A Hollow-Shaped ZIF-8-N-Doped Porous Carbon Fiber for High-Performance Zn-Ion Hybrid Supercapacitors
Abstract
:1. Introduction
2. Methods and Materials
2.1. Materials
2.2. Preparation of ZIF-8 and ZPCNF
2.3. Physical Characterization
2.4. Electrochemical Measurements
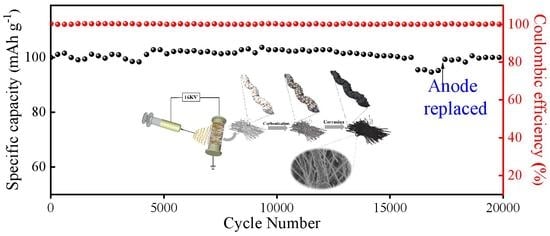
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhao, R.; Elzatahry, A.; Chao, D.; Zhao, D. Making MXene more energetic in aqueous battery. Matter 2022, 5, 8–10. [Google Scholar] [CrossRef]
- Xu, L.; Pan, G.; Yu, C.; Li, J.; Gong, Z.; Lu, T.; Pan, L. Co-doped MnO2 with abundant oxygen vacancies as a cathode for superior aqueous magnesium ion storage. Inorg. Chem. Front. 2023, 10, 1748–1757. [Google Scholar] [CrossRef]
- Zhao, R.; Di, H.; Hui, X.; Zhao, D.; Wang, R.; Wang, C.; Yin, L. Self-assembled Ti3C2 MXene and N-rich porous carbon hybrids as superior anodes for high-performance potassium-ion batteries. Energy Environ. Sci. 2020, 13, 246–257. [Google Scholar] [CrossRef]
- Li, L.; Lu, Y.; Zhang, Q.; Zhao, S.; Hu, Z.; Chou, S.L. Recent progress on layered cathode materials for nonaqueous rechargeable magnesium batteries. Small 2021, 17, 1902767. [Google Scholar] [CrossRef]
- Molaiyan, P.; Reis, G.D.; Karuppiah, D.; Subramaniyam, C.M.; García-Alvarado, F.; Lassi, U. Recent progress in biomass-derived carbon materials for Li-ion and Na-ion batteries—A review. Batteries 2023, 9, 116. [Google Scholar] [CrossRef]
- Simon, P.; Gogotsi, Y. Perspectives for electrochemical capacitors and related devices. Nat. Mater. 2020, 19, 1151–1163. [Google Scholar] [CrossRef]
- Yan, J.; Liu, T.; Liu, X.; Yan, Y.; Huang, Y. Metal-organic framework-based materials for flexible supercapacitor application. Coord. Chem. Rev. 2022, 452, 214300. [Google Scholar] [CrossRef]
- Xie, B.; He, J.; Sun, Y.; Li, S.; Li, J. Hybrid Anionic Electrolytes for the High Performance of Aqueous Zinc-Ion Hybrid Supercapacitors. Energies 2022, 16, 248. [Google Scholar] [CrossRef]
- Chao, D.; Zhou, W.; Ye, C.; Zhang, Q.; Chen, Y.; Gu, L.; Davey, K.; Qiao, S. An Electrolytic Zn-MnO2 Battery for High-Voltage and Scalable Energy Storage. Angew. Chem. Int. Ed. Engl. 2019, 58, 7823–7828. [Google Scholar] [CrossRef]
- Deng, X.; Li, J.; Zhu, S.; Sha, J.; Ma, L.; Zhao, Z. N, O co-doped hierarchical carbon cathode for high-performance Zn-ion hybrid supercapacitors with enhanced pseudocapacitance. J. Mater. Chem. A 2020, 8, 11617–11625. [Google Scholar] [CrossRef]
- Pan, G.; Li, J.; Han, L.; Peng, W.; Xu, X.; Lu, T.; Amin, M.A.; Yamauchi, Y.; Xu, M.; Pan, L. MoS2 nanosheets with expanded interlayer spacing for ultra-stable aqueous Mg-ion hybrid supercapacitor. Inorg. Chem. Front. 2022, 9, 1666–1673. [Google Scholar] [CrossRef]
- Zeng, Y.; Zhang, X.; Qin, R.; Liu, X.; Fang, P.; Zheng, D.; Tong, Y.; Lu, X. Dendrite-Free Zinc Deposition Induced by Multifunctional CNT Frameworks for Stable Flexible Zn-Ion Batteries. Adv. Mater. 2019, 31, e1903675. [Google Scholar] [CrossRef] [PubMed]
- Song, P.; Li, C.; Zhao, N.; Ji, Z.; Zhai, L.; Shen, X.; Liu, Q. Molten salt-confined pyrolysis towards heteroatom-doped porous carbon nanosheets for high-energy-density Zn-ion hybrid supercapacitors. Colloid Interface Sci. 2023, 633, 362–373. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; An, Y.; Dong, S.; Chen, C.; Wu, L.; Sun, Y.; Zhang, X. Progress on zinc ion hybrid supercapacitors: Insights and challenges. Energy Storage Mater. 2020, 31, 252–266. [Google Scholar] [CrossRef]
- Sui, D.; Wu, M.; Shi, K.; Li, C.; Lang, J.; Yang, Y.; Zhang, X.; Yan, X.; Chen, Y. Recent progress of cathode materials for aqueous zinc-ion capacitors: Carbon-based materials and beyond. Carbon 2021, 185, 126–151. [Google Scholar] [CrossRef]
- Tang, B.; Shan, L.; Liang, S.; Zhou, J. Issues and opportunities facing aqueous zinc-ion batteries. Energy Environ. Sci. 2019, 12, 3288–3304. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, L.; Zhang, F.; Ding, X.; Shin, K.; Tang, Y. High-performance Zn-graphite battery based on LiPF6 single-salt electrolyte with high working voltage and long cycling life. J. Energy Chem. 2021, 58, 602–609. [Google Scholar] [CrossRef]
- Zhang, N.; Chen, X.; Yu, M.; Niu, Z.; Cheng, F.; Cheng, J. Materials chemistry for rechargeable zinc-ion batteries. Chem. Soc. Rev. 2020, 49, 4203–4219. [Google Scholar] [CrossRef]
- Huang, J.; Wang, L.; Peng, Z.; Peng, M.; Li, L.; Tang, X.; Xu, Y.; Tang, L.; Yuan, K.; Chen, Y. Minimization of ion transport resistance: Diblock copolymer micelle derived nitrogen-doped hierarchically porous carbon spheres for superior rate and power Zn-ion capacitors. J. Mater. Chem. A 2021, 9, 8435–8443. [Google Scholar] [CrossRef]
- Han, L.; Zhang, X.; Li, J.; Huang, H.; Xu, X.; Liu, X.; Yang, Z.; Xu, M.; Pan, L. Enhanced energy storage of aqueous zinc-carbon hybrid supercapacitors via employing alkaline medium and B, N dual doped carbon cathode. J. Colloid Interface Sci. 2021, 599, 556–565. [Google Scholar] [CrossRef]
- Feng, J.; Wang, Y.; Xu, Y.; Sun, Y.; Tang, Y.; Yan, X. Ion regulation of ionic liquid electrolytes for supercapacitors. Energy Environ. Sci. 2021, 14, 2859–2882. [Google Scholar] [CrossRef]
- Wang, C.; Pei, Z.; Meng, Q.; Zhang, Z.; Sui, X.; Yuan, Z.; Wang, S.; Chen, Y. Toward Flexible Zinc-Ion Hybrid Capacitors with Superhigh Energy Density and Ultralong Cycling Life: The Pivotal Role of ZnCl2 Salt-Based Electrolytes. Angew. Chem. Int. Ed. Engl. 2021, 60, 990–997. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Zhao, M.; Cai, Y.; Zhao, G.; Xie, Y.; Zhang, L.; Zhu, G.; Pan, L. Scalable synthesis of strutted nitrogen doped hierarchical porous carbon nanosheets for supercapacitors with both high gravimetric and volumetric performances. Carbon 2021, 179, 458–468. [Google Scholar] [CrossRef]
- Peng, H.; Yao, B.; Wei, X.; Liu, T.; Kou, T.; Xiao, P.; Zhang, Y.; Li, Y. Pore and Heteroatom Engineered Carbon Foams for Supercapacitors. Adv. Energy Mater. 2019, 9, 1803665. [Google Scholar] [CrossRef]
- Wei, J.; Ding, C.; Zhang, P.; Ding, H.; Niu, X.; Ma, Y.; Li, C.; Wang, Y.; Xiong, H. Robust Negative Electrode Materials Derived from Carbon Dots and Porous Hydrogels for High-Performance Hybrid Supercapacitors. Adv. Mater. 2019, 31, e1806197. [Google Scholar] [CrossRef]
- Thalji, M.R.; Ali, G.A.; Liu, P.; Zhong, Y.; Chong, K. W18O49 nanowires-graphene nanocomposite for asymmetric supercapacitors employing AlCl3 aqueous electrolyte. Chem. Eng. J. 2021, 409, 128216. [Google Scholar] [CrossRef]
- Li, H.; Wu, J.; Wang, L.; Liao, Q.; Niu, X.; Zhang, D.; Wang, K. A zinc ion hybrid capacitor based on sharpened pencil-like hierarchically porous carbon derived from metal–organic framework. Chem. Eng. J. 2022, 428, 131071. [Google Scholar] [CrossRef]
- Zhao, G.; Han, L.; Ning, K.; Zhu, G.; Yang, J.; Wang, H. O doped Tremella-shaped porous carbon for zinc-ion hybrid capacitors with long life and enhanced energy density. Mater. Lett. 2022, 329, 133180. [Google Scholar] [CrossRef]
- Chen, C.; Li, P.; Wang, T.; Wang, S.; Zhang, M. S-Doped Carbon Fibers Uniformly Embedded with Ultrasmall TiO2 for Na+/Li+ Storage with High Capacity and Long-Time Stability. Small 2019, 15, e1902201. [Google Scholar] [CrossRef]
- Chen, C.; Yang, Y.; Tang, X.; Qiu, R.; Wang, S.; Cao, G.; Zhang, M. Graphene-Encapsulated FeS2 in Carbon Fibers as High Reversible Anodes for Na+ /K+ Batteries in a Wide Temperature Range. Small 2019, 15, e1804740. [Google Scholar] [CrossRef]
- Marpaung, F.; Kim, M.; Khan, J.; Konstantinov, K.; Yamauchi, Y.; Hossain, M.; Na, J.; Kim, J. Metal-Organic Framework (MOF)-Derived Nanoporous Carbon Materials. Chem. Asian J. 2019, 14, 1331–1343. [Google Scholar] [CrossRef] [PubMed]
- Mōri, T.; Namba, Y. Crystal structure of diamondlike carbon films prepared by ionized deposition from methane gas. J. Appl. Phys. 1984, 55, 3276–3279. [Google Scholar] [CrossRef]
- Shao, J.; Zhu, G.; Xie, L.; Tao, S.; Zhang, Y.; Zhang, J.; Wang, H.; Zhang, L.; Chen, C. One-step production of N, S co-doped honeycomb-like activated carbon from instant dry yeast for high gravimetric and volumetric performance supercapacitors. Diam. Relat. Mater. 2022, 127, 109165. [Google Scholar] [CrossRef]
- Streletskiy, O.; Zavidovskiy, I.; Sychev, V.; Dudin, A.; Savinov, S.; Pavlikov, A. Magnetron deposition of a-C: ND coatings by nanodiamond transfer: Pulse number impact on aggregation and graphitization. Appl. Phys. A 2021, 128, 1–8. [Google Scholar] [CrossRef]
- Gao, S.; Geng, K.; Liu, H.; Wei, X.; Zhang, M.; Wang, P.; Wang, J. Transforming organic-rich amaranthus waste into nitrogen-doped carbon with superior performance of the oxygen reduction reaction. Energy Environ. Sci. 2015, 8, 221–229. [Google Scholar] [CrossRef]
- Li, W.; Li, M.; Wang, M.; Zeng, L.; Yu, Y. Electrospinning with partially carbonization in air: Highly porous carbon nanofibers optimized for high-performance flexible lithium-ion batteries. Nano Energy 2015, 13, 693–701. [Google Scholar] [CrossRef]
- Yun, Y.; Cho, S.; Shim, J.; Kim, B.; Chang, S.; Beak, S.; Huh, Y.; Tak, V.; Park, Y.; Prak, S.; et al. Microporous carbon nanoplates from regenerated silk proteins for supercapacitors. Adv. Mater. 2013, 25, 1993–1998. [Google Scholar] [CrossRef]
- Schwan, J.; Ulrich, S.; Batori, V.; Ehrhardt, H.; Silva, S. Raman spectroscopy on amorphous carbon films. J. Appl. Phys. 1996, 80, 440–447. [Google Scholar] [CrossRef]
- Kim, C.; Park, S.; Cho, J.; Lee, D.; Park, T.; Lee, W.; Yang, K. Raman spectroscopic evaluation of polyacrylonitrile-based carbon nanofibers prepared by electrospinning. J. Raman Spectrosc. 2004, 35, 928–933. [Google Scholar] [CrossRef]
- Fan, H.; Niu, R.; Duan, J.; Liu, W.; Shen, W. Fe3O4@Carbon Nanosheets for All-Solid-State Supercapacitor Electrodes. ACS Appl. Mater. Interfaces 2016, 8, 19475–19483. [Google Scholar] [CrossRef]
- Ma, C.; Li, Y.; Shi, J.; Song, Y.; Liu, L. High-performance supercapacitor electrodes based on porous flexible carbon nanofiber paper treated by surface chemical etching. Chem. Eng. J. 2014, 249, 216–225. [Google Scholar] [CrossRef]
- Wu, T.; Jing, M.; Yang, L.; Zou, G.; Hou, H.; Zhang, Y.; Zhang, Y.; Cao, X.; Ji, X. Controllable Chain-Length for Covalent Sulfur-Carbon Materials Enabling Stable and High-Capacity Sodium Storage. Adv. Energy Mater. 2019, 9, 1803478. [Google Scholar] [CrossRef]
- Zhao, G.; Xu, X.; Zhu, G.; Shi, J.; Li, Y.; Zhang, S.; Hossain, M.S.A.; Wu, K.C.-W.; Tang, J.; Yamauchi, Y. Flexible nitrogen-doped carbon heteroarchitecture derived from ZIF-8/ZIF-67 hybrid coating on cotton biomass waste with high supercapacitive properties. Microporous Mesoporous Mater. 2020, 303, 110257. [Google Scholar] [CrossRef]
- Olabi, A.G.; Abbas, Q.; Abdelkareem, M.; Alami, A.H.; Mirzaeian, M.; Sayed, E.T. Carbon-based materials for supercapacitors: Recent progress, challenges and barriers. Batteries 2023, 9, 19. [Google Scholar] [CrossRef]
- Xia, J.; Zhang, N.; Chong, S.; Li, D.; Chen, Y.; Sun, C. Three-dimensional porous graphene-like sheets synthesized from biocarbon via low-temperature graphitization for a supercapacitor. Green Chem. 2018, 20, 694–700. [Google Scholar] [CrossRef]
- Xu, B.; Wang, H.; Zhu, Q.; Sun, N.; Anasori, B.; Hu, L.; Wang, F.; Guan, Y.; Gogotsi, Y. Reduced graphene oxide as a multi-functional conductive binder for supercapacitor electrodes. Energy Storage Mater. 2018, 12, 128–136. [Google Scholar] [CrossRef]
- Li, Q.; Xie, W.; Liu, D.; Wang, Q.; He, D. Nitrogen and oxygen co-doped carbon nanofibers with rich sub-nanoscale pores as self-supported electrode material of high-performance supercapacitors. Electrochim. Acta 2016, 222, 1445–1454. [Google Scholar] [CrossRef]
- Chen, L.; Lu, Y.; Yu, L.; Lou, X. Designed formation of hollow particle-based nitrogen-doped carbon nanofibers for high-performance supercapacitors. Energy Environ. Sci. 2017, 10, 1777–1783. [Google Scholar] [CrossRef]
- Cheng, H.; Chen, X.; Yu, H.; Guo, M.; Chang, Y.; Zhang GHierarchically Porous, N. P-Codoped Carbon Materials for High-Performance Supercapacitors. ACS Appl. Energy Mater. 2020, 3, 10080–10088. [Google Scholar] [CrossRef]
- He, H.; Lian, J.; Chen, C.; Xiong, Q.; Zhang, M. Super hydrophilic carbon fiber film for freestanding and flexible cathodes of zinc-ion hybrid supercapacitors. Chem. Eng. J. 2021, 421, 129786. [Google Scholar] [CrossRef]
- Jiang, C.; Zou, Z. Waste polyurethane foam filler-derived mesoporous carbons as superior electrode materials for EDLCs and Zn-ion capacitors. Diam. Relat. Mater. 2020, 101, 107603. [Google Scholar] [CrossRef]
- Liu, Y.; Tan, H.; Tan, Z.; Cheng, X. Rice husk-derived carbon materials for aqueous Zn-ion hybrid supercapacitors. Appl. Surf. Sci. 2023, 608, 155215. [Google Scholar]
- Wang, H.; Huang, J.; Wang, X.; Guo, Z.; Liu, W. Fabrication of TiN/CNTs on carbon cloth substrates via a CVD–ALD method as free-standing electrodes for zinc ion hybrid capacitors. New J. Chem. 2022, 46, 15175–15184. [Google Scholar] [CrossRef]
- Wang, Q.; Wang, S.; Guo, X.; Ruan, L.; Wei, N.; Ma, Y.; Li, J.; Wang, M.; Li, W.; Zeng, W. MXene-Reduced Graphene Oxide Aerogel for Aqueous Zinc-Ion Hybrid Supercapacitor with Ultralong Cycle Life. Adv. Electron. Mater. 2019, 5, 1900537. [Google Scholar] [CrossRef]
- Zhu, Y.; Ye, X.; Jiang, H.; Xie, J.; Yue, Z.; Wang, L.; Wan, Z.; Jia, C.; Yao, X. Controlled swelling of graphene films towards hierarchical structures for supercapacitor electrodes. J. Power Sources 2020, 453, 227851. [Google Scholar] [CrossRef]
- Chen, S.; Ma, L.; Zhang, K.; Kamruzzaman, M.; Zhi, C.; Zapien, J.A. A flexible solid-state zinc ion hybrid supercapacitor based on co-polymer derived hollow carbon spheres. J. Mater. Chem. A 2019, 7, 7784–7790. [Google Scholar] [CrossRef]
- Jian, Z.; Yang, N.; Vogel, M.; Leith, S.; Schulte, A.; Schönherr, H.; Jiao, T.; Zhang, W.; Müller, J.; Butz, B.; et al. Flexible Diamond Fibers for High-Energy-Density Zinc-Ion Supercapacitors. Adv. Energy Mater. 2020, 10, 2002202. [Google Scholar]








Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wei, M.; Jiang, Z.; Yang, C.; Jiang, T.; Zhang, L.; Zhao, G.; Zhu, G.; Yu, L.; Zhu, Y. A Hollow-Shaped ZIF-8-N-Doped Porous Carbon Fiber for High-Performance Zn-Ion Hybrid Supercapacitors. Batteries 2023, 9, 405. https://doi.org/10.3390/batteries9080405
Wei M, Jiang Z, Yang C, Jiang T, Zhang L, Zhao G, Zhu G, Yu L, Zhu Y. A Hollow-Shaped ZIF-8-N-Doped Porous Carbon Fiber for High-Performance Zn-Ion Hybrid Supercapacitors. Batteries. 2023; 9(8):405. https://doi.org/10.3390/batteries9080405
Chicago/Turabian StyleWei, Mingqi, Zhenlong Jiang, Chengcheng Yang, Tao Jiang, Linlin Zhang, Guangzhen Zhao, Guang Zhu, Lianghao Yu, and Yuanyuan Zhu. 2023. "A Hollow-Shaped ZIF-8-N-Doped Porous Carbon Fiber for High-Performance Zn-Ion Hybrid Supercapacitors" Batteries 9, no. 8: 405. https://doi.org/10.3390/batteries9080405