Effect of Soil Use and Coverage on the Spectral Response of an Oxisol in the VIS-NIR-MIR Region
Abstract
:1. Introduction
2. Materials and Methods
2.1. Characterization of the Study Area
2.2. Characterization of Samples
2.3. Acquisition of Spectra
3. Results and Discussion
3.1. Soil Properties
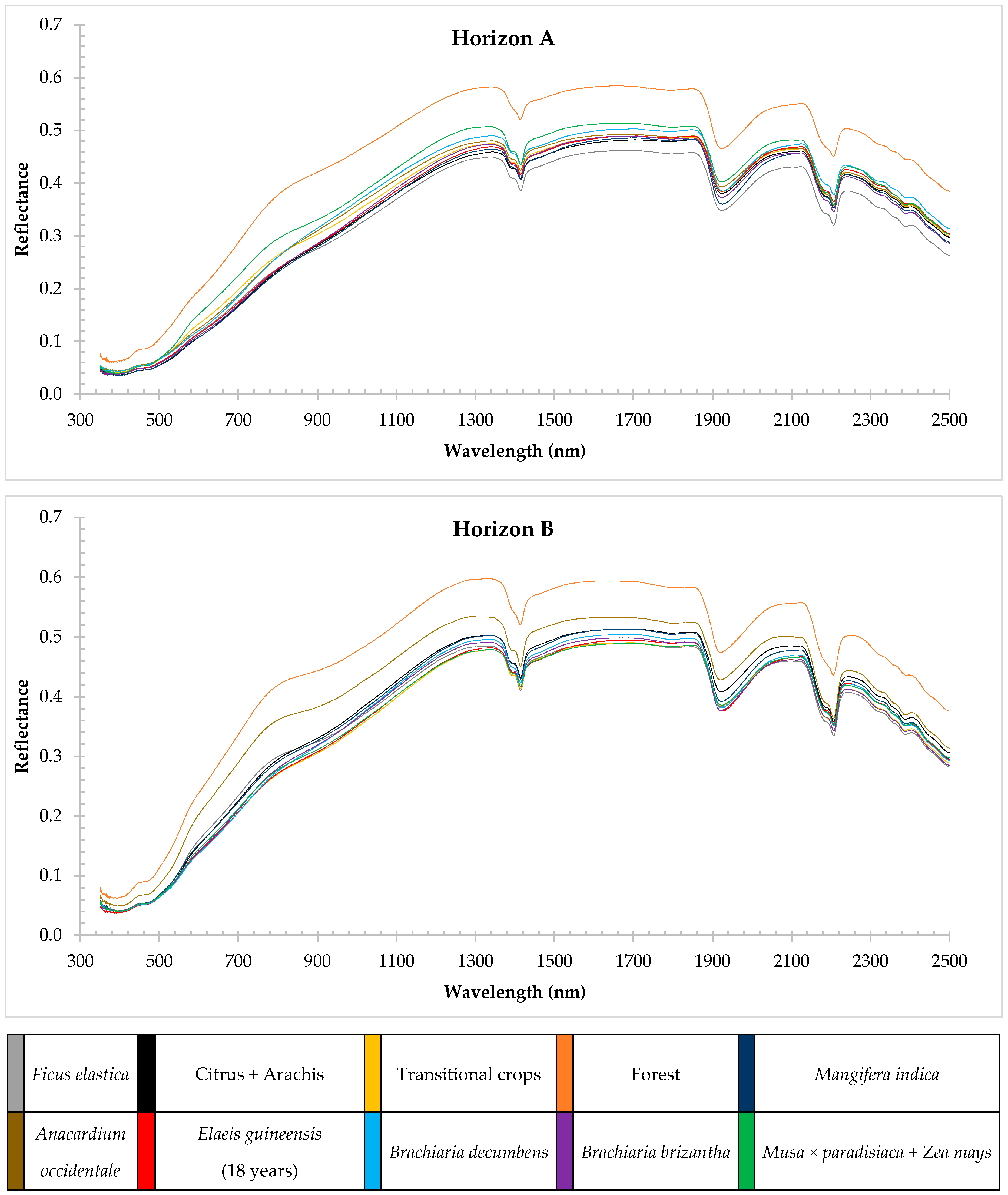
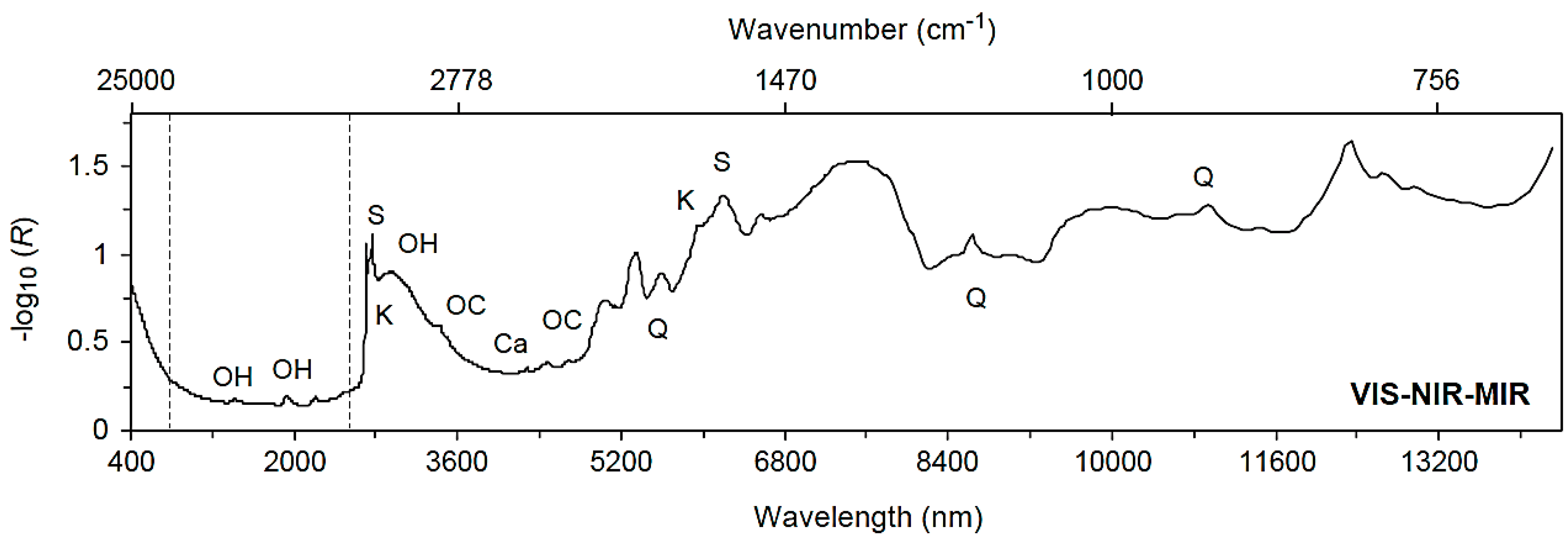
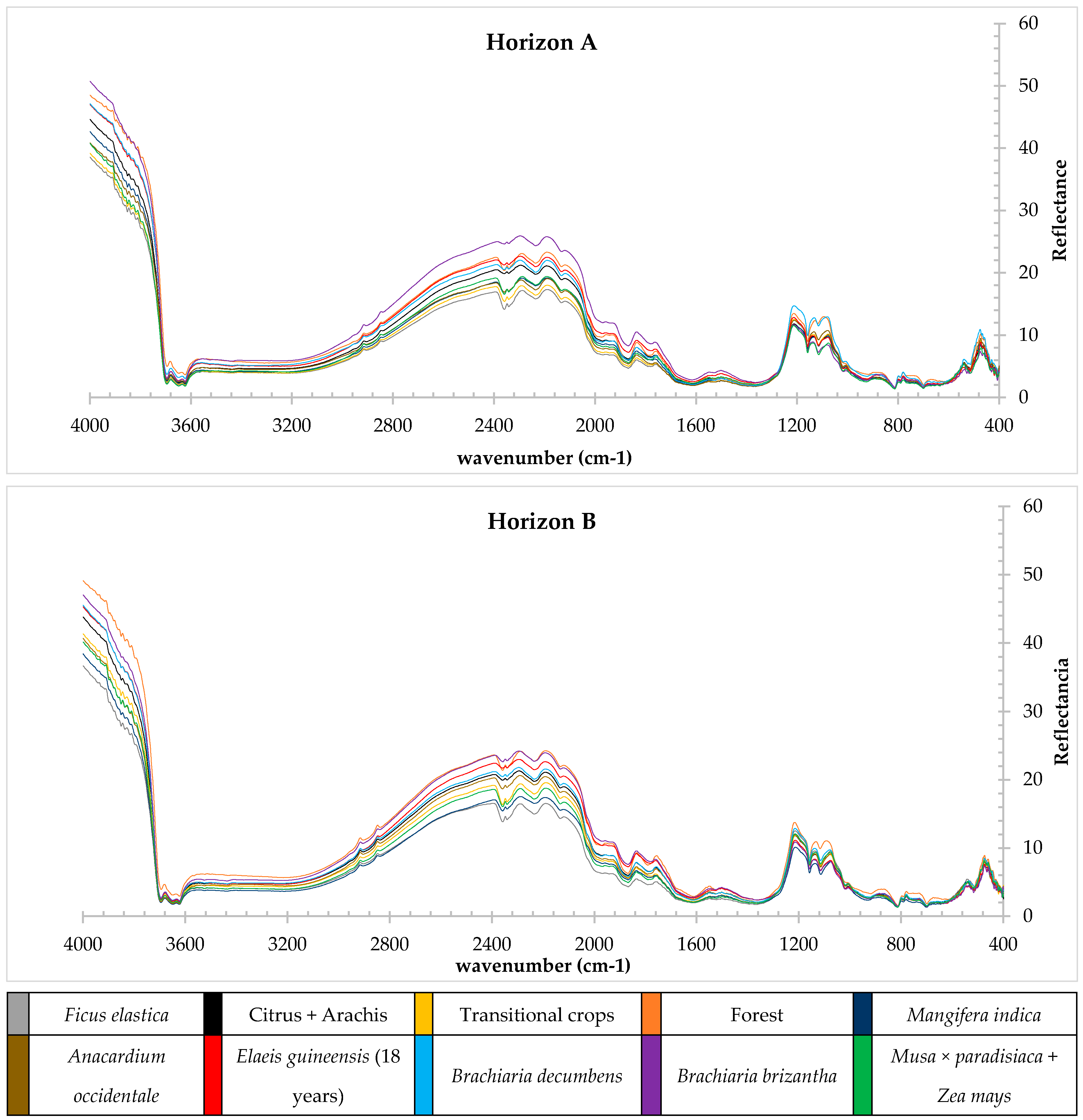
3.2. Spectral Characteristics of Soils
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Yong, H.M.H.; García, A.; Hernandez, A.; Songa, H. Prediction of soil macronutrients content using near-infrared spectroscopy. Comput. Electron. Agric. 2007, 58, 144–153. [Google Scholar]
- Viscarra, R.R. Visible, near infrared, mid infrared or combined diffuse reflectance spectroscopy for simultaneous assessment of various soil properties. Geoderma 2006, 131, 59–75. [Google Scholar] [CrossRef]
- Deaville, E.; Flinn, P. Near-Infrared (NIR) Spectroscopy: An Alternative Approach for the Estimation of Forage Quality and Voluntary Intake; The Centre for Agriculture and Bioscience International (CABI) Publishing: Wallingford, UK, 2000; pp. 301–320. [Google Scholar]
- Reeves III, J.B. Use of Near-Infrared Reflectance Spectroscopy. Farm Animal Metabolism and Nutrition; The Centre for Agriculture and Bioscience International (CABI) Publishing: Wallingford, UK, 2000; Volume 9, pp. 184–209. [Google Scholar]
- Norris, K.; Barnes, R.; Moore, J.; Shenk, J. Predicting forage quality by infrared reflectance spectroscopy. J. Anim. Sci. 1976, 43, 889–897. [Google Scholar] [CrossRef]
- Fahey, G.C.; Hussein, H. Forage quality symposium. Forty years of forage quality research: Accomplishments and impact from an animal nutrition perspective. Crop Sci. 1999, 39, 4–12. [Google Scholar] [CrossRef]
- Williams, P.C.; Norris, K.H. Qualitative applications of near infrared reflectance spectroscopy. In Near-Infrared Technology in the Agricultural and Food Industries; Williams, P., Norris, K., Eds.; American Association of Cereal Chemists: St. Paul, MN, USA, 2009; pp. 241–246. [Google Scholar]
- Viscarra Rossel, R.A.; Lark, R.M. Improved analysis and modelling of soil diffuse reflectance spectra using wavelets. Eur. J. Soil Sci. 2009, 60, 453–464. [Google Scholar] [CrossRef]
- Chodak, M.; Khanna, P.; Horvarth, B.; Beese, F. Near infrared spectroscopy for determination of total and exchangeable cations in geologically heterogeneous forest soils. J. Near Infrared Spectrosc. 2004, 12, 315–324. [Google Scholar] [CrossRef]
- Sorensen, L.; Dalsgaard, S. Determination of clay and other soil properties by near infrared spectroscopy. Soil Sci. Soc. Am. J. 2005, 69, 159–167. [Google Scholar] [CrossRef]
- Cohen, M.; Prenger, J.; DeBusk, W. Visible-Near infrared reflectance spectroscopy for rapid, non-destructive assessment of wetland soil quality. J. Environ. Qual. 2005, 34, 1422–1434. [Google Scholar] [CrossRef] [PubMed]
- Guerrero, C. Near-infrared spectroscopy to estimate the maximum temperatures reached on burned soils. Soil Sci. Soc. Am. J. 2007, 71, 1029–1037. [Google Scholar] [CrossRef]
- Nguyen, T.; Janik, L.; Raupach, M. Diffuse reflectance infrared Fourier transform (DRIFT) spectroscopy in soil studies. Aust. J. Soil Res. 1991, 29, 49–67. [Google Scholar] [CrossRef]
- Ben-Dor, E.; Banin, A. Near infrared analysis (NIRA) as a method to simultaneously evaluate spectral featureless constituents in soils. Soil Sci. 1995, 4, 259–270. [Google Scholar] [CrossRef]
- Scheinost, A.; Chavernas, A.; Barrón, V.; Torrent, J. Use and limitations of second-derivative diffuse reflectance spectroscopy in the visible to near-infrared range to identify and quantify Fe oxides in soils. Clays Clay Miner. 1998, 46, 528–536. [Google Scholar] [CrossRef]
- Minasny, B.; McBratney, A.B. Regression rules as a tool for predicting soil properties from infrared reflectance spectroscopy. Chemom. Intell. Lab. Syst. 2008, 94, 72–79. [Google Scholar] [CrossRef]
- Janik, L. Rapid prediction of soil water retention using mid infrared spectroscopy. Soil Sci. Soc. Am. 2007, 71, 507–514. [Google Scholar] [CrossRef]
- Rodríguez, M.A. Indicadores Ambientales Para el uso del Suelo. 2005. Available online: www.dama.gov.co (accessed on 9 February 2017). [Google Scholar]
- Orlando, O.J.; Sandra, M.J.B.; Gustavo, N.V.L. Caracterización morfo-agronómica y molecular de mandarina ‘Arrayana’ en el piedemonte del Meta (Colombia). Rev. Corpoica Cienc. Tecnol. Agropec. 2012, 13, 5–12. (In Spanish) [Google Scholar]
- Orduz, J.O.; Baquero, J. Aspectos basicos para el cultivo de los cítricos en el piedemonte llanero. Rev. Achagua. 2003, 7, 7–20. (In Spanish) [Google Scholar]
- Rincón, C.A.; Caicedo, G.S. Monitoreo de las condiciones de los suelos establecidos con la asociación maíz/pastos para la recuperación praderas degradadas en el piedemonte llanero. In Proceedings of the En: Memorias del XV Congreso colombiano de la ciencia del suelo, “El suelo: Soporte de la biodiversidad y la producción en los agroecosistemas tropicales”, Pereira, Colombia, 27–29 Octubre 2010. (In Spanish)
- Janeth, G.N. Efecto del Uso y Ocupación en las Propiedades Físicas y Químicas en un Suelo del Piedemonte Llanero. Master’s Thesis, Universidad Nacional de Colombia, Bogota, Colombia, 2014. [Google Scholar]
- Bisquerra, R. Metodología de la Investigación Educativa; La Muralla: Madrid, Spain, 2004; p. 59. (In Spanish) [Google Scholar]
- Norusis, M.J. SPSS 11.0 Guide to Data Analysis; Prentice Hall: Upper Saddle River, NJ, USA, 2002. [Google Scholar]
- Gaviria, S.; Faivre, P. Génesis y evolución mineralógica de oxisoles de los. Anal. Geogr. 2005, 1, 50–64. (In Spanish) [Google Scholar]
- Instituto Geografico Agustín Codazzi (IGAC). Estudio Semidetallado de los Suelos del Centro de Investigaciones Agropecuairas de Carimagua ICA—CIAT; Departamento del Meta. Instituto Geografico Agustín Codazzi, Subdirección de Agrología: Bogota, Colombia, 1983; p. 291. (In Spanish)
- Malagon, C.D. Ensayo sobre tipología de suelos colombianos—Énfasis en génesis y aspectos ambientales. Rev. Acad. Colomb. Cienc. 2003, 27, 319–341. (In Spanish) [Google Scholar]
- Zenaida, L.P.; Alvaro, M.; Mary, H.R.; Carlos, B.; Adriana, O.; Alexis, T.; Carmen, R.; Marcia, T. Cambios en las propiedades quimicas de un suelo de sabana luego de la introducción de pasturas mejoradas. Bioagro 2010, 22, 135–144. (In Spanish) [Google Scholar]
- Garavito, N.F. Introducción a Los Suelos; Instituto Geografico Agustín Codazzi (IGAC): Bogota, Colombia, 2012. (In Spanish)
- Elizabeth, H.; del Carmen, G.M.; Enrique, R.J.; Jorge, A. Caracterización del Suelo y Plantas de un Sitio Contaminado Con Hidrocarburos. Available online: http://www.redalyc.org/articulo.oa?id=57324403 (accessed on 25 September 2014). (In Spanish)
- Rubiano, Y. Sistema Georreferenciado de Indicadores de Calidad del Suelo. Herramienta SIG Para Apoyo a la Planificación, uso y Manejo del Suelo. Ph.D. Thesis, Universidad Nacional de Colombia, Palmira, Colombia, 2005. [Google Scholar]
- GenúI, A.M.; Demattê, J.A.M. Prediction of soil chemical attributes using optical remote sensing. Acta Sci. Agron. 2004, 33, 4. [Google Scholar] [CrossRef] [Green Version]
- Camacho-Tamayo, J.H.; Rubiano, S.Y.; Hurtado, S.M.P. Near-infrared (NIR) diffuse reflectance spectroscopy for the prediction of carbon and nitrogen in an Oxisol. Agron. Colomb. 2014, 32, 86–94. [Google Scholar] [CrossRef]
- Dematte, J.A.M.; Huete, A.R.; Ferreira, L.G., Jr.; Alves, M.C.; Nanni, M.R.; Cerri, C.E. Evaluation of tropical soils through ground and orbital sensors. In Proceedings of the 2nd International Conference Geospatial Information in Agriculture and Forestry Veridian, Lake Buena Vista, FL, USA, 2000.
- Demattê, J.A.M. Characterization and discrimination of soils by their reflected electromagnetic energy. Pesqui. Agropecu. Bras. 2002, 37, 1445–1458. [Google Scholar] [CrossRef]
- Giron-Rios, Y.; Oleschko-Lutkova, K.; Parrot, J.; Hernandez-Alcantara, J.M.; García, E.C.; Velazquez-García, J.J. Analisis fractal de la reflectancia de los suelos salinos. Agrociencia 2009, 43, 403–416. (In Spanish) [Google Scholar]
- Dematteê, J.A.M.; Garcia, G.J. Alteration of soil properties through a weathering sequence as evaluated by spectral reflectance. Soil Sci. Soc. Am. J. 1999, 63, 327–342. [Google Scholar] [CrossRef]
- Formaggio, A.R.; Epiphanio, J.C.N.; Valeriano, M.M.; Oliveira, J.B. Comportamento espectral (450–2450 nm) de solos tropicais de Sao Paulo (Spectral behavior (450–2450 nm) of tropical soils from Sao Paulo State, Brazil). Braz. J. Soil Sci. 1996, 20, 467–474. (In Spanish) [Google Scholar]
- Schreirer, H. Quantitative prediction of chemical soil conditions from multiespectral airbone, ground and laboratory measurements. In Proceedings of the 4th Canadian Symposium on Remote Sensing, Ottawa, QC, Canada, 17–18 May 1977; pp. 107–112.
- Janik, L.J.; Merry, R.H.; Skjemstad, J.O. Can mid infrared diffuse reflectance analysis replace soil extractions? Aust. J. Exp. Agric. 1998, 38, 681–696. [Google Scholar] [CrossRef]
- Singh, A.N.; Dwivedi, R.S. The utility of Landsat imagery as an integral part of the data base for small-scale soil mapping. Int. J. Remote Sens. 1986, 7, 1099–1108. [Google Scholar] [CrossRef]
- McDowell, M.L.; Bruland, G.L.; Deenik, J.L.; Grunwald, S.; Knox, N.M. Soil total carbon analysis in Hawaiian soils with visible, near-infrared and mid-infrared diffuse reflectance spectroscopy. Geoderma 2012, 189–190, 312–320. [Google Scholar] [CrossRef]
- Clark, R.N.; King, T.V.V.; Klejwa, M.; Swayze, G.A. High spectral resolution reflectance spectroscopy of minerals. J. Geophys. Res. 1990, 95, 12653–12680. [Google Scholar] [CrossRef]
- Gaffey, S.J.; McFadden, L.A.; Nash, D.; Pieters, C.M. Ultraviolet, visible, and nearinfrared reflectance spectroscopy: Laboratory spectra of geologic materials. In Remote Geochemical Analysis: Elemental and Mineralogical Composition; Pieters, C.M., Englert, P.A., Eds.; Cambridge University Press: Cambridge, UK, 1993; pp. 43–77. [Google Scholar]
- Stuart, B. Infrared Spectroscopy: Fundamentals and Applications; John Wiley & Sons Ltd.: West Sussex, UK, 2004. [Google Scholar]
- Farmer, V.C. The layer silicates. In The Infrared Spectra of Minerals; Farmer, V.C., Ed.; Bartholomew Press: Surrey, UK, 1974; pp. 331–363. [Google Scholar]
- Moenke, H.H.W. Silica, the three-dimensional silicates, borosilicates, and beryllium silicates. In The Infrared Spectra of Minerals; Farmer, V.C., Ed.; Bartholomew Press: Surrey, UK, 1974; pp. 365–382. [Google Scholar]
- Van der Marel, H.W.; Beutelspacher, H. Atlas of Infrared Spectroscopy of Clay Minerals and Their Admixtures; Elsevier Scientific Publishing Company: Amsterdam, The Netherlands, 1976. [Google Scholar]
- Celi, L.; Schnitzer, M.; Nègre, M. Analysis of carboxyl groups in soil humic acids by wet chemical method, Fourier-transform infrared spectrophotometry and solution-state carbon-13 nuclear magnetic resonance. A comparative study. Soil Sci. 1997, 162, 189–197. [Google Scholar] [CrossRef]
- Ellerbrock, R.H.; Kaiser, M. Stability and composition of different soluble soil organic matter fraction evidence from δ13C and FTIR signatures. Geoderma 2005, 128, 28–37. [Google Scholar] [CrossRef]
- Matějkova, Š.; Šimon, T. Application of FTIR spectroscopy for evaluation of hydrophobic/hydrophilic organic components in arable soils. Plant Soil Environ. 2012, 58, 192–195. [Google Scholar]
| Use/Occupation | Location | Use/Occupation | Location | ||
|---|---|---|---|---|---|
| Latitude | Longitude | Latitude | Longitude | ||
| Transitory crops | 4°03,445′ | 73°28,074′ | Brachiaria decumbens | 4°03,445′ | 73°28,196′ |
| 4°03,998′ | 73°26,674′ | 4°03,397′ | 73°28,189′ | ||
| 4°03,949′ | 73°26,726′ | 4°03,401′ | 73°28,227′ | ||
| 4°03,944′ | 73°26,793′ | 4°03,400′ | 73°28,161′ | ||
| Ficus elastica | 4°03,845′ | 73°26,874′ | Citrus + Arachis | 4°03,649′ | 73°27,446′ |
| 4°03,888′ | 73°26,960′ | 4°03,641′ | 73°27,485′ | ||
| 4°03,868′ | 73°26,960′ | 4°03,626′ | 73°27,568′ | ||
| 4°03,836′ | 73°26,939′ | 4°03,617′ | 73°27,605′ | ||
| Musa × paradisiaca + Zea mays | 4°03,617′ | 73°27,693′ | Mangifera indica | 4°03,423′ | 73°28,036′ |
| 4°03,615′ | 73°27,702′ | 4°03,444′ | 73°28,034′ | ||
| 4°03,613′ | 73°27,717′ | 4°03,448′ | 73°28,001′ | ||
| 4°03,606′ | 73°27,719′ | 4°03,423′ | 73°27,998′ | ||
| Elaeis guineensis (18 years) | 4°03,613′ | 73°27,755′ | Forest cultivation | 4°05,532′ | 73°26,580′ |
| 4°03,568′ | 73°27,757′ | 4°05,532′ | 73°26,592′ | ||
| 4°03,529′ | 73°27,750′ | 4°05,680′ | 73°26,587′ | ||
| 4°03,493′ | 73°27,727′ | 4°05,676′ | 73°26,579′ | ||
| Brachiaria brizantha | 4°03,439′ | 73°28,430′ | Anacardium occidentale | 4°03,385′ | 73°28,461′ |
| 4°03,474′ | 73°28,440′ | 4°03,382′ | 73°28,451′ | ||
| 4°03,480′ | 73°28,398′ | 4°03,395′ | 73°28,445′ | ||
| 4°03,439′ | 73°28,387′ | 4°03,393′ | 73°28,452′ | ||
| Crops | Thickness | pH | Ac. I | P Disp. | CIC | Ca | Mg | K | Na | CO | Sand | Mud | Clay |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (mm) | (cmol/kg) | (%) | |||||||||||
| Ficus elastica | 407.5 | 4.0 | 2.40 | 2.60 | 8.7 | 0.275 | 0.08 | 0.13 | 0.075 | 1.150 | 53.08 | 16.40 | 31.79 |
| Musa × paradisiaca + Zea mays | 332.5 | 4.5 | 1.30 | 13.40 | 7.9 | 0.648 | 0.30 | 0.17 | 0.055 | 1.175 | 47.46 | 16.55 | 36.20 |
| Elaeis guineensis (18 years) | 322.5 | 4.5 | 1.90 | 0.14 | 10.8 | 0.830 | 0.10 | 0.12 | 0.040 | 1.163 | 47.07 | 15.55 | 37.50 |
| Brachiaria brizantha | 255.0 | 5.1 | 1.40 | 45.80 | 8.6 | 1.248 | 0.72 | 0.21 | 0.078 | 1.250 | 46.21 | 17.82 | 36.07 |
| Brachiaria decumbens | 345.0 | 4.6 | 1.30 | 9.40 | 8.9 | 0.873 | 0.07 | 0.24 | 0.108 | 1.088 | 54.41 | 18.68 | 17.92 |
| Citrus + Arachis | 412.5 | 4.7 | 1.60 | 18.30 | 9.3 | 0.610 | 0.17 | 0.26 | 0.123 | 1.193 | 43.23 | 21.07 | 23.58 |
| Mangifera indica | 367.5 | 3.9 | 3.80 | 6.40 | 13.0 | 0.710 | 0.16 | 0.28 | 0.113 | 1.500 | 46.84 | 14.72 | 38.30 |
| Forest cultivation | 252.5 | 4.0 | 0.31 | 1.10 | 11.1 | 0.290 | 0.08 | 0.18 | 0.118 | 1.425 | 40.06 | 24.39 | 29.02 |
| Anacardium occidentale | 277.5 | 4.6 | 1.70 | 11.20 | 13.4 | 0.935 | 0.57 | 0.33 | 0.105 | 1.350 | 43.65 | 18.69 | 41.90 |
| Crops | Thickness | pH | Ac. I | P Disp. | CIC | Ca | Mg | K | Na | CO | Sand | Mud | Clay |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (mm) | (cmol/kg) | (%) | |||||||||||
| Ficus elastica | 240.0 | 4.5 | 1.60 | 0.14 | 8.4 | 0.220 | 0.03 | 0.08 | 0.033 | 0.803 | 51.13 | 15.43 | 37.74 |
| Musa × paradisiaca + Zea mays | 160.0 | 4.6 | 1.50 | 0.00 | 6.7 | 0.295 | 0.04 | 0.12 | 0.055 | 0.795 | 50.35 | 16.17 | 33.37 |
| Elaeis guineensis (18 years) | 215.0 | 4.5 | 2.10 | 0.79 | 10.9 | 1.215 | 0.11 | 0.14 | 0.060 | 1.625 | 54.55 | 16.30 | 31.30 |
| Brachiaria brizantha | 232.5 | 4.6 | 2.70 | 0.79 | 10.4 | 0.370 | 0.14 | 0.11 | 0.070 | 0.798 | 49.54 | 18.50 | 33.29 |
| Brachiaria decumbens | 292.5 | 4.9 | 1.60 | 0.00 | 8.2 | 0.550 | 0.03 | 0.20 | 0.125 | 0.475 | 49.57 | 17.04 | 31.22 |
| Citrus + Arachis | 192.5 | 4.8 | 0.99 | 0.00 | 11.1 | 0.763 | 0.57 | 0.59 | 0.103 | 0.868 | 39.75 | 16.90 | 43.74 |
| Mangifera indica | 237.5 | 4.7 | 2.10 | 0.14 | 8.5 | 0.328 | 0.03 | 0.18 | 0.083 | 0.795 | 40.04 | 17.06 | 38.89 |
| Forest cultivation | 182.5 | 4.3 | 2.90 | 0.00 | 8.9 | 0.240 | 0.05 | 0.13 | 0.103 | 0.675 | 33.39 | 23.58 | 37.87 |
| Anacardium occidentale | 217.5 | 4.5 | 2.70 | 0.57 | 11.1 | 0.405 | 0.12 | 0.26 | 0.088 | 0.855 | 58.18 | 18.37 | 15.91 |
| Crops | Thickness | pH | Ac. I | P Disp. | CIC | Ca | Mg | K | Na | CO | Sand | Mud | Clay |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (mm) | (cmol/kg) | (%) | |||||||||||
| Thickness | 1 | −0.278 | 0.101 | 0.268 | 0.085 | 0.138 | −0.055 | −0.022 | 0.230 | 0.381 | 0.175 | −0.126 | −0.237 |
| pH | 1 | −0.365 | 0.432 | −0.333 | 0.405 | 0.445 | 0.227 | −0.052 | −0.388 | 0.013 | −0.138 | 0.038 | |
| Ac. I | 1 | −0.190 | 0.273 | −0.128 | −0.256 | −0.188 | −0.026 | −0.034 | 0.139 | −0.252 | 0.038 | ||
| P Disp. | 1 | −0.097 | 0.577 * | 0.685 ** | 0.105 | 0.096 | 0.336 | −0.050 | 0.054 | −0.016 | |||
| CIC | 1 | 0.337 | 0.309 | 0.460 | 0.313 | 0.539 * | −0.088 | 0.061 | 0.135 | ||||
| Ca | 1 | 0.625 ** | 0.331 | 0.031 | 0.576 * | 0.152 | −0.251 | 0.070 | |||||
| Mg | 1 | 0.611 ** | 0.110 | 0.289 | −0.228 | −0.049 | 0.389 | ||||||
| K | 1 | 0.518 * | 0.074 | −0.256 | 0.000 | 0.154 | |||||||
| Na | 1 | 0.020 | −0.358 | 0.548 * | −0.246 | ||||||||
| CO | 1 | 0.088 | −0.016 | −0.005 | |||||||||
| Sand | 1 | −0.531 * | −0.566 * | ||||||||||
| Mud | 1 | −0.276 | |||||||||||
| Clay | 1 | ||||||||||||
© 2017 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martín-López, J.M.; Quintero-Arias, G. Effect of Soil Use and Coverage on the Spectral Response of an Oxisol in the VIS-NIR-MIR Region. J. Imaging 2017, 3, 10. https://doi.org/10.3390/jimaging3010010
Martín-López JM, Quintero-Arias G. Effect of Soil Use and Coverage on the Spectral Response of an Oxisol in the VIS-NIR-MIR Region. Journal of Imaging. 2017; 3(1):10. https://doi.org/10.3390/jimaging3010010
Chicago/Turabian StyleMartín-López, Javier M., and Giovanna Quintero-Arias. 2017. "Effect of Soil Use and Coverage on the Spectral Response of an Oxisol in the VIS-NIR-MIR Region" Journal of Imaging 3, no. 1: 10. https://doi.org/10.3390/jimaging3010010






