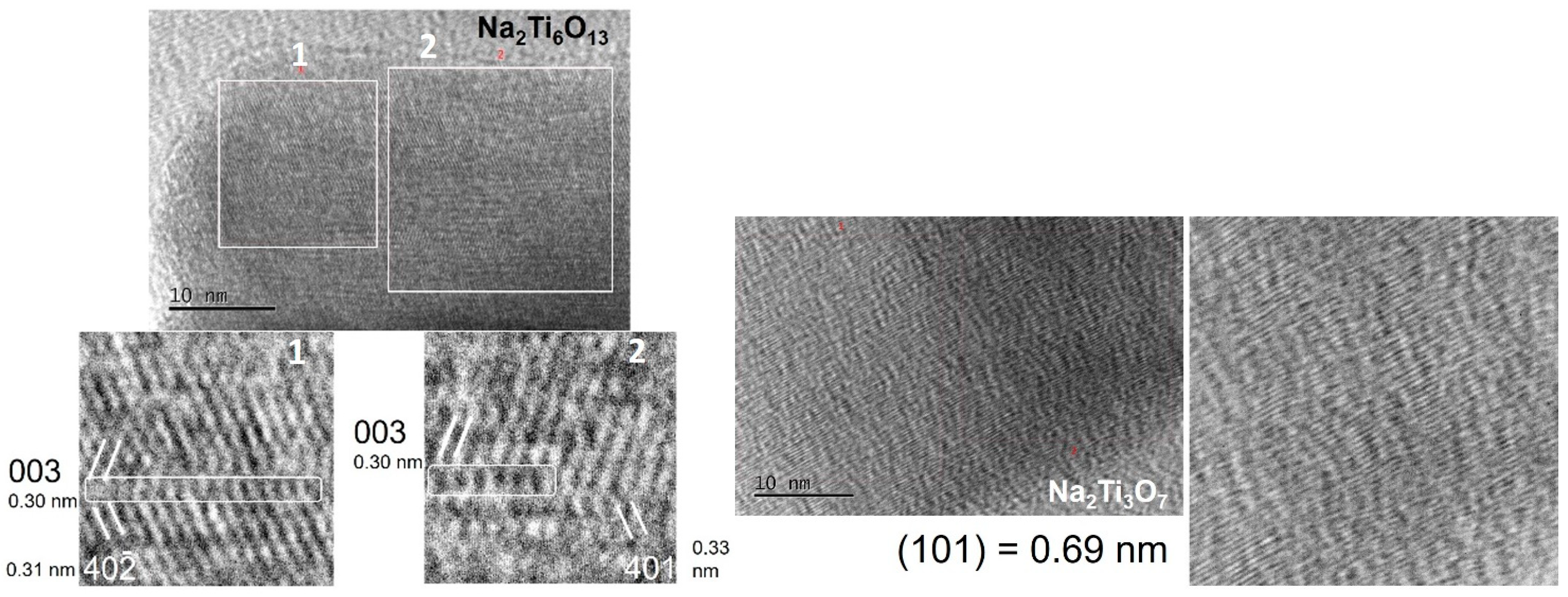
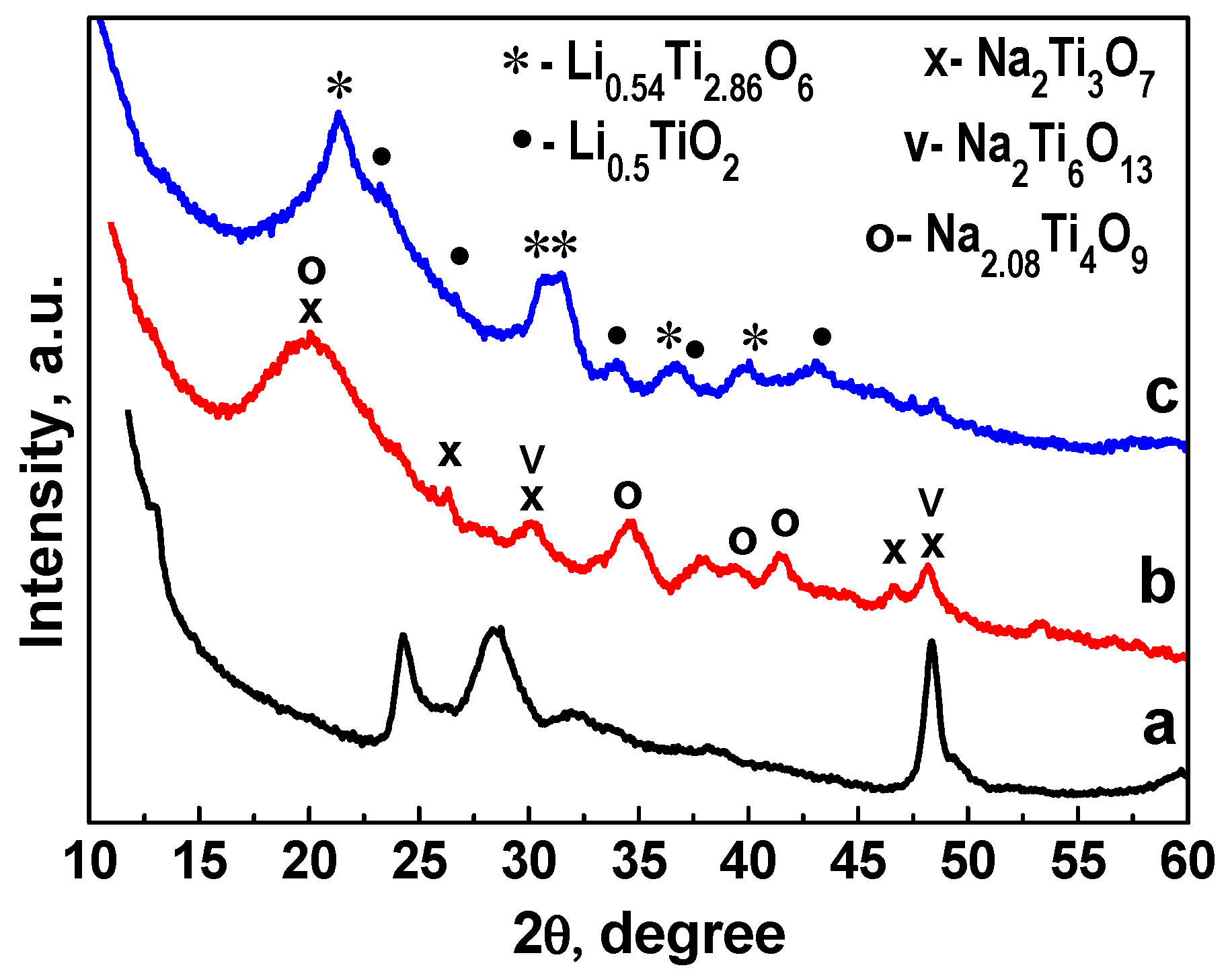
3.1. Synthesis of Sodium Titanate Nanowires
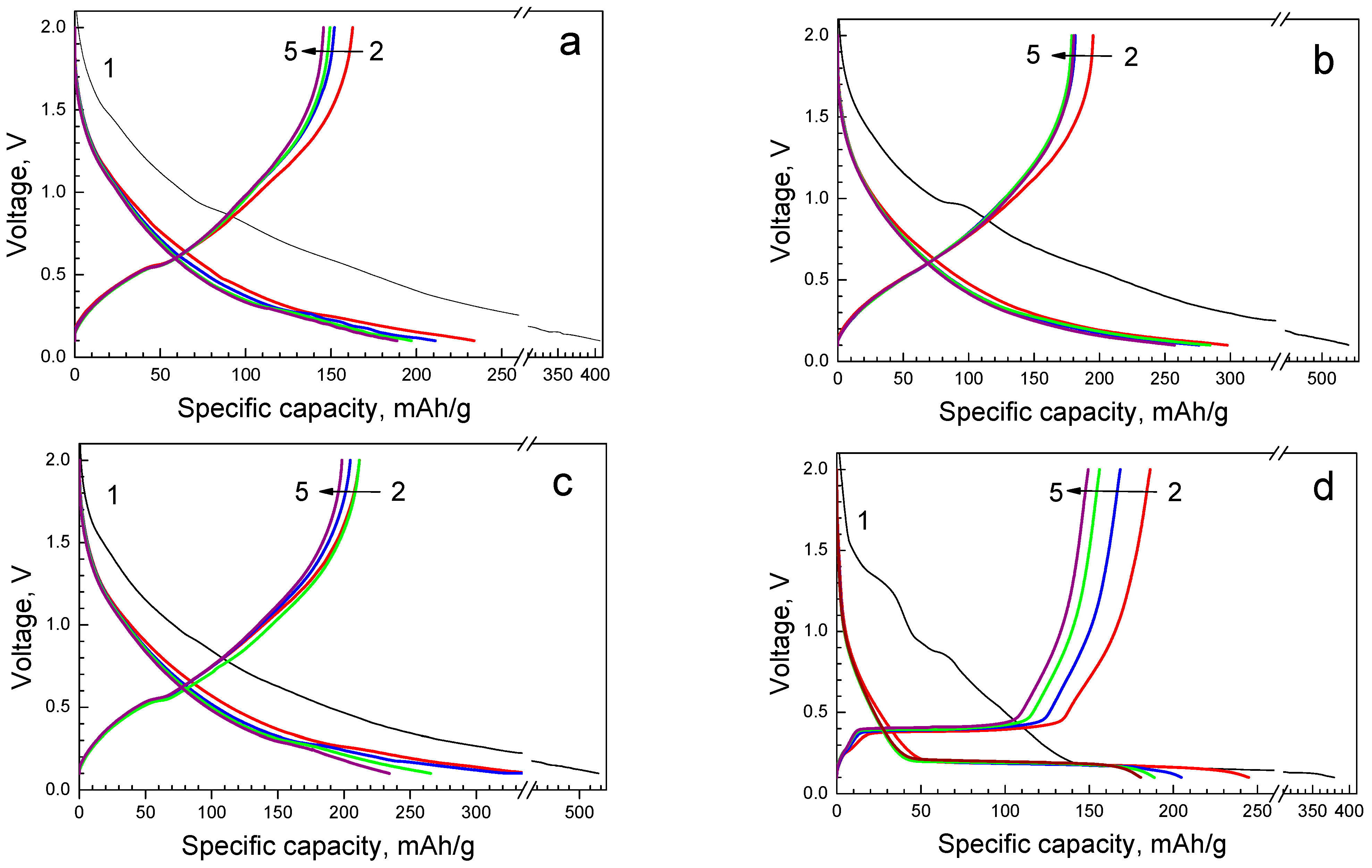
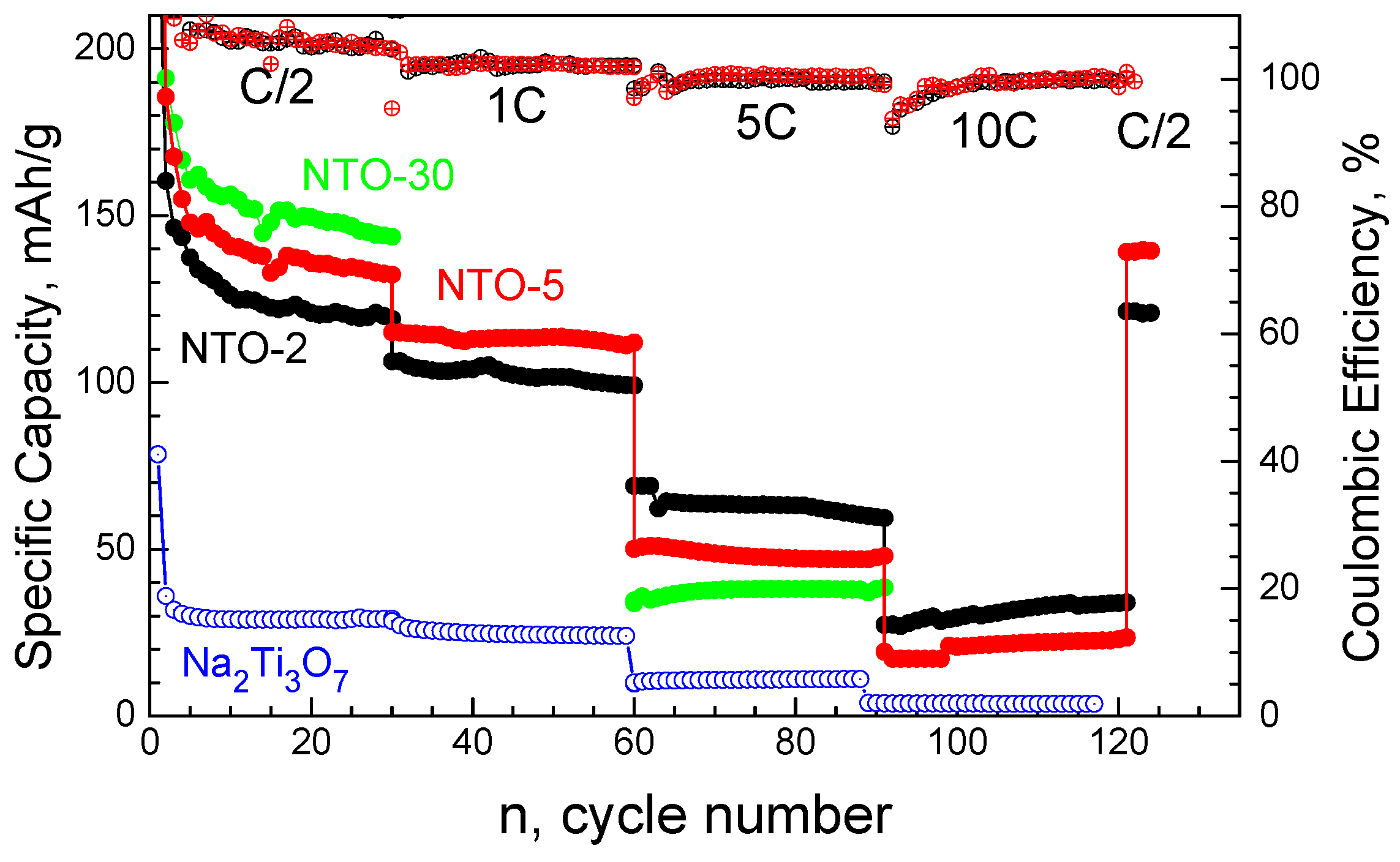
3.2. Sodium Storage Performance of Sodium Titanate Nanowires
3.3. Lithium Storage Performance of Sodium Titanate Nanowires
3.4. Mechanism of Lithium and Sodium Storage into Sodium Titanate Nanowires
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Koleva, V.; Kalapsazova, M.; Marinova, D.; Harizanova, S.; Stoyanova, R. Dual-ion intercalation chemistry enabling hybrid metal-ion batteries. ChemSusChem 2023, 16, e202201442. [Google Scholar] [CrossRef]
- Stoyanova, R.; Koleva, V.; Stoyanova, A. Lithium versus mono/polyvalent ion intercalation: Hybrid metal ion systems for energy storage. Chem. Rec. 2019, 19, 474–501. [Google Scholar] [CrossRef]
- Zhang, W.; Zhang, F.; Ming, F.; Alshareef, H.N. Sodium-ion battery anodes: Status and future trends. EnergyChem 2019, 1, 100012. [Google Scholar] [CrossRef]
- Thompson, M.; Xia, Q.; Hua, Z.; Zhao, X.S. A review on biomass-derived hard carbon materials for sodium-ion batteries. Mater. Adv. 2021, 2, 5881–5905. [Google Scholar] [CrossRef]
- Molaiyan, P.; Simões Dos Reis, G.; Karuppiah, D.; Subramaniyam, C.H.; García-Alvarado, F.; Lassi, U. Recent progress in biomass-derived carbon materials for Li-ion and Na-ion batteries—A review. Batteries 2023, 9, 116. [Google Scholar] [CrossRef]
- Yang, X.; Rogach, A.L. Anodes and Sodium-Free Cathodes in Sodium Ion Batteries. Adv. Energy Mater. 2020, 10, 2000288. [Google Scholar] [CrossRef]
- Lou, S.; Zhao, Y.; Wang, J.; Yin, G.; Du, C.; Sun, X. Ti-based oxide anode materials for advanced electrochemical energy storage: Lithium/sodium ion batteries and hybrid pseudocapacitors. Small 2019, 15, 1904740. [Google Scholar] [CrossRef]
- Wang, Y.; Zhu, W.; Guerfi, A.; Kim, C.; Zaghib, K. Roles of Ti in electrode materials for sodium-ion batteries. Front. Energy Res. 2019, 7, 28. [Google Scholar] [CrossRef]
- Li, X.; Wu, G.; Liu, X.; Li, W.; Li, M. Orderly integration of porous TiO2(B) nanosheets into bunchy hierarchical structure for high- rate and ultralong- lifespan lithium-ion batteries. Nano Energy 2017, 31, 1–8. [Google Scholar] [CrossRef]
- Choi, C.; Ashby, D.S.; Butts, D.M.; DeBlock, R.H.; Wei, Q.; Lau, J.; Dunn, B. Achieving high energy density and high power density with pseudocapacitive materials. Nature Rev. Mater. 2020, 5, 5–19. [Google Scholar] [CrossRef]
- Wei, Z.; Ying, W.; Zongling, H.; Jingze, L. Synthesis of Na2Ti3O7@MWCNTs anode material by spray-drying method of sol-gel precursor for sodium-ion batteries. Sci. Sin. Chim. 2014, 44, 1347–1353. [Google Scholar]
- Dong, S.; Shen, L.; Li, H.; Nie, P.; Zhu, Y.; Sheng, Q.; Zhang, X. Pseudocapacitive behaviours of Na2Ti3O7@CNT coaxial nanocables for high-performance sodium-ion capacitors. J. Mater. Chem. A 2015, 3, 21277–21283. [Google Scholar] [CrossRef]
- Li, Z.; Ye, S.; Wang, W.; Xu, Q.; Liu, H.; Wang, Y.; Xia, Y. Free-standing sandwich-structured flexible film electrode composed of Na2Ti3O7 nanowires@CNT and reduced graphene oxide for advanced sodium-ion batteries. ACS Omega 2017, 2, 5726–5736. [Google Scholar] [CrossRef]
- Xie, F.; Zhang, L.; Su, D.; Jaroniec, M.; Qiao, S.-Z. Na2Ti3O7@Ndoped carbon hollow spheres for sodium-ion batteries with excellent rate performance. Adv. Mater. 2017, 29, 1700989. [Google Scholar] [CrossRef]
- Zhao, M.; Huang, J.; Guo, X.; Chen, H.; Zhao, H.; Dong, L.; Liu, X.-J. Preparation of Na2Ti3O7/titanium peroxide composites and their adsorption property on cationic dyes. J. Chem. 2015, 2015, 363405. [Google Scholar] [CrossRef]
- Ding, C.; Nohira, T.; Hagiwara, R. Electrochemical performance of Na2Ti3O7/C negative electrode in ionic liquid electrolyte for sodium secondary batteries. J. Power Sources 2017, 354, 10–15. [Google Scholar] [CrossRef]
- Zhang, Q.; He, Y.; Mei, P.; Cui, X.; Yang, Y.; Lin, Z. Multi-functional PEDOT-engineered sodium titanate nanowires for sodium–ion batteries with synchronous improvements in rate capability and structural stability. J. Mater. Chem. A 2019, 7, 19241–19247. [Google Scholar] [CrossRef]
- Ko, J.S.; Doan-Nguyen, V.V.T.; Kim, H.-S.; Muller, G.A.; Serino, A.C.; Weiss, P.S.; Dunn, B.S. Na2Ti3O7 nanoplatelets and nanosheets derived from a modifiede process for use as a high-capacity sodium-ion negative electrode. ACS Appl. Mater. Interfaces 2017, 9, 1416–1425. [Google Scholar] [CrossRef]
- Jinghan, Z.; Zeyu, L.; Huaning, J.; Qian, C.; Zhilin, Y.; Xiaokang, G.; Yuying, J.; Yongji, G. Sodium titanate nanowires for Na+-based hybrid energy storage with high power density. SusMat 2022, 2, 720–730. [Google Scholar] [CrossRef]
- Leite, M.M.; Martins, V.L.; Vichi, F.M.; Torresi, R.M. Electrochemistry of sodium titanate nanotubes as a negative electrode for sodium-ion batteries. Electrochim. Acta 2020, 331, 135422. [Google Scholar] [CrossRef]
- Anwer, S.; Huang, Y.; Liu, J.; Liu, J.; Xu, M.; Wang, Z.; Chen, R.; Zhang, J.; Wu, F. Nature-inspired Na2Ti3O7 nanosheets-formed three-dimensional microflowers architecture as a high-performance anode material for rechargeable sodium-ion batteries. ACS Appl. Mater. Interfaces 2017, 9, 11669–11677. [Google Scholar] [CrossRef]
- Tsiamtsouri, M.A.; Allan, P.K.; Pell, A.J.; Stratford, J.M.; Kim, G.; Kerber, R.N.; Magusin, P.C.M.M.; Jefferson, D.A.; Grey, C.P. Exfoliation of layered Na-ion anode material Na2Ti3O7 for enhanced capacity and cyclability. Chem. Mater. 2018, 30, 1505–1516. [Google Scholar] [CrossRef]
- Zhou, G.; Xu, L.; Hu, G.; Mai, L.; Cui, Y. Nanowires for Electrochemical Energy Storage. Chem. Rev. 2019, 119, 11042–11109. [Google Scholar] [CrossRef]
- Zhu, H.; Yang, K.; Lan, H.; Qian, S.; Yu, H.; Yan, L.; Long, N.; Shui, M.; Shu, J. Electrochemical kinetics of Na2Ti3O7 as anode material for lithium-ion batteries. J. Electroanal. Chem. 2017, 788, 203–209. [Google Scholar] [CrossRef]
- Senguttuvan, P.; Rousse, G.; Seznec, V.; Tarascon, J.-M.; Palacín, M.R. Na2Ti3O7: Lowest voltage ever reported oxide insertion electrode for sodium ion batteries. Chem. Mater. 2011, 23, 4109–4111. [Google Scholar] [CrossRef]
- Rudola, A.; Saravanan, K.; Mason, C.W.; Balaya, P. Na2Ti3O7: An intercalation based anode for sodium-ion battery applications. J. Mater. Chem. A 2013, 1, 2653–2662. [Google Scholar] [CrossRef]
- Chiba, K.; Kijima, N.; Takahashi, Y.; Idemoto, Y.; Akimoto, J. Synthesis, structure, and electrochemical Li-ion intercalation properties of Li2Ti3O7 with Na2Ti3O7-type layered structure. Solid State Ion. 2008, 178, 1725–1730. [Google Scholar] [CrossRef]
- Nava-Avendaño, J.; Morales-García, A.; Ponrouch, A.; Rousse, G.; Frontera, C.; Senguttuvan, P.; Tarascon, J.-M.; Arroyo-de Dompablo, M.E.; Rosa Palacín, M. Taking steps forward in understanding the electrochemical behavior of Na2Ti3O7. J. Mater. Chem. A 2015, 3, 22280–22286. [Google Scholar] [CrossRef]
- Dominko, R.; Baudrin, E.; Umek, P.; Arčon, D.; Gaberšček, M.; Jamnik, J. Reversible lithium insertion into Na2Ti6O13 structure. Electrochem. Commun. 2006, 8, 673–677. [Google Scholar] [CrossRef]
- Trinh, N.D.; Crosnier, O.; Schougaard, S.; Brousse, T. Synthesis, characterizations and electrochemical studies of Na2Ti6O13 for sodium ion batteries. ECS Trans. 2012, 35, 91–98. [Google Scholar] [CrossRef]
- Wu, C.; Hua, W.; Zhang, Z.; Zhong, B.; Yang, Z.; Feng, G.; Xiang, W.; Wu, Z.; Guo, X. Design and synthesis of layered Na2Ti3O7 and tunnel Na2Ti6O13 hybrid structures with enhanced electrochemical behavior for sodium-ion batteries. Adv. Sci. 2018, 5, 1800519. [Google Scholar] [CrossRef]
- Cech, O.; Castkova, K.; Chladil, L.; Dohnal, P.; Cudek, P.; Libich, J.; Vanysek, P. Synthesis and characterization of Na2Ti6O13 and Na2Ti6O13/Na2Ti3O7 sodium titanates with nanorod-like structure as negative electrode materials for sodium-ion batteries. J. Energy Storage 2017, 14, 391–398. [Google Scholar] [CrossRef]
- Li, P.; Wang, P.; Qian, S.; Yu, H.; Lin, X.; Shui, M.; Zheng, X.; Long, N.; Shu, J. Synthesis of Na2Ti6O13 nanorods as possible anode materials for rechargeable lithium ion batteries. Electrochim. Acta 2016, 187, 46–54. [Google Scholar] [CrossRef]
- Kataoka, K.; Akimoto, J. Synthesis and electrochemical sodium and lithium insertion properties of sodium titanium oxide with the tunnel type structure. J. Power Sources 2016, 305, 151–155. [Google Scholar] [CrossRef]
- Liu, H.; Yang, D.; Zheng, Z.; Ke, X.; Waclawik, E.; Zhu, H.; Frost, R.L. A Raman spectroscopic and TEM study, on the structural evolution of Na2Ti3O7 during the transition to Na2Ti6O13. J. Raman Spectrosc. 2010, 41, 1331–1337. [Google Scholar] [CrossRef]
- Su, Y.; Balmer, M.L.; Bunker, B.C. Raman spectroscopic studies of silicotitanates. J. Phys. Chem. B 2000, 104, 8160–8169. [Google Scholar] [CrossRef]
- Luo, L.; Zhen, Y.; Lu, Y.; Zhou, K.; Huang, J.; Huang, Z.; Mathur, S.; Hong, Z. Structural evolution from layered Na2Ti3O7 to Na2Ti6O13 nanowires enabling a highly reversible anode for Mg-ion batteries. Nanoscale 2020, 12, 230–238. [Google Scholar] [CrossRef]
- Papp, S.; Kõrösi, L.; Meynen, V.; Cool, P.; Vansant, E.F.; Dékány, I. The influence of temperature on the structural behaviour of sodium tri- and hexa-titanates and their protonated forms. J. Solid State Chem. 2005, 178, 1614–1619. [Google Scholar] [CrossRef]
- Viana, B.C.; Ferreira, O.P.; Souza Filho, A.G.; Hidalgo, A.A.; Filho, J.M.; Alves, O.L. Alkali metal intercalated titanate nanotubes: A vibrational spectroscopy study. Vib. Spectrosc. 2011, 55, 183–187. [Google Scholar] [CrossRef]
- Kim, S.-J.; Yun, Y.-U.; Oh, H.-J.; Hong, S.H.; Roberts, C.A.; Routray, K.; Wachs, I.E. Characterization of hydrothermally prepared titanate nanotube powders by ambient and in situ Raman spectroscopy. J. Phys. Chem. Lett. 2010, 1, 130–135. [Google Scholar] [CrossRef]
- Muñoz-Márquez, M.A.; Zarrabeitia, M.; Castillo-Martínez, E.; Eguía-Barrio, A.; Rojo, T.; Casas-Cabanas, M. Composition and evolution of the solid-electrolyte interphase in Na2Ti3O7 electrodes for Na-ion batteries: XPS and auger parameter analysis. ACS Appl. Mater. Interfaces 2015, 7, 7801–7808. [Google Scholar] [CrossRef]
- Pan, H.; Lu, X.; Yu, X.; Hu, Y.-S.; Li, H.; Yang, X.-Q.; Chen, L. Sodium storage and transport properties in layered Na2Ti3O7 for room-temperature sodium-ion batteries. Adv. Energy Mater. 2013, 3, 1186–1194. [Google Scholar] [CrossRef]
- Ivanova, S.; Zhecheva, E.; Kukeva, R.; Nihtianova, D.; Mihaylov, L.; Atanasova, G.; Stoyanova, R. Layered P3-NaxCo1/3Ni1/3Mn1/3O2 versus Spinel Li4Ti5O12 as a Positive and a Negative Electrode in a Full Sodium–Lithium Cell. ACS Appl. Mater. Interfaces 2016, 8, 17321–17333. [Google Scholar] [CrossRef]
- Shen, K.; Wagemaker, M. Na2+xTi6O13 as potential negative electrode material for Na-ion batteries. Inorg. Chem. 2014, 53, 8250–8256. [Google Scholar] [CrossRef]
- Rubloff, G.W.; Lee, S.B. New science at the meso frontier: Dense nanostructure architectures for electrical energy storage. Curr. Opin. Solid State Mater. Sci. 2015, 19, 227–234. [Google Scholar] [CrossRef]
- Yan, Z.; Liu, L.; Shu, H.; Yang, X.; Wang, H.; Tan, J.; Zhou, Q.; Huang, Z.; Wang, X. A tightly integrated sodium titanate-carbon composite as an anode material for rechargeable sodium ion batteries. J. Power Sources 2015, 274, 8–14. [Google Scholar] [CrossRef]
- Kim, K.-T.; Yu, C.-Y.; Yoon, C.S.; Kim, S.-J.; Sun, Y.-K.; Myung, S.-T. Carbon-coated Li4Ti5O12 nanowires showing high rate capability as an anode material for rechargeable sodium batteries. Nano Energy 2015, 12, 725–734. [Google Scholar] [CrossRef]
- Rousse, G.; Arroyo-de Dompablo, M.E.; Senguttuvan, P.; Ponrouch, A.; Tarascon, J.-M.; Palacín, M.R. Rationalization of intercalation potential and redox mechanism for A2Ti3O7 (A = Li, Na). Chem. Mater. 2013, 25, 4946–4956. [Google Scholar] [CrossRef]
- Dominko, R.; Dupont, L.; Gaberšček, M.; Jamnik, J.; Baudrin, E. Alkali hexatitanates—A2Ti6O13 (A = Na, K) as host structure for reversible lithium insertion. J. Power Sources 2007, 174, 1172–1176. [Google Scholar] [CrossRef]
- Xu, Y.; Bauer, D.; Lübke, M.; Ashton, T.E.; Zong, Y.; Darr, J.A. High-power sodium titanate anodes; a comparison of lithium vs. sodium-ion batteries. J. Power Sources 2018, 408, 28–37. [Google Scholar] [CrossRef]
- Liao, J.-Y.; Smith, T.W.; Pandey, R.P.; He, X.; Chusuei, C.C.; Xing, Y. Substantially enhanced rate capability of lithium storage in Na2Ti6O13 with self-doping and carbon-coating. RSC Adv. 2018, 8, 8929–8936. [Google Scholar] [CrossRef]
- Augustyn, V.; Simon, P.; Dunn, B. Pseudocapacitive oxide materials for high-rate electrochemical energy storage. Energy Environ. Sci. 2014, 7, 1597–1614. [Google Scholar] [CrossRef]









| Samples | C/20—First Discharge Capacity, mAh/g | C/20—First Irreversible Capacity, mAh/g | C/2—First Discharge Capacity, mAh/g | C/2—First Irreversible Capacity, mAh/g |
|---|---|---|---|---|
| NTO-2 | 406 | 244 | 253 | 112 |
| NTO-5 | 565 | 370 | 258 | 106 |
| NTO-30 | 536 | 324 | 300 | 136 |
| Na2Ti3O7 | 380 | 194 | 78 | 51 |
| Samples | C/20—First Discharge Capacity, mAh/g | C/20—First Irreversible Capacity, mAh/g | C/2—First Discharge Capacity, mAh/g | C/2—First Irreversible Capacity, mAh/g |
|---|---|---|---|---|
| NTO-2 | 772 | 497 | 730 | 396 |
| NTO-5 | 988 | 629 | 735 | 388 |
| NTO-30 | 820 | 482 | 616 | 292 |
| Na2Ti3O7 | 244 | 122 | 153 | 71 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stanchovska, S.; Kalapsazova, M.; Harizanova, S.; Koleva, V.; Stoyanova, R. Design of Sodium Titanate Nanowires as Anodes for Dual Li,Na Ion Batteries. Batteries 2023, 9, 271. https://doi.org/10.3390/batteries9050271
Stanchovska S, Kalapsazova M, Harizanova S, Koleva V, Stoyanova R. Design of Sodium Titanate Nanowires as Anodes for Dual Li,Na Ion Batteries. Batteries. 2023; 9(5):271. https://doi.org/10.3390/batteries9050271
Chicago/Turabian StyleStanchovska, Silva, Mariya Kalapsazova, Sonya Harizanova, Violeta Koleva, and Radostina Stoyanova. 2023. "Design of Sodium Titanate Nanowires as Anodes for Dual Li,Na Ion Batteries" Batteries 9, no. 5: 271. https://doi.org/10.3390/batteries9050271






