Gravity Concentration in Urban Mining Applications—A Review
Abstract
:1. Introduction
2. Structure of the Paper
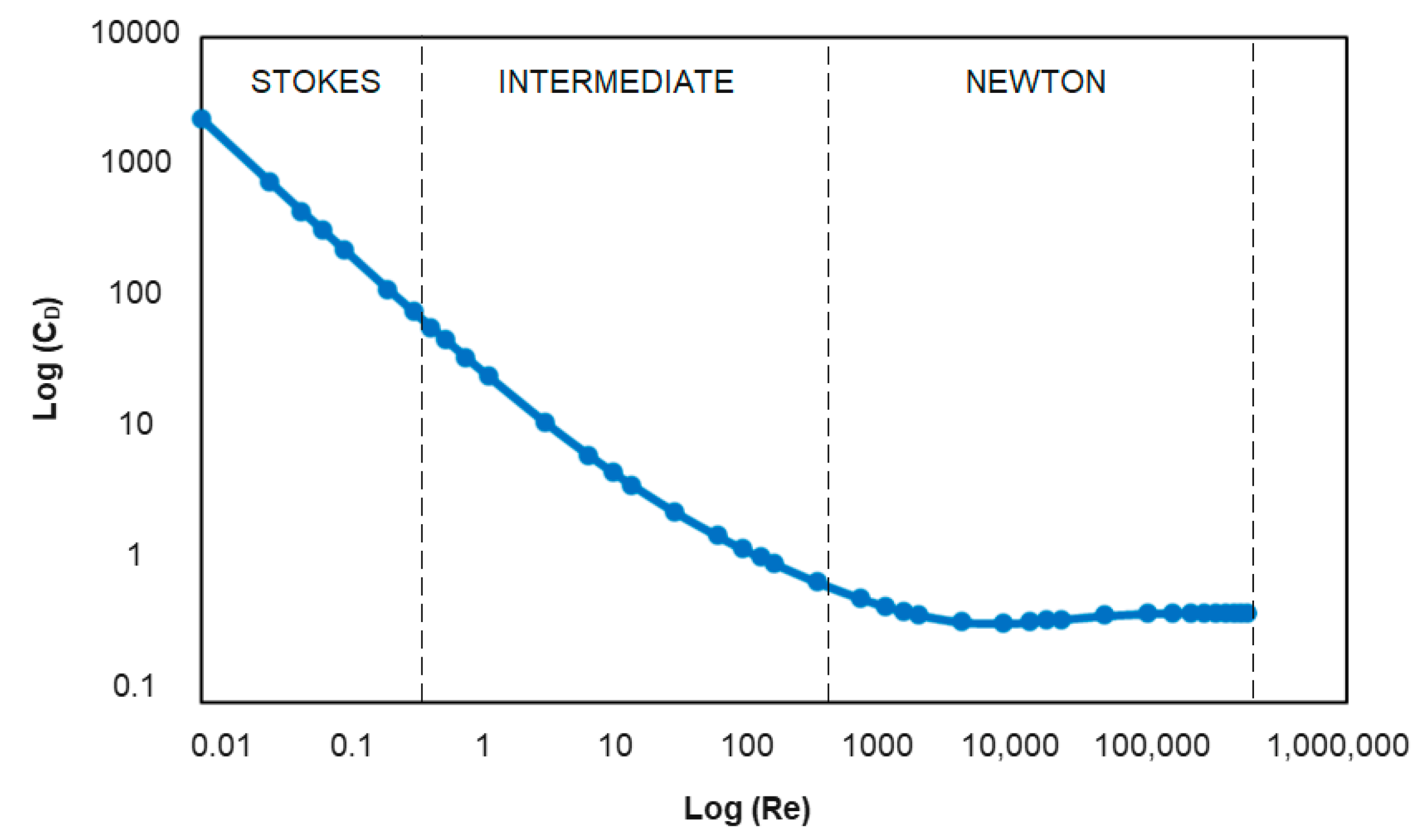
3. Gravity Concentration Fundamentals
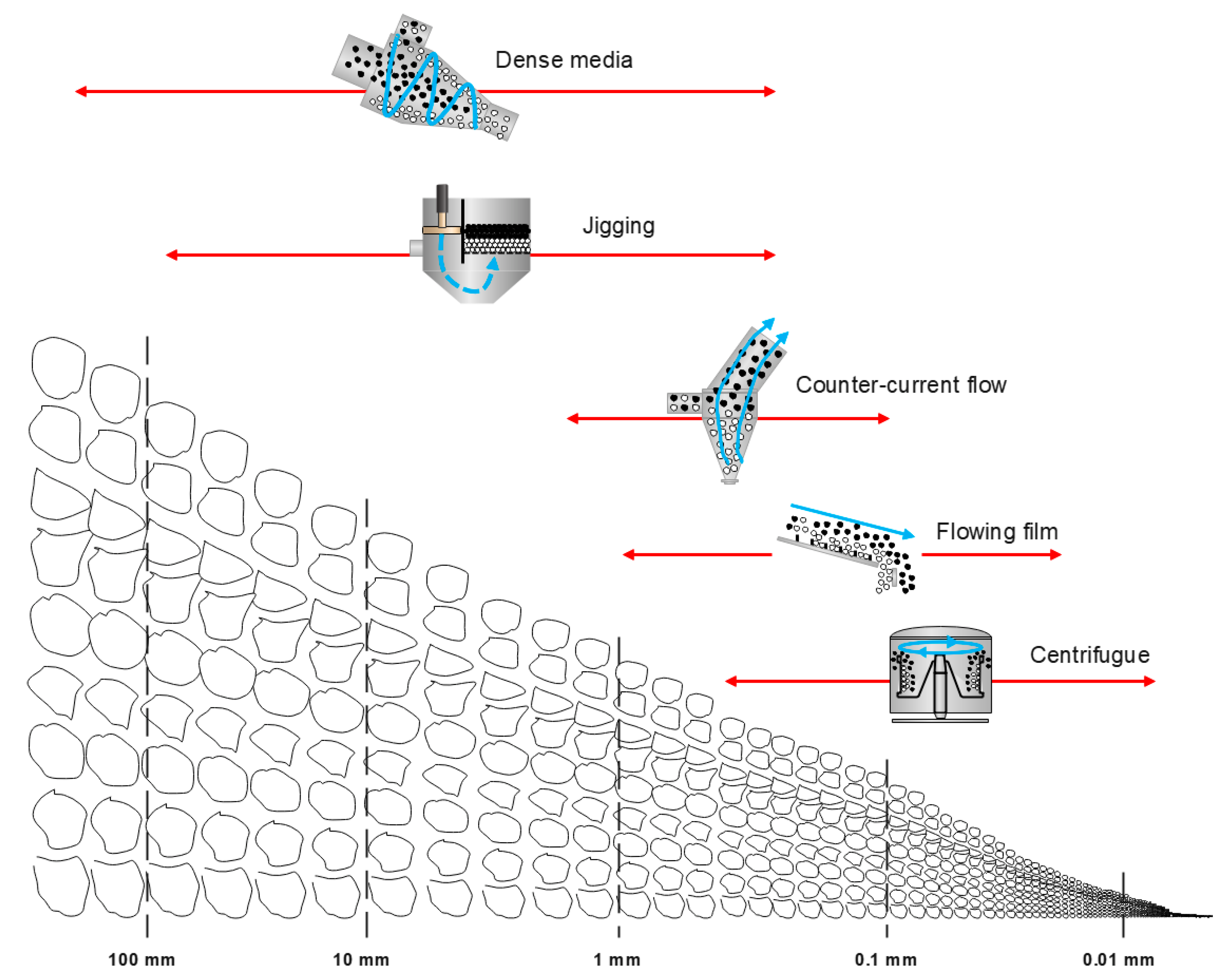
4. Gravity Concentration Techniques
4.1. Dense Media Separation
4.1.1. General Characteristics
4.1.2. Equipment
4.1.3. Advantages and Disadvantages
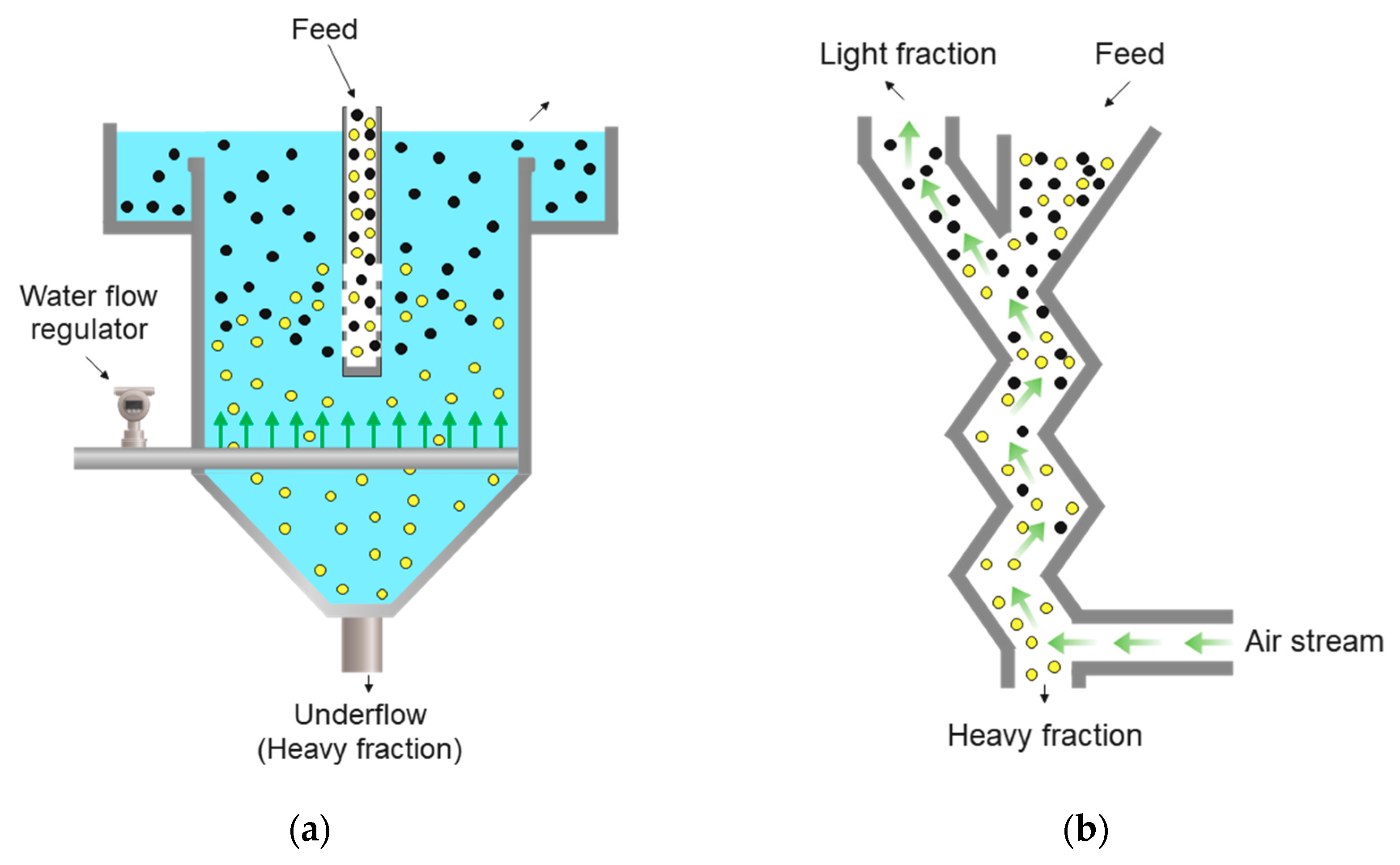
4.2. Counter-Current Flow Separation
4.2.1. General Characteristics
4.2.2. Equipment
4.2.3. Advantages and Disadvantages
4.3. Jigging
4.3.1. General Characteristics
4.3.2. Equipment
4.3.3. Advantages and Disadvantages
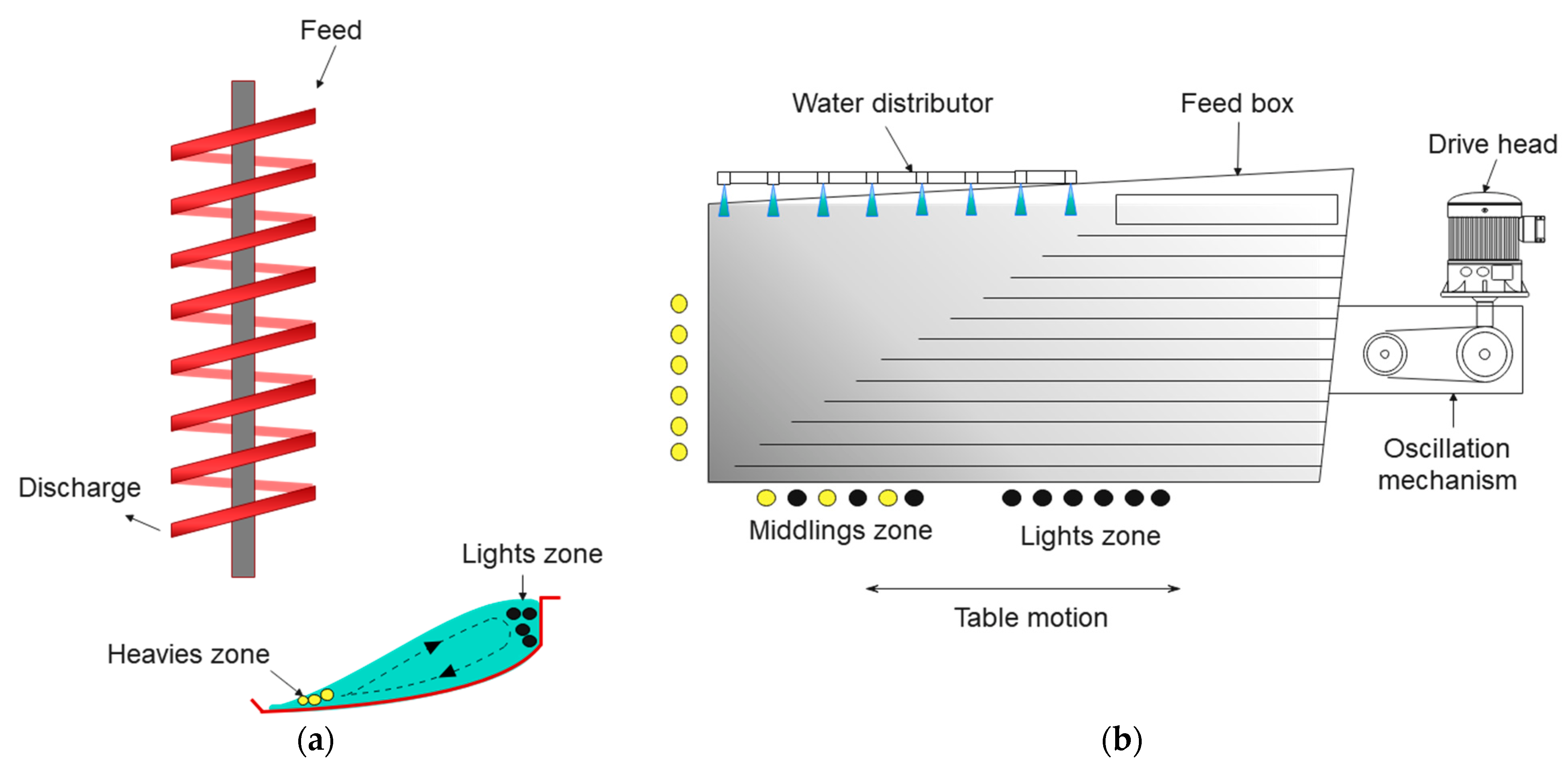
4.4. Flowing-Film Separation
4.4.1. General Characteristics
4.4.2. Equipment
4.4.3. Advantages and Disadvantages
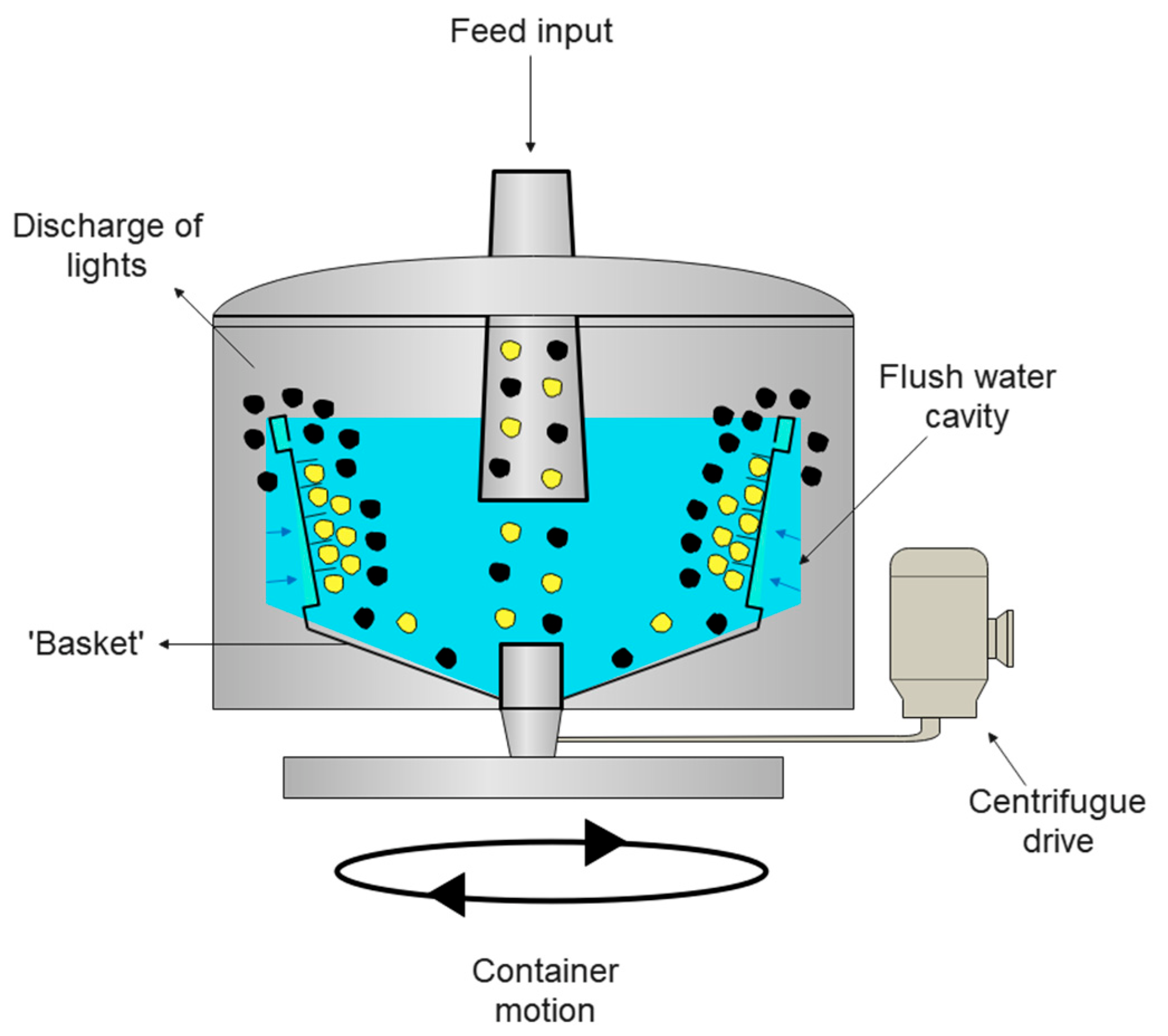
4.5. Centrifugal Separation
4.5.1. General Characteristics
4.5.2. Equipment
4.5.3. Advantages and Disadvantages
5. Urban Mining Applications
5.1. Plastic Wastes
5.2. Construction and Demolition Wastes (CDW)
5.3. Waste of Electrical and Electronic Equipment (WEEE)
6. Challenges and Opportunities
6.1. Complex and Heterogeneous Nature of the Feed Waste
6.2. Competition from Alternative Technologies
6.3. Additional Challenges and Possibilities
- -
- Water Management: Many gravity separators demand substantial water resources for their operation, ranging from some few liters per minute to hundreds of cubic meters per hour. Certain techniques, such as centrifugal separation, can necessitate dozens of cubic meters of water per ton of solid material processed [28]. As the number and scale of recycling facilities continue to grow, the competition for accessing water resources from communities and other industries will likely drive the need for water recycling systems within recycling plants. Given the potential economic challenges associated with water recycling, this is expected to encourage the adoption of low-water-consuming and dry separation technologies. Consequently, there will likely be an increased demand for the development of new prototypes for dry gravity separation, leading to the next point.
- -
- Tailored Recycling Equipment: Most instances of gravity separation in waste recycling involve the direct use of equipment initially designed for ore processing, under the assumption that solid waste, as granular material, can be treated in the same manner as ores. However, as previously discussed in Section 6.1, this may not be the case. The development of new separators designed specifically for solid waste recycling could potentially enhance the application of gravity separation in urban mining, as demonstrated by the successful customization of jigs for plastic separation (see Section 4.3). Efforts in this direction can already be found in the literature, such as the adaptation of dry jigging for multicomponent separation of CDW [61] and the development of gas-solid fluidized bed separators (using silica sand as a ‘separating medium’) for waste plastic separation [168,169]. Studies in this regard are still limited but hold significant potential for breakthroughs.
- -
- Supergravity Separation: An innovative approach to metals recycling is the so-called ‘supergravity separation’, which involves utilizing extremely high centrifugal fields (up to 1000 G) to separate metals and alloys based on their varying densities and melting points, whether in molten–solid or molten–molten systems. Meng et al. [170]) achieved successful separation of Cu and Zn from PCBs using a centrifugal apparatus situated within a heating furnace. The setup employed graphite crucibles as ‘separation containers’, subjecting the process to 1000 G and temperatures of 1300 °C, resulting in excellent recoveries and metal grades exceeding 78%. Meng et al. [171] achieved comparable positive outcomes in the separation of contaminant metals (Fe, Mn, Si, Zn, etc.) from scrap Al–Mg alloys using a heated insulating centrifugal tank operating at 500 °C and 600 G. Given the operation with molten metals and by offering the possibility to achieve high metallic grades, supergravity separation may represent a potential expansion of gravity separation beyond its conventional boundaries, entering the domain of extractive metallurgy.
7. Conclusions
- -
- Gravity separation is mostly utilized for sorting mixed solid waste into separate categories, such as plastics, ceramics, and metals, which can then serve as feedstock for subsequent processing.
- -
- Most plastics, due to their low density and hydrophobic properties, may be considered as the urban mining equivalent of coal in mineral processing. Therefore, methods like jigging, counter-current flow separation, and dense media separation can effectively separate polymers when the material feed is adequately liberated. Centrifuge gravity separation also shows potential for isolating microplastics from soil and sediments.
- -
- Gravity concentration is promising for producing recycled aggregates from construction and demolition waste (CDW), especially for coarse aggregates (>4.75 mm). However, processing fine CDW (<4.75 mm) remains underexplored, possibly due to direct applications in backfilling and geotechnical fields.
- -
- Gravity concentration can yield metallic concentrates from discarded printed circuit boards (PCBs) by controlling separation density, resulting in separate streams of plastics, metals, and aluminum/glass/ceramics. Wet tabling and centrifuge separation are suitable for processing the fine fraction where metal liberation occurs.
- -
- Challenges associated with applying gravity separation in recycling processes include the heterogeneity of waste materials, the presence of contaminants, and the need for cost-effective strategies like waste diversion and automated control systems for stable operations.
- -
- Gravity separation is increasingly challenged by two fronts: sensor-based sorting (SBS) in the processing of coarse materials and froth flotation in the treatment of fine-sized materials. However, there is an intermediate size range (about 0.75–5 mm) where these techniques face technical difficulties and in which gravity separation typically excels, particularly for treating construction and demolition waste (CDW) and microplastics.
- -
- Water management and consumption will play an increasingly significant role in future applications, potentially driving the development of new dry separators and innovative techniques like ‘supergravity separation’.
Funding
Data Availability Statement
Conflicts of Interest
References
- Amon, D.J.; Gollner, S.; Morato, T.; Smith, C.R.; Chen, C.; Christiansen, S.; Currie, B.; Drazen, J.C.; Fukushima, T.; Gianni, M. Assessment of scientific gaps related to the effective environmental management of deep-seabed mining. Mar. Policy 2022, 138, 105006. [Google Scholar] [CrossRef]
- Hu, S.; Tao, C.; Liao, S.; Zhu, C.; Qiu, Z. Transformation of minerals and mobility of heavy metals during oxidative weathering of seafloor massive sulfide and their environmental significance. Sci. Total Environ. 2022, 819, 153091. [Google Scholar] [CrossRef] [PubMed]
- Nakajima, Y.; Yamabe, Y.; Fujita, T.; Dodbiba, G. Beneficiation of Seafloor Massive Sulfides by Liquid–Liquid Extraction. J. Offshore Mech. Arct. Eng. 2022, 144, 011501. [Google Scholar] [CrossRef]
- Reis, G.S.d.; Quattrone, M.; Ambrós, W.M.; Grigore Cazacliu, B.; Hoffmann Sampaio, C. Current applications of recycled aggregates from construction and demolition: A review. Materials 2021, 14, 1700. [Google Scholar] [CrossRef]
- U.S. Geological Survey. Mineral Commodity Summaries 2021; U.S. Geological Survey: Reston, Virginia, 2021; p. 200.
- Da Silva, S.R.; Andrade, J.J.d.O. A review on the effect of mechanical properties and durability of concrete with construction and demolition waste (CDW) and fly ash in the production of new cement concrete. Sustainability 2022, 14, 6740. [Google Scholar] [CrossRef]
- Delobel, F.; Bulteel, D.; Mechling, J.; Lecomte, A.; Cyr, M.; Rémond, S. Application of ASR tests to recycled concrete aggregates: Influence of water absorption. Constr. Build. Mater. 2016, 124, 714–721. [Google Scholar] [CrossRef]
- Meng, Y.; Ling, T.-C.; Mo, K.H. Recycling of wastes for value-added applications in concrete blocks: An overview. Resour. Conserv. Recycl. 2018, 138, 298–312. [Google Scholar] [CrossRef]
- Jeon, S.; Ito, M.; Tabelin, C.B.; Pongsumrankul, R.; Kitajima, N.; Park, I.; Hiroyoshi, N. Gold recovery from shredder light fraction of E-waste recycling plant by flotation-ammonium thiosulfate leaching. Waste Manag. 2018, 77, 195–202. [Google Scholar] [CrossRef]
- Wieszczycka, K.; Tylkowski, B.; Staszak, K. Metals in Wastes; De Gruyter: Berlin, Germany, 2018. [Google Scholar]
- Nakamura, T.; Halada, K. Urban Mining Systems; Springer: Berlin/Heidelberg, Germany, 2015. [Google Scholar]
- Sverdrup, H.U.; Ragnarsdottir, K.V.; Koca, D. An assessment of metal supply sustainability as an input to policy: Security of supply extraction rates, stocks-in-use, recycling, and risk of scarcity. J. Clean. Prod. 2017, 140, 359–372. [Google Scholar] [CrossRef]
- Zhao, Y.; Li, J. Sensor-Based Technologies in Effective Solid Waste Sorting: Successful Applications, Sensor Combination, and Future Directions. Environ. Sci. Technol. 2022, 56, 17531–17544. [Google Scholar] [CrossRef]
- Burt, R.O. Gravity Concentration Technology; Elsevier: Amsterdam, The Netherlands, 1984. [Google Scholar]
- Hori, K.; Tsunekawa, M.; Ueda, M.; Hiroyoshi, N.; Ito, M.; Okada, H. Development of a New Gravity Separator for Plastics—A Hybrid-Jig—. Mater. Trans. 2009, 50, 2844–2847. [Google Scholar] [CrossRef]
- Phengsaart, T.; Manositchaikul, C.; Srichonphaisarn, P.; Juntarasakul, O.; Maneeintr, K.; Jeon, S.; Park, I.; Tabelin, C.B.; Hiroyoshi, N.; Ito, M. Reverse hybrid jig separation efficiency estimation of floating plastics using apparent specific gravity and concentration criterion. Adv. Green Sustain. Chem. Phys. Technol. Resour. Recycl. Solid Wastes 2023, 16648714, 57. [Google Scholar] [CrossRef]
- Gadotti, G.I.; Hornke, N.F.; Cavalcante, J.A.; da Silva, J.G.; Gonçalves, V.P.; Capilheira, A.F. Efficiency of the gravity table in the processing of coriander seeds. Hortic. Bras. 2020, 38, 211–216. [Google Scholar] [CrossRef]
- Majekodunmi, S.O. A review on centrifugation in the pharmaceutical industry. Am. J. Biomed. Eng. 2015, 5, 67–78. [Google Scholar]
- Wills, B.A.; Finch, J. Wills’ Mineral Processing Technology: An Introduction to the Practical aspects of Ore Treatment and Mineral Recovery; Butterworth-Heinemann: Oxford, UK, 2015. [Google Scholar]
- Rhodes, M.J. Introduction to Particle Technology; John Wiley & Sons: Hoboken, NJ, USA, 2008. [Google Scholar]
- Coulson, J.; Richardson, J. Chemical Engineering-Particle Technology and Separation Processes; RK Butterworth: Oxford, England, 1998; Volume 2. [Google Scholar]
- Haider, A.; Levenspiel, O. Drag coefficient and terminal velocity of spherical and nonspherical particles. Powder Technol. 1989, 58, 63–70. [Google Scholar] [CrossRef]
- Von Rittinger, P.R. Lehrbuch der Aufbereitungskunde: In Ihrer Neuesten Entwicklung Und AUSBILDUNG Systematisch Dargestellt; Ernst & Korn: Berlin, Germany, 1867. [Google Scholar]
- Taggart, A.F. Handbook of Mineral Dressing; Wiley: New York, USA, 1945; Volume 1. [Google Scholar]
- Gupta, A.; Yan, D.S. Mineral Processing Design and Operations: An Introduction; Elsevier: Amsterdam, The Netherlands, 2016. [Google Scholar]
- Revuelta, M.B. Mineral Resources: From Exploration to Sustainability Assessment; Springer: Berlin/Heidelberg, Germany, 2017. [Google Scholar]
- Cazacliu, B.; Sampaio, C.H.; Miltzarek, G.; Petter, C.; Le Guen, L.; Paranhos, R.; Huchet, F.; Kirchheim, A.P. The potential of using air jigging to sort recycled aggregates. J. Clean. Prod. 2014, 66, 46–53. [Google Scholar] [CrossRef]
- Sampaio, C.H.; Tavares, L.M.M. Beneficiamento Gravimétrico: Uma Introdução Aos Processos de Concentração Mineral e Reciclagem de Materiais Por Densidade; Editora da UFRGS: Porto Alegre, Brazil, 2005. [Google Scholar]
- Allen, T. Particle Size Measurement; Springer: Berlin/Heidelberg, Germany, 2013. [Google Scholar]
- Pita, F.; Castilho, A. Influence of shape and size of the particles on jigging separation of plastics mixture. Waste Manag. 2016, 48, 89–94. [Google Scholar] [CrossRef]
- Burt, R. A review of gravity concentration techniques for processing fines. Prod. Process. Fine Part. 1988, 375–385. [Google Scholar] [CrossRef]
- Hunter, R.J. Foundations of Colloid Science; Oxford University Press: Oxford, UK, 2001. [Google Scholar]
- Chaves, A.P.; Chaves Filho, R.C. Separação Densitária; Oficina de Textos: São Paulo, Brazil, 2013. [Google Scholar]
- Banerjee, P.; Rao, T.; Govindarajan, B.; Bapat, J.; Chatterjee, S.; Barnwal, J.; Rao, P. A plant comparison of the vorsyl separator and dense medium cyclone in the treatment of Indian coals. Int. J. Miner. Process. 2003, 69, 101–114. [Google Scholar] [CrossRef]
- Gent, M.R.; Menendez, M.; Toraño, J.; Isidro, D.; Torno, S. Cylinder cyclone (LARCODEMS) density media separation of plastic wastes. Waste Manag. 2009, 29, 1819–1827. [Google Scholar] [CrossRef] [PubMed]
- Biddulph, M.W. Design of vertical air classifiers for municipal solid waste. Can. J. Chem. Eng. 1987, 65, 571–580. [Google Scholar] [CrossRef]
- Kaas, A.; Mütze, T.; Peuker, U.A. Review on zigzag air classifier. Processes 2022, 10, 764. [Google Scholar] [CrossRef]
- Cossu, R.; Lai, T. Automotive shredder residue (ASR) management: An overview. Waste Manag. 2015, 45, 143–151. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Lee, H. Separation of auto shredder residue materials using an air table to achieve highly efficient recycling rate. Sep. Sci. Technol. 2021, 56, 2449–2457. [Google Scholar] [CrossRef]
- Duan, C.; He, Y.; Zhao, Y.; Wen, X.; Wang, H.; Song, S. The fundamental study on the reutilization of electronic scrap by passive pulsed air classifiers. In Proceedings of the 2005 IEEE International Symposium on Electronics and the Environment, New Orleans, LA, USA, 16–19 May 2005; pp. 107–115. [Google Scholar]
- Eswaraiah, C.; Kavitha, T.; Vidyasagar, S.; Narayanan, S. Classification of metals and plastics from printed circuit boards (PCB) using air classifier. Chem. Eng. Process. Process Intensif. 2008, 47, 565–576. [Google Scholar] [CrossRef]
- He, J.; He, Y.; Zhou, N.; Duan, C.; Wang, S.; Zhang, H. A Novel Flowsheet for the Recycling of Valuable Constituents from Waste Printed Circuit Boards. In Interdisciplinary Research and Applications in Bioinformatics, Computational Biology, and Environmental Sciences; IGI Global: Hershey, PA, USA, 2011; pp. 296–306. [Google Scholar]
- Grotjohann, P.; Snoby, R. Allflux separator—A new way to process heavy minerals. Min. Metall. Explor. 1999, 16, 25–28. [Google Scholar] [CrossRef]
- Kohmuench, J.; Mankosa, M.; Honaker, R.; Bratton, R. Applications of the CrossFlow teeter-bed separator in the US coal industry. Min. Metall. Explor. 2006, 23, 187–195. [Google Scholar]
- Honaker, R.; Dunne, R.; Galvin, K. Density-based separation innovations in coal and minerals processing applications. In Mineral Processing and Extractive Metallurgy-100 Years of Innovation; Society for Mining, Metallurgy & Exploration (SME): Englewood, CO, USA, 2013. [Google Scholar]
- Nunna, V.; Hapugoda, S.; Pownceby, M.; Sparrow, G. Application of a Floatex density separator for iron recovery from Pilbara iron ore plant rejects. Miner. Process. Extr. Met. Rev. 2022, 2022, 1–8. [Google Scholar] [CrossRef]
- Nguyentranlam, G.; Galvin, K. Particle classification in the reflux classifier. Miner. Eng. 2001, 14, 1081–1091. [Google Scholar] [CrossRef]
- Rodrigues, A.F.; Junior, H.D.; Rodrigues, O.M.; Zhou, J.; Galvin, K.P. Gravity separation of fine itabirite iron ore using the Reflux Classifier–Part I–Investigation of continuous steady state separations across a wide range of parameters. Miner. Eng. 2023, 201, 108187. [Google Scholar] [CrossRef]
- Lukas, E.; Roloff, C.; van Wachem, B.; Thévenin, D. Experimental investigation of the grade efficiency of a zigzag separator. Powder Technol. 2020, 369, 38–52. [Google Scholar] [CrossRef]
- Mann, H.; Roloff, C.; Hagemeier, T.; Thévenin, D.; Tomas, J. Model-based experimental data evaluation of separation efficiency of multistage coarse particle classification in a zigzag apparatus. Powder Technol. 2017, 313, 145–160. [Google Scholar] [CrossRef]
- He, Y.; Wang, H.; Duan, C.; Song, S. Airflow fields simulation on passive pulsing air classifiers. J. South. Afr. Inst. Min. Metall. 2005, 105, 525–531. [Google Scholar]
- Chen-long, D.; Ya-qun, H.; Yue-min, Z.; Jing-feng, H.; Bao-feng, W. Development and application of the active pulsing air classification. Procedia Earth Planet. Sci. 2009, 1, 667–672. [Google Scholar] [CrossRef]
- Duan, C.; Li, H.; He, J.; Zhao, Y.; Dong, L.; Lv, K.; He, Y. Experimental and numerical simulation of spent catalyst separation in an active pulsing air classifier. Sep. Sci. Technol. 2015, 50, 633–645. [Google Scholar] [CrossRef]
- Hu, X.; Calo, J. Plastic particle separation via liquid-fluidized bed classification. AIChE J. 2006, 52, 1333–1342. [Google Scholar] [CrossRef]
- Baigabelov, A.; Das, A.; Young, C. Integrated density concentration and surface treatment for selective separation of plastics from a mixture. J. Mater. Cycles Waste Manag. 2021, 23, 2162–2178. [Google Scholar] [CrossRef]
- Ambrós, W.M. Jigging: A review of fundamentals and future directions. Minerals 2020, 10, 998. [Google Scholar] [CrossRef]
- Mayer, F. Fundamentals of a potential theory of the jigging process. In Proceedings of the 7th International Mineral Processing Congress, New York, NY, USA, 20–24 September 1964; pp. 75–86. [Google Scholar]
- King, R. A Quantitative Model for Gravity Separation Unit Operations That Rely on Stratification. In Proceedings of the Twentieth International Symposium on the Application of Computers and Mathematics in the Mineral Industries (APCOM), Johannesburg, South Africa, 19–23 October 1987; pp. 147–151. [Google Scholar]
- Tavares, L.; King, R. A useful model for the calculation of the performance of batch and continuous jigs. Coal Prep. 1995, 15, 99–128. [Google Scholar] [CrossRef]
- Ito, M.; Tsunekawa, M.; Ishida, E.; Kawai, K.; Takahashi, T.; Abe, N.; Hiroyoshi, N. Reverse jig separation of shredded floating plastics—Separation of polypropylene and high density polyethylene. Int. J. Miner. Process. 2010, 97, 96–99. [Google Scholar] [CrossRef]
- Ambros, W.M.; Sampaio, C.H.; Cazacliu, B.G.; Miltzarek, G.L.; Miranda, L.R. Usage of air jigging for multi-component separation of construction and demolition waste. Waste Manag. 2017, 60, 75–83. [Google Scholar] [CrossRef]
- Vegas, I.; Broos, K.; Nielsen, P.; Lambertz, O.; Lisbona, A. Upgrading the quality of mixed recycled aggregates from construction and demolition waste by using near-infrared sorting technology. Constr. Build. Mater. 2015, 75, 121–128. [Google Scholar] [CrossRef]
- Hoover, H.; Hoover, L.H. De re metallica; Courier Corporation: North Chelmsford, MA, USA, 1950. [Google Scholar]
- Bazin, C.; Sadeghi, M.; Roy, P.; Bourassa, M.; Cataford, D.; Lavoie, F.; Rochefort, C.; Gosselin, C.; Renaud, M.; Mahieu, G. Simulation of an iron ore concentration circuit using mineral size recovery curves of industrial spirals. In Proceedings of the 46th Canadian Mineral Processors, CIM, Ottawa, ON, Canada, 21–23 January 2014. [Google Scholar]
- Sivamohan, R.; Forssberg, E. Principles of spiral concentration. Int. J. Miner. Process. 1985, 15, 173–181. [Google Scholar] [CrossRef]
- Ye, G.; Ma, L.; Alberini, F.; Xu, Q.; Huang, G.; Yu, Y. Numerical studies of the effects of design parameters on flow fields in spiral concentrators. Int. J. Coal Prep. Util. 2022, 42, 67–81. [Google Scholar] [CrossRef]
- Boisvert, L.; Sadeghi, M.; Bazin, C. Investigation of residence time and fluid volume in spiral concentrators. Miner. Eng. 2023, 202, 108272. [Google Scholar] [CrossRef]
- Haldar, S.K. Mineral Exploration: Principles and Applications; Elsevier: Amsterdam, The Netherlands, 2018. [Google Scholar]
- Veiga, M.M.; Gunson, A.J. Gravity concentration in artisanal gold mining. Minerals 2020, 10, 1026. [Google Scholar] [CrossRef]
- Chelgani, S.C.; Neisiani, A.A. Dry Mineral Processing; Springer: Berlin/Heidelberg, Germany, 2022. [Google Scholar]
- Back, S.; Ueda, K.; Sakanakura, H. Determination of metal-abundant high-density particles in municipal solid waste incineration bottom ash by a series of processes: Sieving, magnetic separation, air table sorting, and milling. Waste Manag. 2020, 112, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Akbari, H.; Ackah, L.; Mohanty, M. Performance optimization of a new air table and flip-flow screen for fine particle dry separation. Int. J. Coal Prep. Util. 2020, 40, 581–603. [Google Scholar] [CrossRef]
- Keshun, Y.; Chengyu, W.; Huizhong, L. Research on intelligent implementation of the beneficiation process of shaking table. Miner. Eng. 2023, 199, 108108. [Google Scholar] [CrossRef]
- Keshun, Y.; Huizhong, L. Intelligent deployment solution for tabling adapting deep learning. IEEE Access 2023, 11, 22201–22208. [Google Scholar] [CrossRef]
- Knelson, B. The Knelson concentrator. Metamorphosis from crude beginning to sophisticated world wide acceptance. Miner. Eng. 1992, 5, 1091–1097. [Google Scholar] [CrossRef]
- Koppalkar, S.K. Effect of Operating Variables in Knelson Concentrators: A Pilot-Scale Study; McGill University: Montreal, QC, Canada, 2010. [Google Scholar]
- Chen, Q.; Yang, H.-y.; Tong, L.-l.; Niu, H.-q.; Zhang, F.-s.; Chen, G.-m. Research and application of a Knelson concentrator: A review. Miner. Eng. 2020, 152, 106339. [Google Scholar] [CrossRef]
- Greenwood, M.; Langlois, R.; Waters, K. The potential for dry processing using a Knelson Concentrator. Miner. Eng. 2013, 45, 44–46. [Google Scholar] [CrossRef]
- Zhou, M.; Kökkılıç, O.; Langlois, R.; Waters, K.E. Size-by-size analysis of dry gravity separation using a 3-in. Knelson Concentrator. Miner. Eng. 2016, 91, 42–54. [Google Scholar] [CrossRef]
- Farajzadeh, S.; Chehreh Chelgani, S. Gravity separation by falcon concentrator-an over review. Sep. Sci. Technol. 2022, 57, 2145–2164. [Google Scholar] [CrossRef]
- Kroll-Rabotin, J.-S.; Bourgeois, F.; Climent, É. Physical analysis and modeling of the Falcon concentrator for beneficiation of ultrafine particles. Int. J. Miner. Process. 2013, 121, 39–50. [Google Scholar] [CrossRef]
- Das, A.; Sarkar, B. Advanced gravity concentration of fine particles: A review. Miner. Process. Extr. Metall. Rev. 2018, 39, 359–394. [Google Scholar] [CrossRef]
- Tucker, P. Modelling the Kelsey centrifugal jig. Miner. Eng. 1995, 8, 333–336. [Google Scholar] [CrossRef]
- Laplante, A.; Gray, S. Advances in gravity gold technology. Dev. Miner. Process. 2005, 15, 280–307. [Google Scholar]
- Tejaswini, M.; Pathak, P.; Gupta, D. Sustainable approach for valorization of solid wastes as a secondary resource through urban mining. J. Environ. Manag. 2022, 319, 115727. [Google Scholar] [CrossRef]
- Hussain, C.M.; Hait, S. Advanced Organic Waste Management: Sustainable Practices and Approaches; Elsevier: Amsterdam, The Netherlands, 2022. [Google Scholar]
- Schlesinger, M.E. Aluminum Recycling; CRC Press: Boca Raton, FL, USA, 2006. [Google Scholar]
- Pita, F.; Castilho, A. Separation of plastics by froth flotation. The role of size, shape and density of the particles. Waste Manag. 2017, 60, 91–99. [Google Scholar] [CrossRef]
- Gschwenter, V.L.; Tubino, R.M.; Ambrós, W.M.; Miltzarek, G.L.; Sampaio, C.H.; Moncunill, J.O.; Cazacliu, B.G.; Dal Molin, D.C. Production of high-quality coarse recycled aggregates through a two-stage jigging process. Minerals 2022, 12, 532. [Google Scholar] [CrossRef]
- Sampaio, C.H.; Cazacliu, B.G.; Miltzarek, G.L.; Huchet, F.; Le Guen, L.; Petter, C.O.; Paranhos, R.; Ambrós, W.M.; Oliveira, M.L.S. Stratification in air jigs of concrete/brick/gypsum particles. Constr. Build. Mater. 2016, 109, 63–72. [Google Scholar] [CrossRef]
- Torgal, F.P.; Ding, Y. Handbook of Recycled Concrete and Demolition Waste; Elsevier: Amsterdam, The Netherlands, 2013. [Google Scholar]
- Zhang, Y.; Wang, Q.; Yalikun, N.; Wang, H.; Wang, C.; Jiang, H. A comprehensive review of separation technologies for waste plastics in urban mine. Resour. Conserv. Recycl. 2023, 197, 107087. [Google Scholar] [CrossRef]
- de Buzin, P.J.W.K.; Ambrós, W.M.; de Brum, I.A.S.; Tubino, R.M.C.; Hoffmann Sampaio, C.; Oliva Moncunill, J. Development of a Physical Separation Route for the Concentration of Base Metals from Old Wasted Printed Circuit Boards. Minerals 2021, 11, 1014. [Google Scholar] [CrossRef]
- Yao, Y.; He, J.; Yang, B.; Zhao, Y.; Zhu, L. Study on particle characteristics and metal distribution of waste printed circuit boards based on a shear crusher. Powder Technol. 2023, 415, 118103. [Google Scholar] [CrossRef]
- Agarwal, V.; Halli, P.; Helin, S.; Tesfaye, F.; Lundstrom, M. Electrohydraulic fragmentation of aluminum and polymer fractions from waste pharmaceutical blisters. ACS Sustain. Chem. Eng. 2020, 8, 4137–4145. [Google Scholar] [CrossRef]
- Şahin, G.G.; Karaboyacı, M. Process and machinery design for the recycling of tetra pak components. J. Clean. Prod. 2021, 323, 129186. [Google Scholar] [CrossRef]
- Gent, M.R.; Menendez, M.; Toraño, J.; Diego, I. Recycling of plastic waste by density separation: Prospects for optimization. Waste Manag. Res. 2009, 27, 175–187. [Google Scholar] [CrossRef]
- Serranti, S.; Bonifazi, G. Techniques for separation of plastic wastes. In Use of Recycled Plastics in Eco-efficient Concrete; Elsevier: Amsterdam, The Netherlands, 2019; pp. 9–37. [Google Scholar]
- Bauer, M.; Lehner, M.; Schwabl, D.; Flachberger, H.; Kranzinger, L.; Pomberger, R.; Hofer, W. Sink–float density separation of post-consumer plastics for feedstock recycling. J. Mater. Cycles Waste Manag. 2018, 20, 1781–1791. [Google Scholar] [CrossRef]
- Fu, S.; Qian, Y.; Yuan, H.; Fang, Y. Effect of cone angles of a hydrocyclone for the separation of waste plastics with low value of density difference. Waste Manag. 2022, 140, 183–192. [Google Scholar] [CrossRef]
- Richard, G.M.; Mario, M.; Javier, T.; Susana, T. Optimization of the recovery of plastics for recycling by density media separation cyclones. Resour. Conserv. Recycl. 2011, 55, 472–482. [Google Scholar] [CrossRef]
- Ito, M.; Saito, A.; Murase, N.; Phengsaart, T.; Kimura, S.; Tabelin, C.B.; Hiroyoshi, N. Development of suitable product recovery systems of continuous hybrid jig for plastic-plastic separation. Miner. Eng. 2019, 141, 105839. [Google Scholar] [CrossRef]
- La Marca, F.; Moroni, M.; Cherubini, L.; Lupo, E.; Cenedese, A. Separation of plastic waste via the hydraulic separator Multidune under different geometric configurations. Waste Manag. 2012, 32, 1306–1315. [Google Scholar] [CrossRef]
- Tatemoto, Y.; Michikoshi, T.; Higashino, T.; Maeda, S.; Maeda, S.; Bando, Y. Behavior of Pieces of Plastic Sheet in Solid-Liquid Fluidized Bed with Stirring. Chem. Eng. Technol. 2012, 35, 1872–1878. [Google Scholar] [CrossRef]
- Bonifazi, G.; Serranti, S.; Potenza, F.; Luciani, V.; Di Maio, F. Gravity packaging final waste recovery based on gravity separation and chemical imaging control. Waste Manag. 2017, 60, 50–55. [Google Scholar] [CrossRef]
- Dodbiba, G.; Shibayama, A.; Miyazaki, T.; Fujita, T. Separation performance of PVC and PP plastic mixture using air table. Phys. Sep. Sci. Eng. 2003, 12, 71–86. [Google Scholar] [CrossRef]
- Carvalho, M.; Agante, E.; Durão, F. Recovery of PET from packaging plastics mixtures by wet shaking table. Waste Manag. 2007, 27, 1747–1754. [Google Scholar] [CrossRef] [PubMed]
- Wolff, S.; Kerpen, J.; Prediger, J.; Barkmann, L.; Müller, L. Determination of the microplastics emission in the effluent of a municipal waste water treatment plant using Raman microspectroscopy. Water Res. X 2019, 2, 100014. [Google Scholar] [CrossRef] [PubMed]
- Wu, R.-T.; Cai, Y.-F.; Xing, S.-C.; Yang, Y.-W.; Mi, J.-D.; Liao, X.-D. A novel method for extraction of polypropylene microplastics in swine manure. Environ. Sci. Pollut. Res. 2021, 28, 13021–13030. [Google Scholar] [CrossRef]
- Grause, G.; Kuniyasu, Y.; Chien, M.-F.; Inoue, C. Separation of microplastic from soil by centrifugation and its application to agricultural soil. Chemosphere 2022, 288, 132654. [Google Scholar] [CrossRef]
- Menegaki, M.; Damigos, D. A review on current situation and challenges of construction and demolition waste management. Curr. Opin. Green Sustain. Chem. 2018, 13, 8–15. [Google Scholar] [CrossRef]
- Waskow, R.P. Avaliação técnica, ambiental e econômica do uso do jigue a ar na reciclagem do resíduo da construção e demolição (RCD) brasileiro. Master’s Thesis, 2019. Available online: http://hdl.handle.net/10183/215017 (accessed on 1 July 2023).
- Coelho, A.; De Brito, J. Economic viability analysis of a construction and demolition waste recycling plant in Portugal–part I: Location, materials, technology and economic analysis. J. Clean. Prod. 2013, 39, 338–352. [Google Scholar] [CrossRef]
- Hiete, M. Waste management plants and technology for recycling construction and demolition (C&D) waste: State-of-the-art and future challenges. In Handbook of Recycled Concrete and Demolition Waste; Elsevier: Amsterdam, The Netherlands, 2013; pp. 53–75. [Google Scholar]
- Jiménez, J.; Agrela, F.; Ayuso, J.; López, M. A comparative study of recycled aggregates from concrete and mixed debris as material for unbound road sub-base. Mater. De Construcción 2011, 61, 289–302. [Google Scholar] [CrossRef]
- Lovato, P.S. Verificação dos parâmetros de controle de agregados reciclados de resíduos de construção e demolição para utilização em concreto. Master’s Thesis. 2007. Available online: http://hdl.handle.net/10183/10609 (accessed on 15 July 2023).
- C33-03; A Standard Spesification for Concrete Agregates. American Society for Testing and Materials. ASTM International: West Conshohocken, PA, USA, 2003.
- Técnicas, A.-A.B.d.N. NBR 7211; Agregados Para Concreto–Especificação. Brazilian National Standards Organization: Rio de Janeiro, Brazil, 2009.
- Jungmann, A. Building rubble treatment using the alljig in Europe and USA [Bauschuttaufbereitung mit alljig-Setzmaschinen in Europa und USA]. Aufbereit Tech. Miner. Process 1997, 38, 543–549. [Google Scholar]
- Xing, W. Quality Improvement of Granular Secondary Raw Building Materials by Separation and Cleansing Techniques. Ph.D. Thesis. 2004. Available online: http://resolver.tudelft.nl/uuid:a6d0a00b-9093-40a0-ae56-5ed481398d9a (accessed on 17 October 2023).
- Ambrós, W.M.; Sampaio, C.H.; Cazacliu, B.G.; Conceição, P.N.; dos Reis, G.S. Some observations on the influence of particle size and size distribution on stratification in pneumatic jigs. Powder Technol. 2019, 342, 594–606. [Google Scholar] [CrossRef]
- Waskow, R.P.; Dos Santos, V.L.; Ambrós, W.M.; Sampaio, C.H.; Passuello, A.; Tubino, R.M. Optimization and dust emissions analysis of the air jigging technology applied to the recycling of construction and demolition waste. J. Environ. Manag. 2020, 266, 110614. [Google Scholar] [CrossRef]
- Hoffmann Sampaio, C.; Cazacliu, B.G.; Ambrós, W.M.; Kronbauer, M.A.; Tubino, R.M.; Dal Molin, D.C.; Oliva, J.; Miltzarek, G.L.; Waskow, R.P.; Dos Santos, V.L. Demolished concretes recycling by the use of pneumatic jigs. Waste Manag. Res. 2020, 38, 392–399. [Google Scholar] [CrossRef]
- Khoury, E.; Ambrós, W.; Cazacliu, B.; Sampaio, C.H.; Remond, S. Heterogeneity of recycled concrete aggregates, an intrinsic variability. Constr. Build. Mater. 2018, 175, 705–713. [Google Scholar] [CrossRef]
- Agarwal, A.; Datta, M.; Ramana, G.; Soni, N.K.; Satyakam, R. Assessment of Using Soil-Sized Material from Construction and Demolition Waste as an Earthfill. In Proceedings of the Indian Geotechnical Conference, Tamilnadu, India, 16–18 December 2021; pp. 253–260. [Google Scholar]
- Agarwal, A.; Ramana, G.; Datta, M.; Soni, N.K.; Satyakam, R. Environmental assessment of unprocessed sand-sized construction & demolition waste for geotechnical reuse. J. Clean. Prod. 2022, 363, 132504. [Google Scholar]
- Ulsen, C.; Kahn, H.; Hawlitschek, G.; Masini, E.A.; Angulo, S.C. Separability studies of construction and demolition waste recycled sand. Waste Manag. 2013, 33, 656–662. [Google Scholar] [CrossRef]
- Paranhos, R.S.; Sampaio, C.H.; Cazacliu, B.G.; Neto, R.O.; Liendo, M.A. Jigs, Hydrocyclones and Sensor-Based Sorting to Value Recycled Aggregate. In Proceedings of the 3rd Pan American Materials Congress, San Diego, CA, USA, 26 February–2 March 2017; pp. 215–225. [Google Scholar]
- Directive, E. Directive 2012/19/EU of the European Parliament and of the Council of 4 July 2012 on waste electrical and electronic equipment, WEEE. Off. J. Eur. Union L 2012, 197, 38–71. [Google Scholar]
- Council, E.A.S.A. Priorities for Critical Materials for a Circular Economy; ESAC Secretariat; German National Academy of Sciences: Halle, Germany, 2016. [Google Scholar]
- Kaya, M. Electronic Waste and Printed Circuit Board Recycling Technologies; Springer: Berlin/Heidelberg, Germany, 2019. [Google Scholar]
- Wang, J.; Guo, J.; Xu, Z. An environmentally friendly technology of disassembling electronic components from waste printed circuit boards. Waste Manag. 2016, 53, 218–224. [Google Scholar] [CrossRef]
- He, W.; Li, G.; Ma, X.; Wang, H.; Huang, J.; Xu, M.; Huang, C. WEEE recovery strategies and the WEEE treatment status in China. J. Hazard. Mater. 2006, 136, 502–512. [Google Scholar] [CrossRef]
- Kumar, V.; Gupta, M. Comparative study of different natural fibre printed circuit board (PCB) composites. Mater. Today Proc. 2021, 44, 2097–2101. [Google Scholar] [CrossRef]
- Gutiérrez-Gutiérrez, S.C.; Coulon, F.; Jiang, Y.; Wagland, S. Rare earth elements and critical metal content of extracted landfilled material and potential recovery opportunities. Waste Manag. 2015, 42, 128–136. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Shrivastava, P.; Gao, Z.; Zhang, H.-C. Printed circuit board recycling: A state-of-the-art survey. IEEE Trans. Electron. Packag. Manuf. 2004, 27, 33–42. [Google Scholar]
- Duan, C.; Wen, X.; Shi, C.; Zhao, Y.; Wen, B.; He, Y. Recovery of metals from waste printed circuit boards by a mechanical method using a water medium. J. Hazard. Mater. 2009, 166, 478–482. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, E.F.; De Rossi, A.; Rovaris, B.; Valerio, A.; de Oliveira, D.; Hotza, D. Cleaner pre-concentration of metals from printed circuit board waste using novel dense liquid medium based on sodium silicate. Waste Biomass Valorization 2021, 12, 4081–4087. [Google Scholar] [CrossRef]
- Sarvar, M.; Salarirad, M.M.; Shabani, M.A. Characterization and mechanical separation of metals from computer Printed Circuit Boards (PCBs) based on mineral processing methods. Waste Manag. 2015, 45, 246–257. [Google Scholar] [CrossRef]
- Ogunniyi, I.; Vermaak, M.K.G.; Groot, D. Chemical composition and liberation characterization of printed circuit board comminution fines for beneficiation investigations. Waste Manag. 2009, 29, 2140–2146. [Google Scholar] [CrossRef] [PubMed]
- Yazıcı, E.; Deveci, H.; Alp, I.; Akcil, A.; Yazıcı, R. Characterisation of computer printed circuit boards for hazardous properties and beneficiation studies. In Proceedings of the XXV International Mineral Processing Congress (IMPC), Brisbane, QLD, Australia, 6–10 September 2010. [Google Scholar]
- Barnwal, A.; Dhawan, N. Recovery of copper values from discarded random access memory cards via fluidization and thermal exposure. J. Clean. Prod. 2020, 256, 120516. [Google Scholar] [CrossRef]
- Bilesan, M.R.; Makarova, I.; Wickman, B.; Repo, E. Efficient separation of precious metals from computer waste printed circuit boards by hydrocyclone and dilution-gravity methods. J. Clean. Prod. 2021, 286, 125505. [Google Scholar] [CrossRef]
- Veit, H.M.; Juchneski, N.C.d.F.; Scherer, J. Use of gravity separation in metals concentration from printed circuit board scraps. Rem: Rev. Esc. De Minas 2014, 67, 73–79. [Google Scholar] [CrossRef]
- Tanısalı, E.; Özer, M.; Burat, F. Precious metals recovery from waste printed circuit boards by gravity separation and leaching. Miner. Process. Extr. Metall. Rev. 2021, 42, 24–37. [Google Scholar] [CrossRef]
- de Oliveira, C.M.; Bellopede, R.; Tori, A.; Zanetti, G.; Marini, P. Gravity and Electrostatic Separation for Recovering Metals from Obsolete Printed Circuit Board. Materials 2022, 15, 1874. [Google Scholar] [CrossRef]
- Dinç, N.İ.; Tosun, A.U.; Baştürkcü, E.; Özer, M.; Burat, F. Recovery of valuable metals from WPCB fines by centrifugal gravity separation and froth flotation. J. Mater. Cycles Waste Manag. 2022, 24, 224–236. [Google Scholar] [CrossRef]
- Chaves, A.P. Manuseio de Sólidos Granulados: Coleção Teoria e Prática do Tratamento de Minérios-Volume 5; Oficina de Textos: São Paulo, Brazil, 2015. [Google Scholar]
- Schofield, C. Raw Material Stacking, Reclaiming and Homogenisation. In Proceedings of the Symposium Series; Institute of Chemical Engineers: Rugby, England, 1981. [Google Scholar]
- Brooks, L.; Gaustad, G.; Gesing, A.; Mortvedt, T.; Freire, F. Ferrous and non-ferrous recycling: Challenges and potential technology solutions. Waste Manag. 2019, 85, 519–528. [Google Scholar] [CrossRef]
- Sbárbaro, D.; Del Villar, R. Advanced Control and Supervision of Mineral Processing Plants; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2010. [Google Scholar]
- Ambrós, W.M.; Cazacliu, B.G.; Sampaio, C.H. Wall effects on particle separation in air jigs. Powder Technol. 2016, 301, 369–378. [Google Scholar] [CrossRef]
- Cai, R.; Qiu, J. Position and posture determination of a large dense object in a fluidized bed. Flow Meas. Instrum. 2016, 51, 40–48. [Google Scholar] [CrossRef]
- Robben, C.; Wotruba, H. Sensor-based ore sorting technology in mining—Past, present and future. Minerals 2019, 9, 523. [Google Scholar] [CrossRef]
- Beigbeder, J.; Perrin, D.; Mascaro, J.-F.; Lopez-Cuesta, J.-M. Study of the physico-chemical properties of recycled polymers from waste electrical and electronic equipment (WEEE) sorted by high resolution near infrared devices. Resour. Conserv. Recycl. 2013, 78, 105–114. [Google Scholar] [CrossRef]
- Küppers, B.; Schloegl, S.; Oreski, G.; Pomberger, R.; Vollprecht, D. Influence of surface roughness and surface moisture of plastics on sensor-based sorting in the near infrared range. Waste Manag. Res. 2019, 37, 843–850. [Google Scholar] [CrossRef] [PubMed]
- Ruj, B.; Pandey, V.; Jash, P.; Srivastava, V. Sorting of plastic waste for effective recycling. Int. J. Appl. Sci. Eng. Res. 2015, 4, 564–571. [Google Scholar]
- Hoffmann Sampaio, C.; Ambrós, W.M.; Cazacliu, B.G.; Oliva Moncunill, J.; Veras, M.M.; Miltzarek, G.L.; Silva, L.F.; Kuerten, A.S.; Liendo, M.A. Construction and demolition waste recycling through conventional jig, air jig, and sensor-based sorting: A comparison. Minerals 2021, 11, 904. [Google Scholar] [CrossRef]
- Serranti, S.; Gargiulo, A.; Bonifazi, G. Classification of polyolefins from building and construction waste using NIR hyperspectral imaging system. Resour. Conserv. Recycl. 2012, 61, 52–58. [Google Scholar] [CrossRef]
- Küppers, B.; Seidler, I.; Koinig, G.; Pomberger, R.; Vollprecht, D. Influence of throughput rate and input composition on sensor-based sorting efficiency. Detritus 2020, 9, 59–67. [Google Scholar] [CrossRef]
- Peukert, D.; Xu, C.; Dowd, P. A Review of Sensor-Based Sorting in Mineral Processing: The Potential Benefits of Sensor Fusion. Minerals 2022, 12, 1364. [Google Scholar] [CrossRef]
- Ogunniyi, I.; Vermaak, M. Froth flotation for beneficiation of printed circuit boards comminution fines: An overview. Miner. Process. Extr. Metall. Rev. 2009, 30, 101–121. [Google Scholar] [CrossRef]
- Kökkılıç, O.; Mohammadi-Jam, S.; Chu, P.; Marion, C.; Yang, Y.; Waters, K.E. Separation of plastic wastes using froth flotation–an overview. Adv. Colloid Interface Sci. 2022, 308, 102769. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, Y.; Wang, C. Surface modification and selective flotation of waste plastics for effective recycling—A review. Sep. Purif. Technol. 2019, 226, 75–94. [Google Scholar] [CrossRef]
- Wang, C.-q.; Wang, H.; Fu, J.-g.; Liu, Y.-n. Flotation separation of waste plastics for recycling—A review. Waste Manag. 2015, 41, 28–38. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, B.; Claveau-Mallet, D.; Hernandez, L.M.; Xu, E.G.; Farner, J.M.; Tufenkji, N. Separation and analysis of microplastics and nanoplastics in complex environmental samples. Acc. Chem. Res. 2019, 52, 858–866. [Google Scholar] [CrossRef]
- Herrera, A.; Garrido-Amador, P.; Martínez, I.; Samper, M.D.; López-Martínez, J.; Gómez, M.; Packard, T.T. Novel methodology to isolate microplastics from vegetal-rich samples. Mar. Pollut. Bull. 2018, 129, 61–69. [Google Scholar] [CrossRef] [PubMed]
- Xia, D.; Zhang, F.-S. A novel dry cleaning system for contaminated waste plastic purification in gas-solid media. J. Clean. Prod. 2018, 171, 1472–1480. [Google Scholar] [CrossRef]
- Yoshida, M.; Nakatsukasa, S.; Nanba, M.; Gotoh, K.; Zushi, T.; Kubo, Y.; Oshitani, J. Decrease of Cl contents in waste plastics using a gas–solid fluidized bed separator. Adv. Powder Technol. 2010, 21, 69–74. [Google Scholar] [CrossRef]
- Meng, L.; Zhong, Y.; Guo, L.; Wang, Z.; Chen, K.; Guo, Z. Recovery of Cu and Zn from waste printed circuit boards using super-gravity separation. Waste Manag. 2018, 78, 559–565. [Google Scholar] [CrossRef] [PubMed]
- Meng, L.; Wang, Z.; Wang, L.; Guo, L.; Guo, Z. Novel and efficient purification of scrap Al-Mg alloys using supergravity technology. Waste Manag. 2021, 119, 22–29. [Google Scholar] [CrossRef] [PubMed]









| Material | Density (g/cm3) | Reference |
|---|---|---|
| Aluminum | 2.70 | Schlesinger [87] |
| Copper | 8.97 | Schlesinger [87] |
| Lead | 11.30 | Schlesinger [87] |
| Tin | 7.28 | Schlesinger [87] |
| Brass | 8.40 | Schlesinger [87] |
| Stainless Steel | 7.90 | Schlesinger [87] |
| Zinc | 7.14 | Schlesinger [87] |
| Polystyrene (PS) | 1.047 | Pita and Castilho [88] |
| Polymethyl methacrylate (PMMA) | 1.204 | Pita and Castilho [88] |
| Polyvinyl chloride (PVC) | 1.209–1.326 | Pita and Castilho [30] |
| Polyethylene terephthalate (PET) | 1.364–1.372 | Pita and Castilho [30] |
| Ceramic (2 MPa) | 2.22 | Gschwenter et al. [89] |
| Ceramic (7 MPa) | 2.35 | Gschwenter et al. [89] |
| Concrete (16 MPa) | 2.46 | Gschwenter et al. [89] |
| Concrete (54 MPa) | 2.76 | Gschwenter et al. [89] |
| Mortar | 2.04 | Gschwenter et al. [89] |
| Gypsum | 1.86 | Sampaio et al. [90] |
| Fiberboard | 0.60 | Torgal and Ding [91] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ambrós, W.M. Gravity Concentration in Urban Mining Applications—A Review. Recycling 2023, 8, 85. https://doi.org/10.3390/recycling8060085
Ambrós WM. Gravity Concentration in Urban Mining Applications—A Review. Recycling. 2023; 8(6):85. https://doi.org/10.3390/recycling8060085
Chicago/Turabian StyleAmbrós, Weslei M. 2023. "Gravity Concentration in Urban Mining Applications—A Review" Recycling 8, no. 6: 85. https://doi.org/10.3390/recycling8060085





