Electro-Thermal Analysis of a Pouch–Type Lithium–Ion Battery with a High Discharge Rate for Urban Air Mobility
Abstract
:1. Introduction
2. Methodology
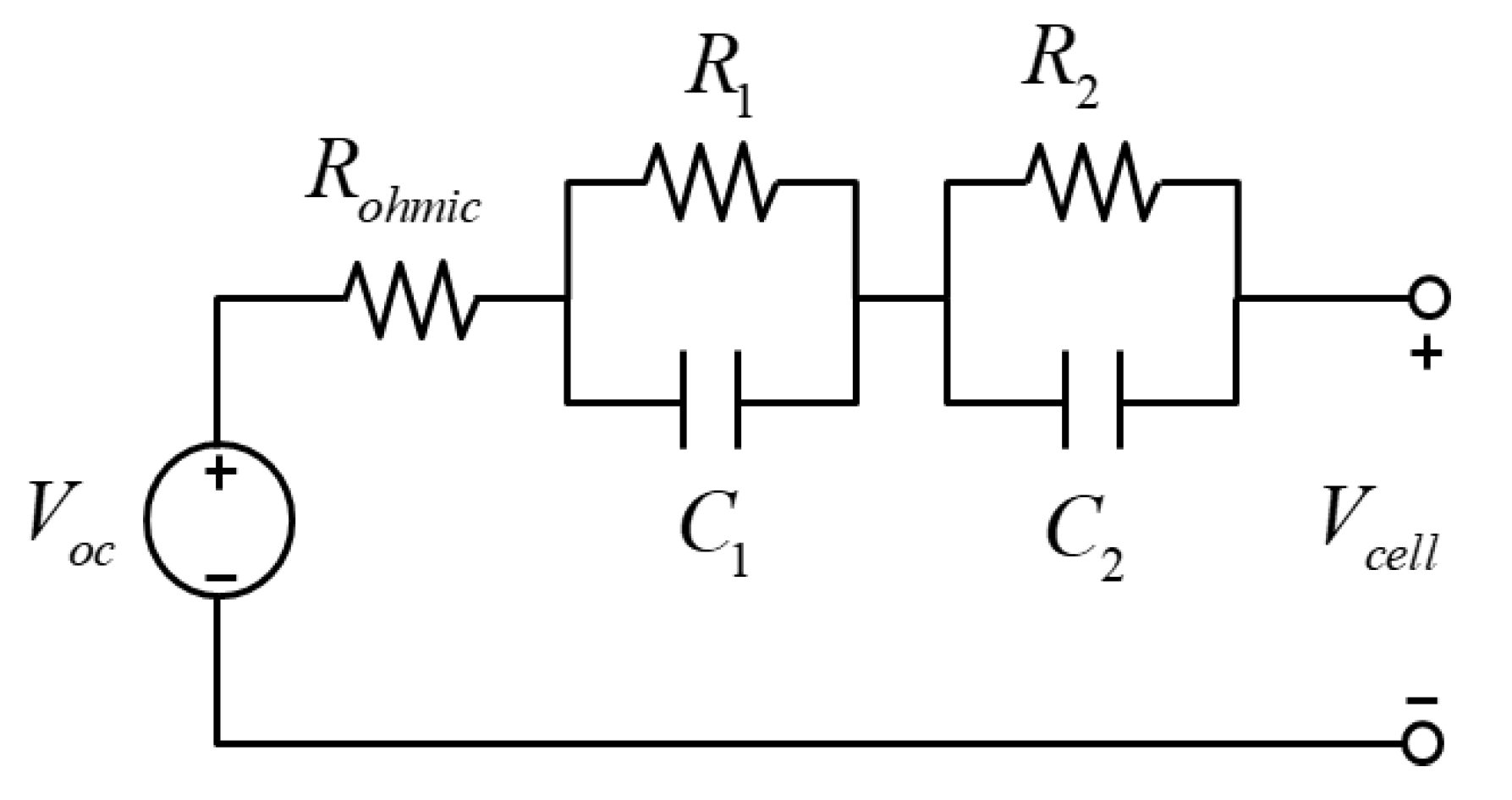
2.1. Model of a Pouch–Type Lithium–Ion Battery
2.2. Governing Equation and Simulation Setup
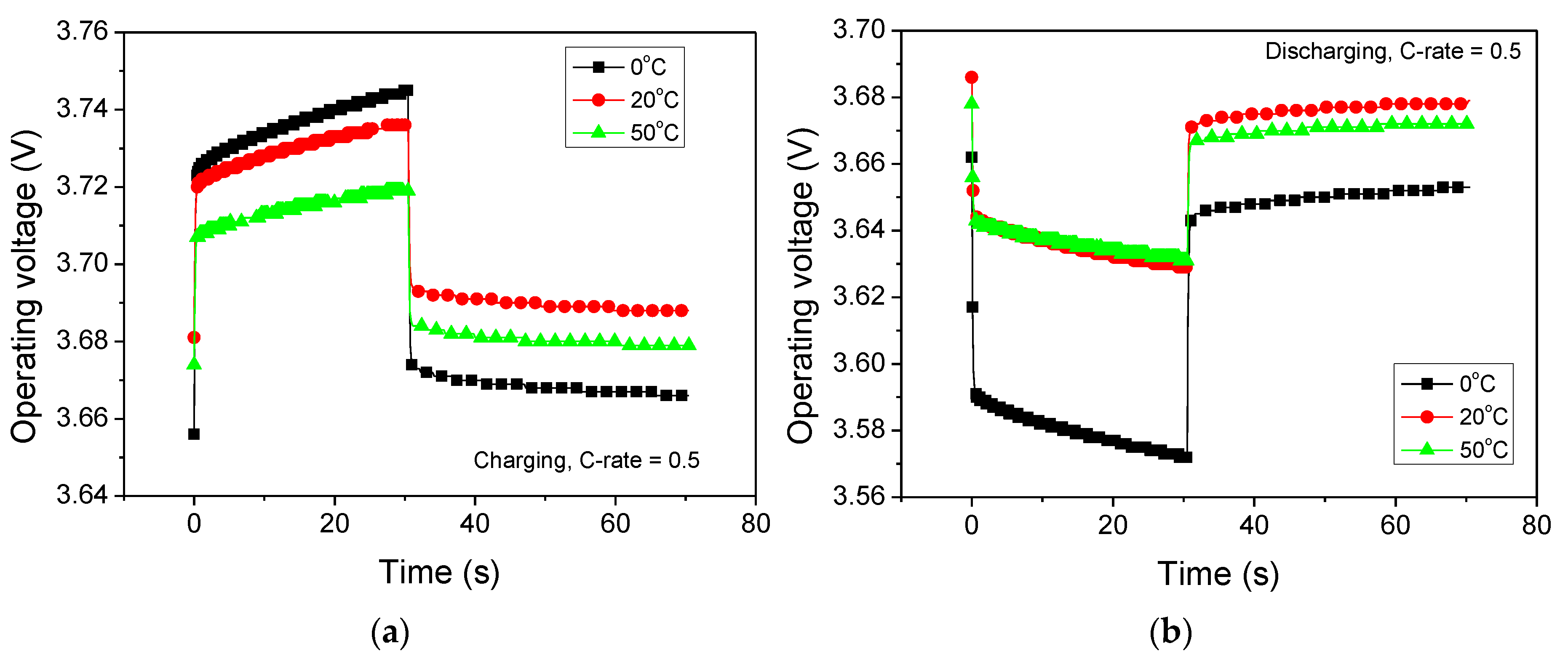
3. Results and Discussion
4. Conclusions
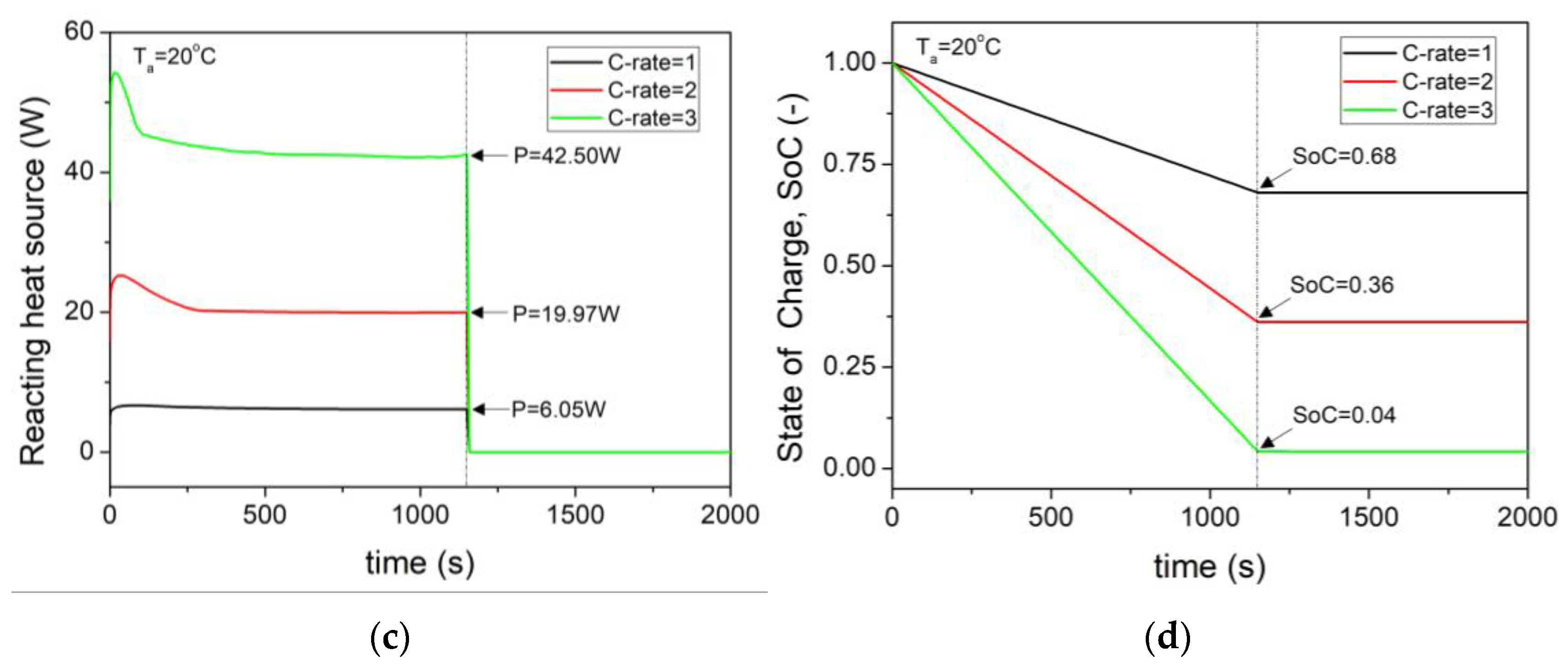
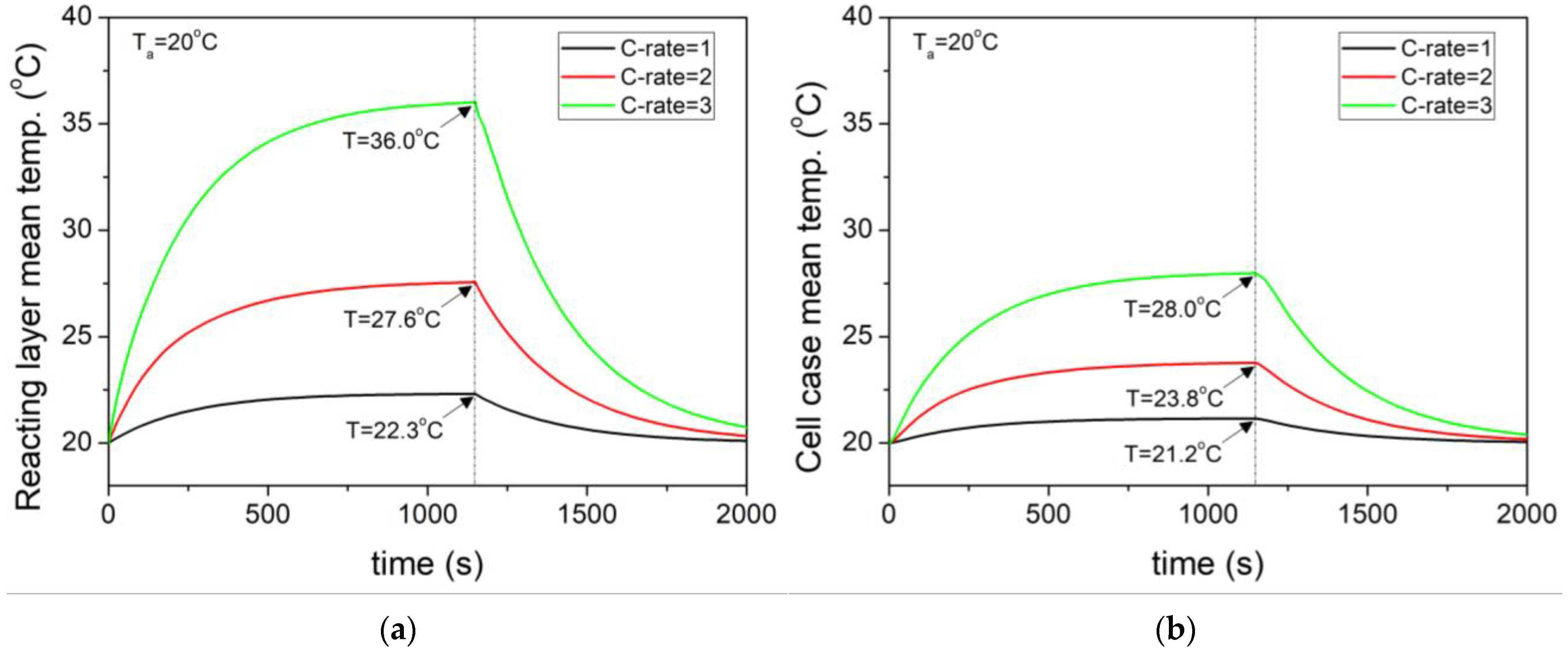
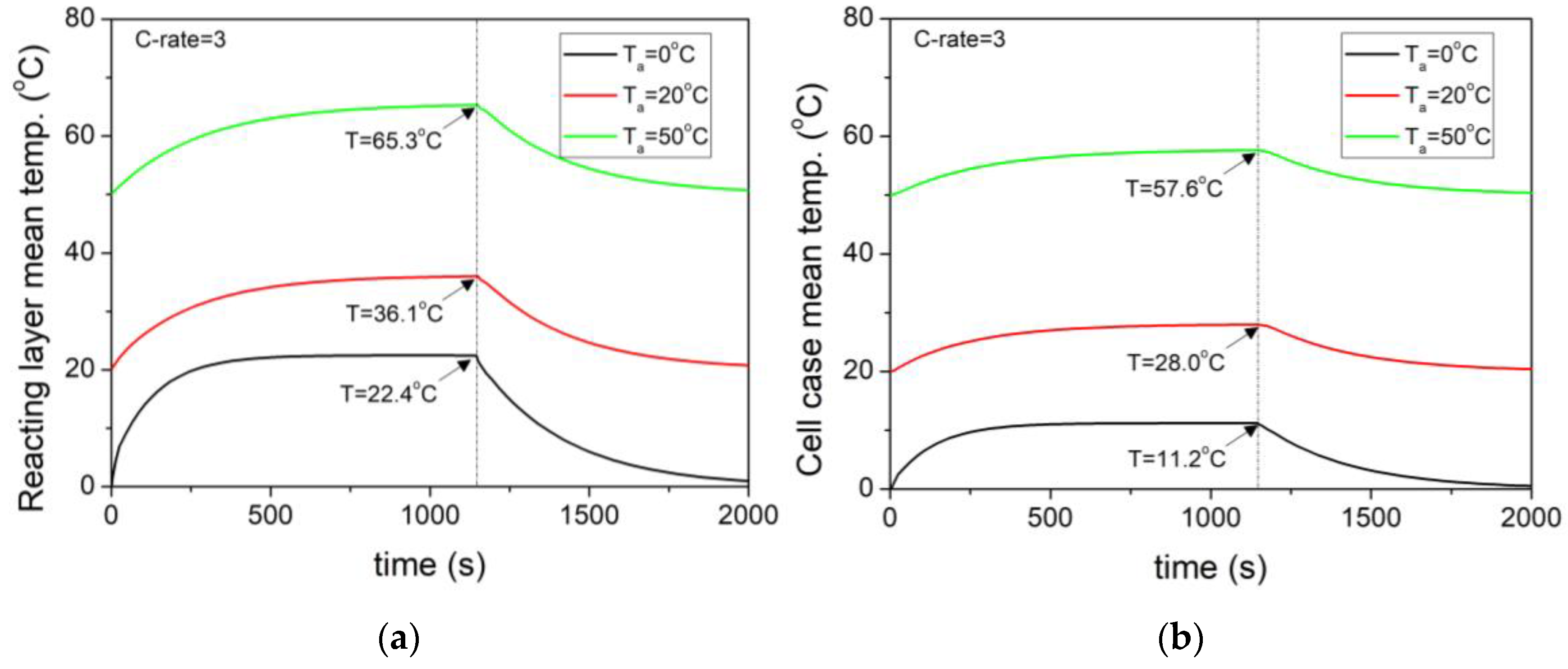
- At an external temperature of 20 °C, the heat generation increased proportionally to the square of the current as the C–rate increased. For 3C, the reaction heat source was 45.5 W, and the average internal temperature of the cell was 36 °C.
- Even at the same 3C, as the external temperature decreased to 0 °C, the increase in internal resistance led to a higher reaction heat source of 58.27 W, which was 36.9% higher than that at 20 °C.
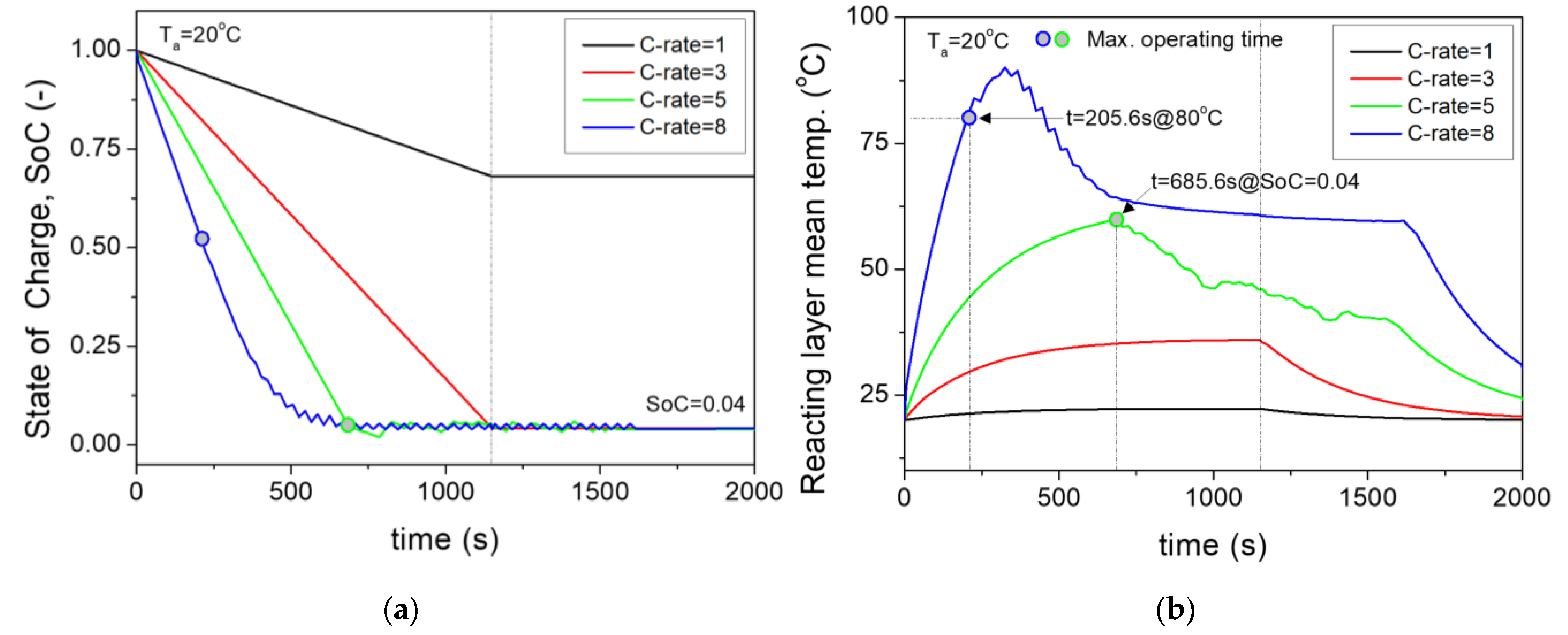
- The cell in this study was designed with a maximum operating condition of an internal cell temperature of 80 °C. At 5C, the maximum operating time was 685.6 s. At this point, the average internal temperature of the cell was 59.8 °C, allowing for normal operation. When the C–rate of the battery cell reached 8C, which was the momentary maximum high–discharge condition, the temperature rose sharply before the reached 0. With an internal average cell temperature of 80 °C, the maximum operating time became 205.6 s. This met the design requirements for UAM in this study.
Funding
Data Availability Statement
Conflicts of Interest
References
- Garrow, L.A.; German, B.J.; Leonard, C.E. Urban air mobility: A Comprehensive Review and Comparative Analysis with Autonomous and Electric Ground Transportation for Informing Future Research. Transp. Res. Part C Emerg. Technol. 2021, 132, 103377. [Google Scholar] [CrossRef]
- Qiao, X.; Chen, G.; Lin, W.; Zhou, J. The Impact of Battery Performance on Urban Air Mobility Operations. Aerospace 2023, 10, 631. [Google Scholar] [CrossRef]
- Liu, T.; Yang, X.G.; Ge, S.; Leng, Y.; Wang, C.Y. Ultrafast Charging of Energy-Dense Lithium-ion Batteries for Urban Air Mobility. eTransportation 2021, 7, 100103. [Google Scholar] [CrossRef]
- Yi, X.; Rao, A.M.; Zhou, J. Trimming the Degrees of Freedom via a K+ Flux Rectifier for Safe and Long-Life Potassium-Ion Batteries. Nano-Micro Lett. 2023, 15, 200. [Google Scholar] [CrossRef]
- Sanad, M.M.; Meselhy, N.K.; El-Boraey, H.A. Surface Protection of NMC811 Cathode Material via ZnSnO3 Perovskite Film for Enhanced Electrochemical Performance in Rechargeable Li-ion batteries. Colloids Surf. A Physicochem. Eng. Asp. 2023, 672, 131748. [Google Scholar] [CrossRef]
- Sanad, M.M.; Meselhy, N.K.; El-Boraey, H.A.; Toghan, A. Controllable engineering of new ZnAl2O4-decorated LiNi0·8Mn0·1Co0·1O2 Cathode Materials for High Performance Lithium-ion Batteries. J. Mater. Res. Technol. 2023, 23, 1528–1542. [Google Scholar] [CrossRef]
- Srinivasan, V.; Wang, C.Y. Analysis of Electrochemical and Thermal Behavior of Li-Ion Cells. J. Electrochem. Soc. 2002, 150, 98–106. [Google Scholar] [CrossRef]
- Zhang, X. Thermal Analysis of a Cylindrical Lithium-ion Battery. Electrochim. Acta 2011, 56, 1246–1255. [Google Scholar] [CrossRef]
- Jeon, D.H. Numerical Modeling of Lithium Ion Battery for Predicting Thermal Behavior in a Cylindrical Cell. Curr. Appl. Phys. 2014, 14, 196–205. [Google Scholar] [CrossRef]
- Lechermann, L.; Kleiner, J.; Komsiyska, L.; Hinterberger, M.; Endisch, C. A Comparative Study of Data-driven Electro-thermal Models for Reconfigurable Lithium-ion Batteries in Real-time Applications. J. Energy Storage 2023, 65, 107188. [Google Scholar] [CrossRef]
- Dubarry, M.; Vuillaume, N.; Liaw, B.Y. From Single Cell Model to Battery Pack Simulation for Li-ion Batteries. J. Power Sources 2007, 186, 500–507. [Google Scholar] [CrossRef]
- Chiang, Y.H.; Sean, W.Y.; Ke, J.C. Online Estimation of Internal Resistance and Open-Circuit Voltage of Lithium-Ion Batteries in Electric Vehicles. J. Power Sources 2011, 196, 3921–3932. [Google Scholar] [CrossRef]
- Dubarry, M.; Liaw, B.Y. Development of A Universal Modeling Tool for Rechargeable Lithium Batteries. J. Power Sources 2007, 174, 856–860. [Google Scholar] [CrossRef]
- Hu, Y.; Yurkovich, S.; Guezennec, Y.; Yurkovich, B.J. A Technique for Dynamic Battery Model identification in Automotive Applications Using Linear Parameter Varying Structures. Control Eng. Pract. 2009, 17, 1190–1201. [Google Scholar] [CrossRef]
- Andre, D.; Meiler, M.; Steiner, K.; Wimmer, C.; Soczka-Guth, T.; Sauer, D.U. Characterization of High-Power Lithium-Ion Batteries by Electrochemical Impedance Spectroscopy. I. Experimental Investigation. J. Power Sources 2011, 196, 5334–5341. [Google Scholar] [CrossRef]
- Li, X.; He, F.; Ma, L. Thermal Management of Cylindrical Batteries Investigated using Wind Tunnel Testing and Computational Fluid Dynamics Simulation. J. Power Sources 2013, 238, 395–402. [Google Scholar] [CrossRef]
- Cicconi, P.; Landi, D.; Germani, M. Thermal Analysis and Simulation of a Li-ion Battery Pack for a Lightweight Commercial EV. Appl. Energy 2017, 192, 159–177. [Google Scholar] [CrossRef]
- Kim, Y.; Mohan, S.; Siegel, J.B.; Stefanopoulou, A.G.; Ding, Y. The Estimation of Temperature Distribution in Cylindrical Battery Cells Under Unknown Cooling Conditions. IEEE Trans. Control Syst. Technol. 2014, 22, 2277–2286. [Google Scholar]
- Saw, L.H.; Ye, Y.; Tay, A.A.O. Electro-thermal Characterization of Lithium Iron Phosphate Cell with Equivalent Circuit Modeling. Energy Convers. Manag. 2014, 87, 367–377. [Google Scholar] [CrossRef]
- Wang, Z.; Ma, J.; Zhang, L. Finite Element Thermal Model and Simulation for a Cylindrical Li-Ion Battery. IEEE Access 2017, 5, 15372–15379. [Google Scholar] [CrossRef]
- Huang, B.; Hu, M.; Chen, L.; Jin, G.; Liao, S.; Fu, C.; Wang, D.; Cao, K. A Novel Electro-Thermal Model of Lithium-Ion Batteries Using Power as the Input. Electronics 2021, 10, 2753. [Google Scholar] [CrossRef]
- Barcellona, S.; Colnago, S.; Montrasio, P.; Piegari, L. Integrated Electro-Thermal Model for Li-Ion Battery Packs. Electronics 2022, 11, 1537. [Google Scholar] [CrossRef]
- AVL. FIRE M User Manual 2020R 2. Available online: https://www.avl.com/cruise-m (accessed on 11 August 2022).
- Doh, C.H.; Ha, Y.C.; Eom, S.W. Entropy Measurement of a Large Format Lithium Ion Battery and Its Application to Calculate Heat Generation. Electrochim. Acta 2019, 309, 382–391. [Google Scholar] [CrossRef]





| Electro–Chemical Model | Electro–Thermal Model |
|---|---|
|
|
| Items | Specification |
|---|---|
| Nominal capacity | 40 Ah |
| Nominal voltage | 3.7 V |
| Energy density | 230 Wh/kg |
| Maximum charge voltage | 4.25 V |
| Cut–off voltage | 2.75 V |
| Material system | Cathode: NMC–811 Anode: Graphite Electrolyte: Carbonate–based |
| Standard discharging method | Const. current/const. voltage |
| Maximum continuous discharge current | 5C |
| Positive Collector | Negative Collector | Reaction Layer | Cell Case | |
|---|---|---|---|---|
| Material | Aluminum | Coper | Cell | Aluminum |
| Density (kg/m3) | 2700 | 8960 | 2400 | 2700 |
| Specific heat capacity (J/(kg·K)) | 1500 | |||
| Thermal conductivity (W/(m·K)) | 236 | 401 | 0.5/30/30 | 236 |
| Electrical conductivity (A/(V·m)) | 5.96 × 107 | 5.96 × 107 | 1 × 109 | 5.96 × 107 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, G. Electro-Thermal Analysis of a Pouch–Type Lithium–Ion Battery with a High Discharge Rate for Urban Air Mobility. Batteries 2023, 9, 476. https://doi.org/10.3390/batteries9090476
Lee G. Electro-Thermal Analysis of a Pouch–Type Lithium–Ion Battery with a High Discharge Rate for Urban Air Mobility. Batteries. 2023; 9(9):476. https://doi.org/10.3390/batteries9090476
Chicago/Turabian StyleLee, Geesoo. 2023. "Electro-Thermal Analysis of a Pouch–Type Lithium–Ion Battery with a High Discharge Rate for Urban Air Mobility" Batteries 9, no. 9: 476. https://doi.org/10.3390/batteries9090476






