Recovery of Graphite from Spent Lithium-Ion Batteries
Abstract
:1. Introduction
2. Results and Discussion
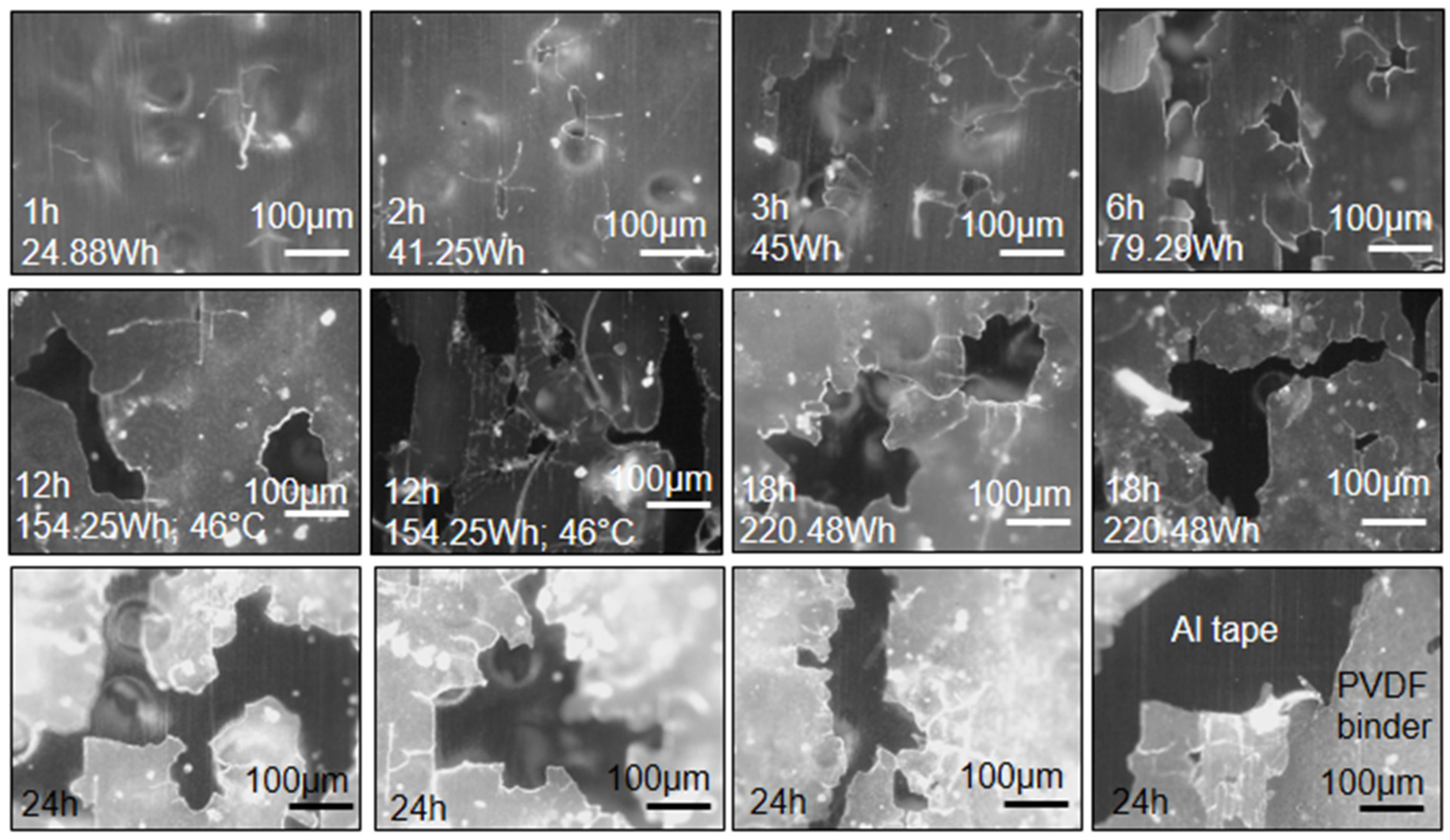
2.1. Polyvinylidene Fluoride (PVDF) Binder Removal Trials
2.2. Graphite Recovery Process
3. Methodology
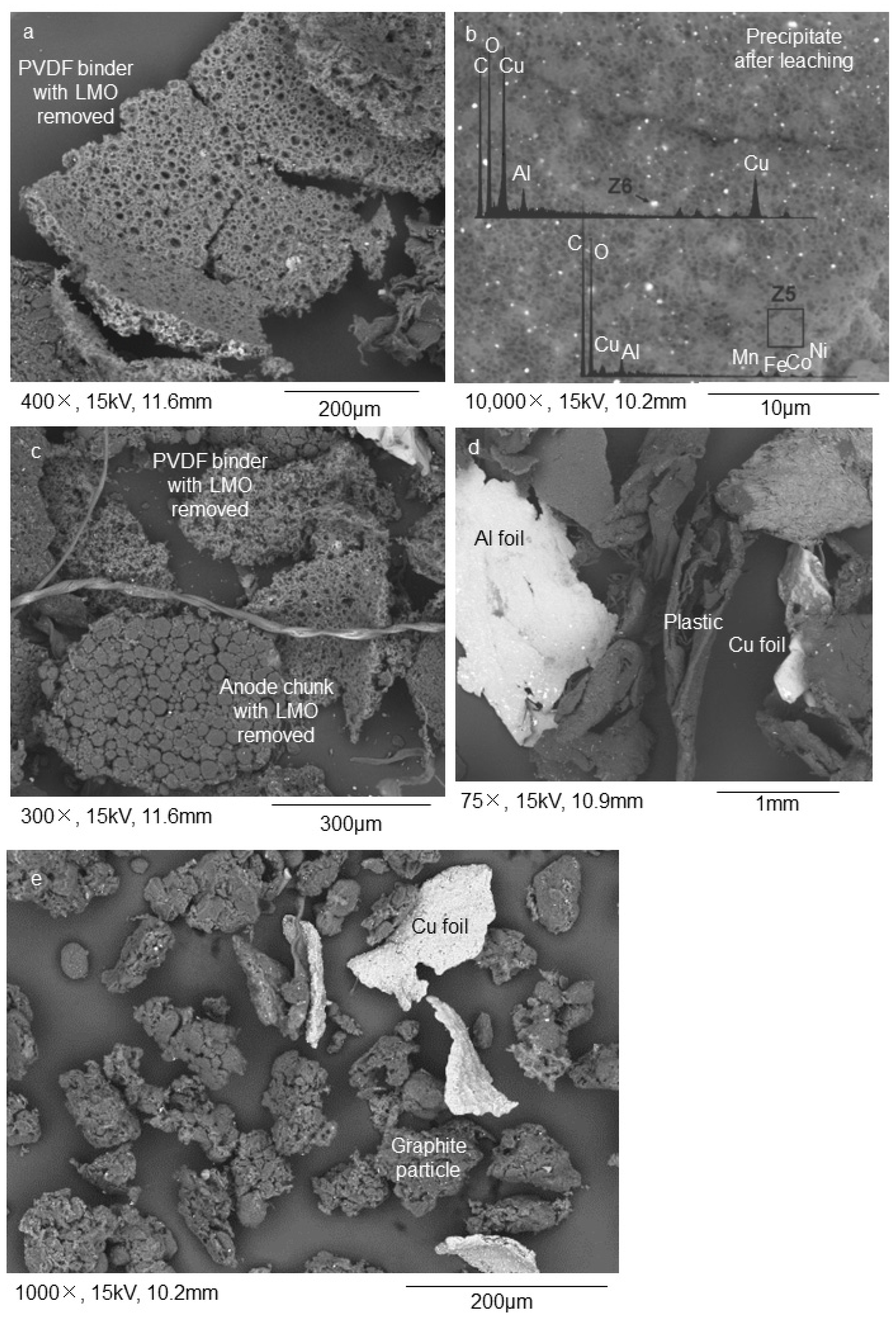
3.1. Sample Characterization
- graphite (includes dense and flake morphotypes);
- binder mixture (binder and small impurities embedded in the binder);
- foils (Al and Cu);
- organic carbon (plastics and other carbonaceous material that is fluorescent under ultraviolet light);
- LMO (includes blue spherical particles described as Li-Mn-O, white spherical particles described as Li-Ni-Co-O and Li-Ni-Mn-Co-O, and blue angular particles described as Li-Co-O);
- other (includes Fe and Si).
3.2. Trials for Removing PVDF Binder
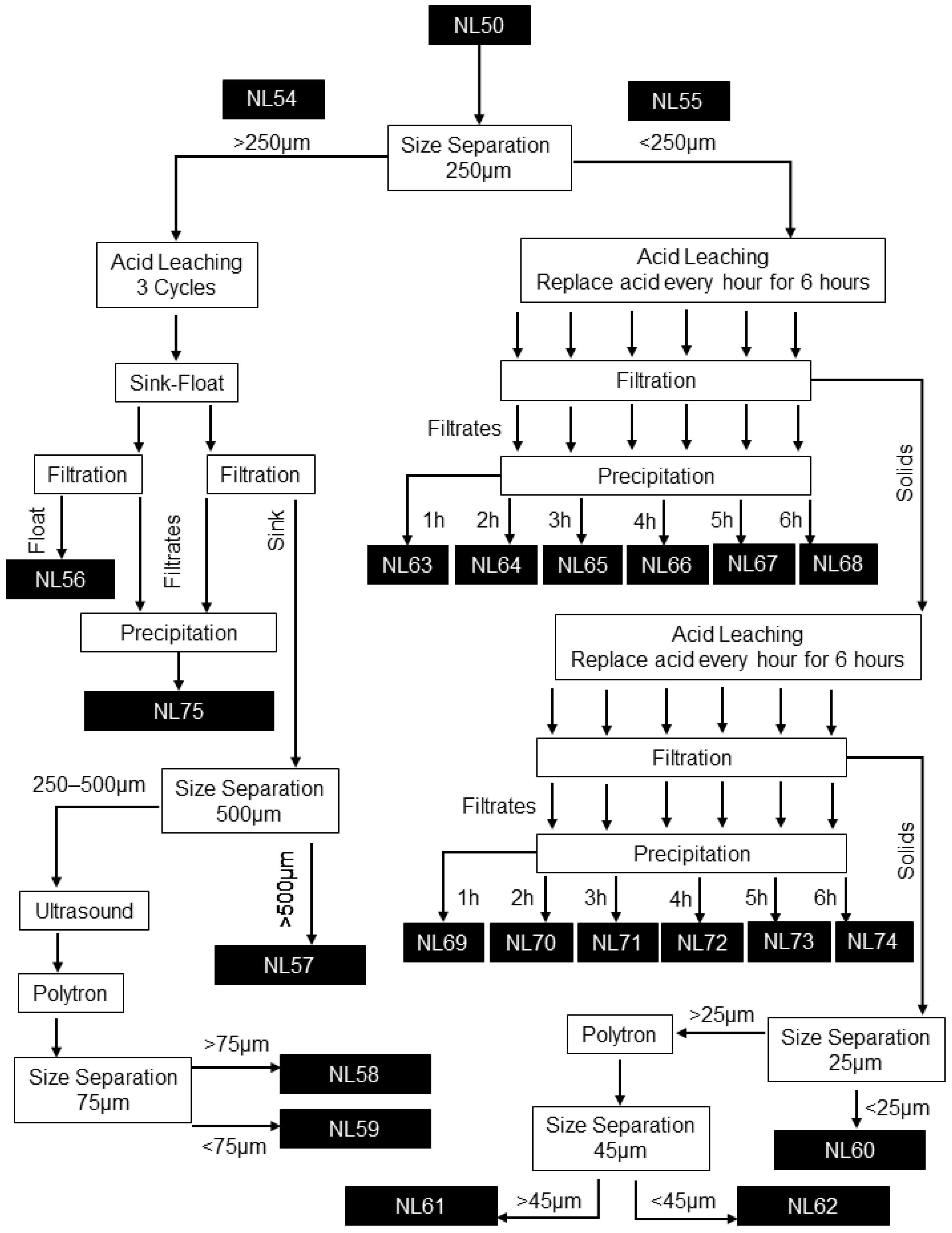
3.3. Graphite Recovery Process Flow Diagram
4. Conclusions
- a > 500 µm concentrate of Al and Cu foil fragments (18.8 wt.% yield);
- usage of ultrasound to tear and peel PVDF binder from LIB and to exfoliate particle surfaces to increase separation efficiency;
- <25 µm, <45 µm and <75 µm graphite-rich products with 88 wt.%, 85 wt.% and 74 wt.% total carbon and 5.6, 7.2, and 1.5 wt.% yields, respectively;
- precipitated products enriched in Li, Co, Mn, and Ni.
- Future tests include:
- improvement of the products’ yields;
- testing of graphite for possible re-use in manufacturing new LIB and/or other applications;
- testing of the precipitated products as carbon polymers and for recovering Li, Co, Mn, and Ni; and
- testing Al and Cu recovery through an electrostatic separator.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Velázquez-Martínez, O.; Valio, J.; Santasalo-Aarnio, A.; Reuter, M.; Serna-Guerrero, R. A critical review of lithium-ion battery recycling processes from a circular economy perspective. Batteries 2019, 5, 68. [Google Scholar] [CrossRef]
- Zhang, Z.; He, W.; Li, G.; Xia, J.; Hu, H.; Huang, J. Ultrasound-assisted Hydrothermal Renovation of LiCoO2 from the Cathode of Spent Lithium-ion Batteries. Int. J. Electrochem. Sci. 2014, 9, 3691–3700. [Google Scholar]
- Silveira, A.; Santana, M.; Tanabe, E.; Bertuol, D. Recovery of valuable materials from spent lithium ion batteries using electrostatic separation. Int. J. Miner. Process. 2017, 169, 91–98. [Google Scholar] [CrossRef]
- Bertuol, D.A.; Toniasso, C.; Jiménez, B.M.; Meili, L.; Dotto, G.L.; Tanabe, E.H.; Aguiar, M.L. Application of spouted bed elutriation in the recycling of lithium ion batteries. J. Power Sources 2015, 275, 627–632. [Google Scholar] [CrossRef]
- Zhang, W.; Xu, C.; He, W.; Li, G.; Huang, J. A review on management of spent lithium ion batteries and strategy for resource recycling of all components from them. Waste Manag. Res. J. Sustain. Circ. Econ. 2017, 36, 99–112. [Google Scholar] [CrossRef]
- Zhu, S.; He, W.; Li, G.; Zhou, X.; Huang, J.; Zhang, X. Recovering copper from spent Lithium ion battery by a mechanical separation process. In Proceedings of the 2011 International Conference on Materials for Renewable Energy & Environment, Shangai, China, 20–22 May 2011; pp. 1008–1012. [Google Scholar] [CrossRef]
- Vanderbruggen, A.; Sygusch, J.; Rudolph, M.; Serna-Guerrero, R. A contribution to understanding the flotation behavior of lithium metal oxides and spheroidized graphite for lithium-ion battery recycling. Colloids Surfaces A Physicochem. Eng. Asp. 2021, 626, 127111. [Google Scholar] [CrossRef]
- Yu, J.; He, Y.; Ge, Z.; Li, H.; Xie, W.; Wang, S. A promising physical method for recovery of LiCoO 2 and graphite from spent lithium-ion batteries: Grinding flotation. Sep. Purif. Technol. 2018, 190, 45–52. [Google Scholar] [CrossRef]
- Javorsky da Costa, A.; Matos, J.F.; Bernardes, A.M.; Müller, I.L. Beneficiation of cobalt, copper and aluminium from wasted lithium-ion batteries by mechanical processing. Int. J. Miner. Process 2015, 145, 77–82. [Google Scholar] [CrossRef]
- Jin, Y.J.; Mei, G.J.; Li, S.Y. Leaching of cobalt from LiCoO2 cathode in spent lithium-ion batteries with sulfuric acid by ultrasonic. Hydrometall. China 2006, 25, 97–99. (In Chinese) [Google Scholar]
- Meng, F.; Liu, Q.; Kim, R.; Wang, J.; Liu, G.; Ghahreman, A. Selective recovery of valuable metals from industrial waste lithium-ion batteries using citric acid under reductive conditions: Leaching optimization and kinetic analysis. Hydrometallurgy 2019, 191, 105160. [Google Scholar] [CrossRef]
- Xu, M.; Kang, S.; Jiang, F.; Yan, X.; Zhu, Z.; Zhao, Q.; Teng, Y.; Wang, Y. A process of leaching recovery for cobalt and lithium from spent lithium-ion batteries by citric acid and salicylic acid. RSC Adv. 2021, 11, 27689. [Google Scholar]
- Pant, D.; Dolker, T. Green and facile method for the recovery of spent Lithium Nickel Manganese Cobalt Oxide (NMC) based Lithium ion batteries. J. Waste Manag. 2017, 60, 689–695. [Google Scholar]
- Bahaloo-Horeh, N.; Mousavi, S.M. Enhanced recovery of valuable metals from spent lithium-ion batteries through optimization of organic acids produced by Aspergillus niger. Waste Manag. 2017, 60, 666–679. [Google Scholar] [CrossRef]
- Zhang, G.; He, Y.; Wang, H.; Feng, Y.; Xie, W.; Zhu, X. Application of mechanical crushing combined with pyrolysis-enhanced flotation technology to recover graphite and LiCoO2 from spent lithium-ion batteries. J. Clean. Prod. 2019, 231, 1418–1427. [Google Scholar] [CrossRef]
- Mousa, E.; Hu, X.; Ånnhagen, L.; Ye, G.; Cornelio, A.; Fahimi, A.; Bontempi, E.; Frontera, P.; Badenhorst, C.; Santos, A.C.; et al. Characterization and Thermal Treatment of the Black Mass from Spent Lithium-Ion Batteries. Sustainability 2023, 15, 15. [Google Scholar] [CrossRef]
- Lee, C.K.; Rhee, K.-I. Preparation of LiCoO2 from spent lithium-ion batteries. J. Power Sources 2002, 109, 17–21. [Google Scholar] [CrossRef]
- He, Y.; Yuan, X.; Zhang, G.; Wang, H.; Zhang, T.; Xie, W.; Li, L. A critical review of current technologies for the liberation of electrode materials from foils in the recycling process of spent lithium-ion batteries. Sci. Total Environ. 2021, 766, 142382. [Google Scholar] [CrossRef]
- Chen, X.; Ma, H.; Luo, C.; Zhou, T. Recovery of valuable metals from waste cathode materials of spent lithium-ion batteries using mild phosphoric acid. J. Hazard. Mater. 2017, 326, 77–86. [Google Scholar] [CrossRef]
- Li, L.; Qu, W.; Zhang, X.; Lu, J.; Chen, R.; Wu, F.; Amine, K. Succinic acid-based leaching system: A sustainable process for recovery of valuable metals from spent Li-ion batteries. J. Power Sources 2015, 282, 544–551. [Google Scholar] [CrossRef]
- Sandhya, C.P.; John, B.; Gouri, C. Synthesis, characterization and electrochemical evaluation of mixed oxides of nickel and cobalt from spent lithium-ion cells. RSC Adv. 2016, 6, 114192–114197. [Google Scholar]
- Xin, Y.; Guo, X.; Chen, S.; Wang, J.; Wu, F.; Xin, B. Bioleaching of valuable metals Li, Co, Ni and Mn from spent electric vehicle Li-ion batteries for the purpose of recovery. J. Clean. Prod. 2016, 116, 249–258. [Google Scholar] [CrossRef]
- Song, D.; Wang, X.; Zhou, E.; Hou, P.; Guo, F.; Zhang, L. Recovery and heat treatment of the Li(Ni1/3Co1/3Mn1/3)O2 cathode scrap material for lithium ion battery. J. Power Sources 2013, 232, 348–352. [Google Scholar] [CrossRef]
- Xu, Y.; Song, D.; Li, L.; An, C.; Wang, Y.; Jiao, L.; Yuan, H. A simple solvent method for the recovery of LixCoO2 and its applications in alkaline rechargeable batteries. J. Power Sources 2014, 252, 286–291. [Google Scholar] [CrossRef]
- Song, X.; Hu, T.; Liang, C.; Long, H.L.; Zhou, L.; Song, W.; You, L.; Wu, Z.S.; Liu, J.W. Direct regeneration of cathode materials from spent lithium iron phosphate batteries using a solid phase sintering method. RSC Adv. 2017, 7, 4783–4790. [Google Scholar] [CrossRef]
- He, L.-P.; Sun, S.-Y.; Mu, Y.-Y.; Song, X.-F.; Yu, J.-G. Recovery of Lithium, Nickel, Cobalt, and Manganese from Spent Lithium-Ion Batteries Using l-Tartaric Acid as a Leachant. ACS Sustain. Chem. Eng. 2017, 5, 714–721. [Google Scholar] [CrossRef]
- Nayaka, G.P.; Pai, K.V.; Manjanna, J.; Keny, S.J. Use of mild organic acid reagents to recover the Co and Li from spent Li-ion batteries. Waste Manag. 2016, 51, 234–238. [Google Scholar] [CrossRef]
- Yang, L.; Xi, G.; Lou, T.; Wang, X.; Wang, J.; He, Y. Preparation and magnetic performance of Co0.8Fe2.2O4 by a sol–gel method using cathode materials of spent Li-ion batteries. Ceram. Int. 2016, 42, 1897–1902. [Google Scholar] [CrossRef]
- Zhang, G.; He, Y.; Feng, Y.; Wang, H.; Zhu, X. Pyrolysis-ultrasonic-assisted flotation technology for recovering graphite and LiCoO2 from spent Lithium-ion battery. ASC Sustain. Chem. Eng. 2018, 6, 10896–10904. [Google Scholar]
- Liu, J.; Wang, H.; Hu, T.; Bai, X.; Wang, S.; Xie, W.; Hao, J.; He, Y. Recovery of LiCoO2 and graphite from spent lithium-ion batteries by cryogenic grinding and froth flotation. Miner. Eng. 2020, 148, 106223. [Google Scholar] [CrossRef]
- Yang, L.; Xu, G.; Feng, Q.; Li, Y.; Zhao, E.; Ma, J.; Fan, S.; Li, X. Separation and recovery of carbon powder in anodes from spent lithium-ion batteries to synthesize graphene. Sci. Rep. 2019, 9, 9823. [Google Scholar] [CrossRef]
- ISO (International Organisation for Standardisation). ISO 7404-2 Methods for the Petrographic Analysis of Coals. Part 2: Methods of Preparing Coal Samples; ISO: Geneva, Switzerland, 2009. [Google Scholar]
- Gottlieb, P.; Wilkie, G.; Sutherland, D.; Ho-Tun, E.; Suthers, S.; Perera, K.; Jenkins, B.; Spencer, S.; Butcher, A.; Rayner, J. Using quantitative electron microscopy for process mineralogy applications. JOM 2000, 52, 24–25. [Google Scholar] [CrossRef]
- Goodall, W.R.; Scales, P.J.; Butcher, A.R. The use of QEMSCAN and diagnostic leaching in the characterisation of visible gold in complex ores. Miner. Eng. 2005, 18, 877–886. [Google Scholar] [CrossRef]
- Goodall, W.R.; Scales, P.J. An overview of the advantages and disadvantages of the determination of gold mineralogy by automated mineralogy. Miner. Eng. 2007, 20, 506–517. [Google Scholar] [CrossRef]
- Rollinson, G.K.; Andersen, J.C.Ø.; Stickland, R.J.; Boni, M.; Fairhurst, R. Characterisation of non-sulphide zinc deposits using QEMSCAN®. Miner. Eng. 2011, 24, 778–787. [Google Scholar] [CrossRef]
- Dadé, M.; Wallmach, T.; Laugier, O. Detailed Microparticle Analyses Providing Process Relevant Chemical and Microtextural Insights into the Black Mass. Minerals 2022, 12, 119. [Google Scholar] [CrossRef]
- Handel, P.; Stangl, C.; God, C.; Filzwieser, M.; Uhlig, F.; Schroettner, H.; Koller, S. About Microwave Solvothermal Synthesis of Solid Solutions LiFexMn1-xPO4/C and Their Electrochemical Properties. ECS Trans. 2015, 66, 127–137. [Google Scholar] [CrossRef]
- Santee, S.G.; Ravdel, B.; Gulbinska, M.K.; Gnanaraj, J.S.; DiCarlo, J.F. Optimizing electrodes for lithiu-ion cells. In Lithium-Ion Battery Materials and Engineering—Current Topics and Problems from the Manufacturing Perspective; Gulbinska, M.K., Ed.; Springer: London, UK, 2014; pp. 63–88. [Google Scholar] [CrossRef]
- Wood, D.L.; Quass, J.D.; Li, J.; Ahmed, S.; Ventola, D.; Daniel, C. Technical and economic analysis of solvent-based lithium-ion electrode drying with water and NMP. Dry. Technol. 2017, 36, 234–244. [Google Scholar] [CrossRef]


| NL50 | NL54 | NL55 | NL56 | NL57 | NL58 | NL59 | NL60 | NL61 | NL62 | NL63 | NL64 | NL65 | NL66 | NL67 | NL68 | NL69 | NL70 | NL71 | NL72 | NL73 | NL74 | NL75 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| XRF—elemental composition (normalized %) | |||||||||||||||||||||||
| Cu | 23.06 | 40.13 | 3.90 | 3.43 | 76.91 | 62.76 | 61.25 | 0.98 | 2.16 | 1.98 | 7.60 | 9.97 | 8.59 | 6.04 | 7.71 | 2.96 | 0.60 | 0.65 | 0.50 | 0.00 | 0.00 | 0.00 | 19.43 |
| Ni | 31.52 | 22.58 | 41.49 | 20.61 | 5.42 | 10.03 | 13.44 | 6.79 | 12.65 | 9.28 | 14.22 | 44.26 | 49.73 | 57.86 | 51.39 | 37.06 | 66.61 | 59.75 | 49.17 | 42.38 | 31.58 | 26.95 | 26.77 |
| Co | 12.28 | 8.29 | 17.23 | 5.54 | 1.31 | 2.66 | 1.99 | 35.60 | 13.43 | 20.96 | 6.65 | 19.09 | 25.04 | 19.43 | 21.07 | 13.83 | 20.98 | 22.65 | 25.59 | 29.14 | 33.08 | 35.93 | 10.97 |
| Si | 1.94 | 2.51 | 1.70 | 48.75 | 4.78 | 8.74 | 12.15 | 20.14 | 25.55 | 23.98 | 2.69 | 0.00 | 0.00 | 0.00 | 0.00 | 29.64 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Fe | 6.38 | 5.92 | 5.28 | 4.87 | 1.77 | 1.41 | 2.69 | 20.61 | 13.76 | 24.51 | 9.28 | 5.07 | 3.58 | 4.32 | 5.14 | 4.94 | 0.99 | 2.16 | 5.02 | 0.00 | 15.04 | 0.00 | 22.03 |
| Mn | 20.93 | 14.61 | 27.29 | 4.65 | 1.13 | 2.03 | 0.48 | 1.92 | 4.72 | 2.82 | 55.38 | 17.91 | 7.87 | 7.34 | 7.19 | 4.94 | 9.54 | 12.08 | 13.55 | 13.25 | 0.00 | 0.00 | 18.87 |
| XRF—oxide composition (normalized %) | |||||||||||||||||||||||
| SiO2 | 4.79 | 4.01 | 5.89 | 60.09 | 4.08 | 12.91 | 21.44 | 31.74 | 35.43 | 32.76 | 4.74 | 0.00 | 0.00 | 0.00 | 0.00 | 79.27 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Al2O3 | 46.34 | 69.07 | 15.69 | 7.54 | 89.48 | 71.68 | 63.91 | 27.56 | 23.34 | 25.81 | 10.80 | 15.38 | 28.57 | 37.74 | 20.41 | 6.10 | 17.14 | 19.42 | 17.86 | 0.00 | 0.00 | 0.00 | 20.78 |
| FeO | 9.45 | 5.64 | 10.97 | 3.58 | 0.93 | 1.25 | 2.87 | 19.54 | 11.46 | 19.85 | 10.54 | 15.38 | 28.57 | 18.87 | 20.41 | 6.10 | 11.43 | 9.71 | 17.86 | 41.67 | 90.91 | 0.00 | 39.62 |
| MnO | 31.10 | 14.01 | 56.87 | 3.48 | 0.58 | 1.81 | 0.51 | 1.85 | 3.92 | 2.32 | 62.96 | 52.69 | 41.43 | 41.51 | 36.73 | 7.93 | 70.86 | 69.90 | 62.50 | 54.17 | 0.00 | 0.00 | 34.03 |
| ICP-OES (mg/kg) | |||||||||||||||||||||||
| Li | 19,850 | 12,888 | 25,272 | 2057 | 1469 | 1791 | 782 | 765 | 883 | 545 | 2710 | 500 | 229 | 204 | 151 | 108 | 677 | 288 | 101 | 34 | 25 | 11 | 4877 |
| NL50 | NL54 | NL55 | NL56 | NL57 | NL58 | NL59 | NL60 | NL61 | NL62 | |
|---|---|---|---|---|---|---|---|---|---|---|
| Total carbon analysis (wt.%) | ||||||||||
| Total carbon | 35.27 | 22.24 | 38.67 | 80.01 | 28.08 | 56.74 | 73.96 | 85.36 | 80.30 | 88.01 |
| TGA (wt.%) | ||||||||||
| Graphitic carbon | - | - | - | - | - | 30.37 | 66.67 | 83.33 | 53.45 | 65.13 |
| Organic carbon | - | - | - | - | - | 35.08 | 17.59 | 16.67 | 45.40 | 24.34 |
| Non-combustible | - | - | - | - | - | 34.55 | 15.74 | 0.00 | 1.15 | 10.53 |
| Petrographic analysis (vol.%) | ||||||||||
| Graphitic carbon | 39.8 | 15.6 | 49.0 | 17.5 | 11.8 | 25.9 | 60.9 | 78.5 | 26.7 | 57.6 |
| Binder-mixture | 20.0 | 24.2 | 14.9 | 33.6 | 23.8 | 35.8 | 32.4 | 20.8 | 39.6 | 36.2 |
| Organic carbon | 7.1 | 12.7 | 2.3 | 28.9 | 7.3 | 20.7 | 2.0 | 0.5 | 24.2 | 4.7 |
| LMO | 23.1 | 18.4 | 31.3 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| Foils | 5.8 | 15.7 | 0.4 | 0.0 | 52.7 | 3.6 | 2.9 | 0.0 | 0.2 | 0.2 |
| Other | 4.2 | 13.4 | 2.1 | 20.0 | 4.4 | 14.0 | 1.7 | 0.2 | 9.3 | 1.3 |
| NL56 | NL57 | NL59 | NL60 | NL61 | NL62 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| % | ||||||||||||
| <50 µm | >50 µm | <50 µm | >50 µm | <50 µm | >50 µm | <50 µm | >50 µm | <50 µm | >50 µm | <50 µm | >50 µm | |
| Anode | 0.61 | 0.00 | 0.09 | 0.00 | 0.17 | 0.00 | 0.23 | 0.00 | 0.50 | 0.00 | 0.29 | 0.00 |
| Cathode | 7.36 | 1.46 | 13.73 | 2.14 | 7.83 | 3.67 | 16.81 | 2.01 | 8.44 | 1.66 | 7.23 | 0.34 |
| Current collectors | 1.61 | 1.72 | 16.78 | 34.16 | 13.95 | 11.11 | 0.11 | 1.31 | 0.49 | 0.89 | 0.17 | 0.26 |
| Separator | 2.22 | 0.76 | 4.00 | 1.61 | 2.49 | 0.12 | 2.04 | 0.05 | 3.44 | 4.01 | 1.68 | 0.03 |
| Unspecified | 31.51 | 14.10 | 22.76 | 53.96 | 52.60 | 55.32 | 55.65 | 91.45 | 69.10 | 69.01 | 75.54 | 91.73 |
| Others | 56.70 | 81.96 | 42.63 | 8.14 | 22.96 | 29.78 | 25.16 | 5.19 | 18.03 | 24.42 | 15.09 | 7.64 |
| Components | Description | |
|---|---|---|
| Anode | Graphite | Carbon but with rounded shape, usually <25 μm each grain, with PVDF binder attached. |
| Cathode | LiCoO2 LiCoNiO2 LiMn2O4 LiNi0.5Mn1.5O4 LiNixMnyCozO2 LiFePO4 | LMO and Li electrolyte, which includes F. |
| Current collectors | Cu foil Al foil | Bright and usually long and folded. Al foils are used as support for LMO and Cu for graphite. This includes Cu, Cu-Al in foils, and wires and an Al-Ni-Cu phase. |
| Separator | Polymers | P phases; includes PVDF binder with F signature, separator membranes, and casing plastics. |
| Unspecified | AlO | This category includes mainly Al oxide and phases of Al with another element. Al oxide is a thin layer of material where we can find graphite, LMO, and other. |
| Other | AlSi | A miscellaneous association of materials including SiO2 glass, silicates, phosphates, and many other inorganic substances. These are materials resistant to citric acid, but their density is variable. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Badenhorst, C.; Kuzniarska-Biernacka, I.; Guedes, A.; Mousa, E.; Ramos, V.; Rollinson, G.; Ye, G.; Valentim, B. Recovery of Graphite from Spent Lithium-Ion Batteries. Recycling 2023, 8, 79. https://doi.org/10.3390/recycling8050079
Badenhorst C, Kuzniarska-Biernacka I, Guedes A, Mousa E, Ramos V, Rollinson G, Ye G, Valentim B. Recovery of Graphite from Spent Lithium-Ion Batteries. Recycling. 2023; 8(5):79. https://doi.org/10.3390/recycling8050079
Chicago/Turabian StyleBadenhorst, Charlotte, Iwona Kuzniarska-Biernacka, Alexandra Guedes, Elsayed Mousa, Violeta Ramos, Gavin Rollinson, Guozhu Ye, and Bruno Valentim. 2023. "Recovery of Graphite from Spent Lithium-Ion Batteries" Recycling 8, no. 5: 79. https://doi.org/10.3390/recycling8050079