The Dose Optimization and Evaluation of Image Quality in the Adult Brain Protocols of Multi-Slice Computed Tomography: A Phantom Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Image Acquisition Protocols
2.2. Data Acquisition and Image Quality Evaluation
2.3. Catphan 700 Phantom
2.4. CT Number Accuracy and Linearity
2.5. The High-Contrast Spatial Resolution and Modulation Transfer Function (MTF)
2.6. Low-Contrast Detectability and Contrast-to-Noise Ratio (CNR)
2.7. Image Noise, Uniformity, and Mean CT Number
2.8. Radiation Dose
2.9. Statistical Analysis
3. Results
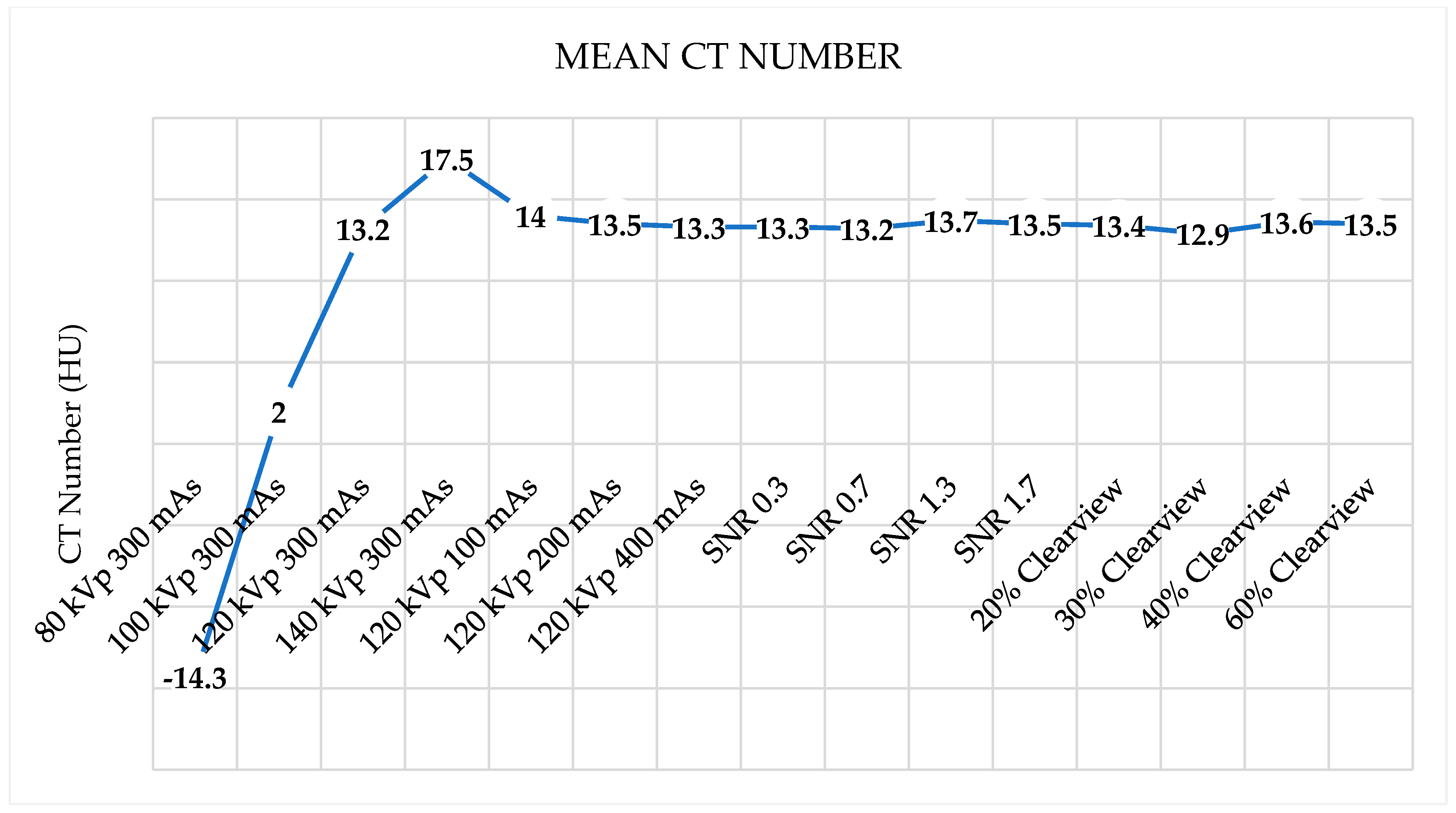
3.1. CT Number Accuracy
3.2. Modulation Transfer Function (MTF)
3.3. High-Contrast Spatial Resolution
3.4. Low-Contrast Detectability
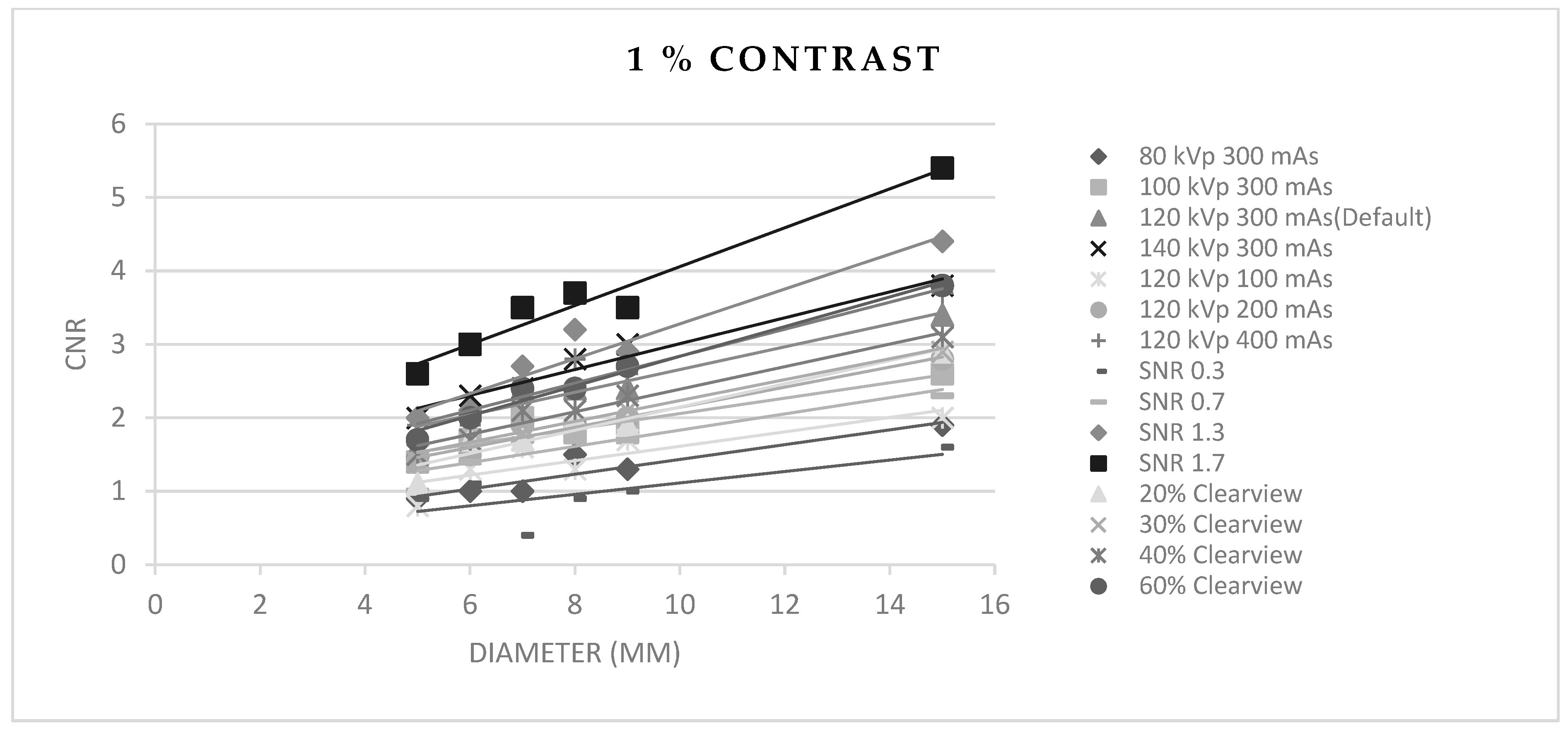
3.5. Contrast-to-Noise Ratio (CNR)
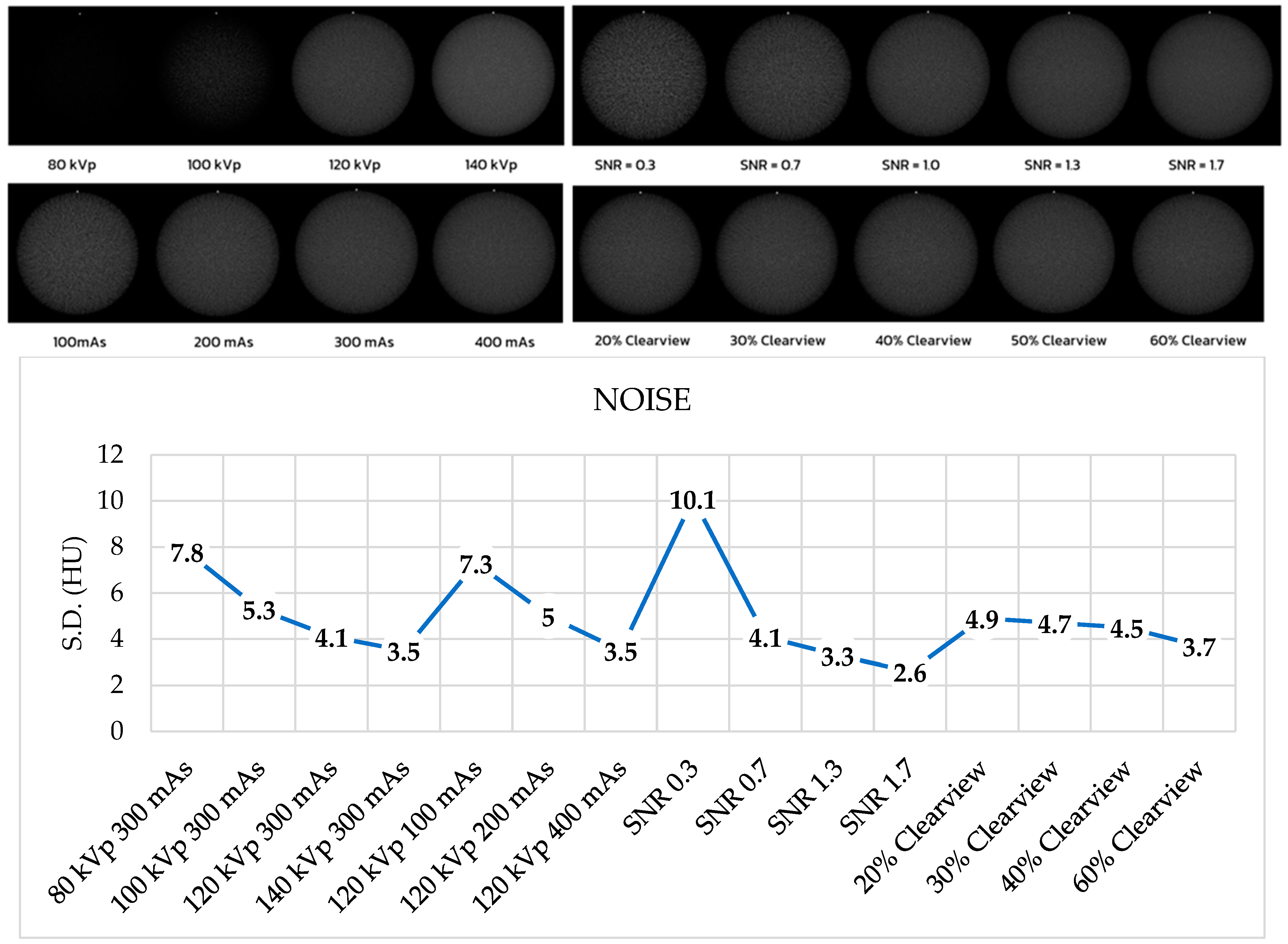
3.6. Image Noise, Uniformity, and Mean CT Number
3.7. Radiation Dose
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Baselet, B.; Rombouts, C.; Benotmane, A.M.; Baatout, S.; Aerts, A. Cardiovascular diseases related to ionizing radiation: The risk of low-dose exposure. Int. J. Mol. Med. 2016, 38, 1623–1641. [Google Scholar] [CrossRef] [PubMed]
- Baskan, O.; Erol, C.; Ozbek, H.; Paksoy, Y. Effect of radiation dose reduction on image quality in adult head CT with noise-suppressing reconstruction system with a 256 slice MDCT. J. Appl. Clin. Med. Phys. 2015, 16, 285–296. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Schoepf, U.J.; Wu, R.; Reddy, R.P.; Zhang, C.; Yu, W.; Liu, Y.; Zhang, Z. Image quality and radiation dose of low dose coronary CT angiography in obese patients: Sinogram affirmed iterative reconstruction versus filtered back projection. Eur. J. Radiol. 2012, 81, 3141–3145. [Google Scholar] [CrossRef]
- Wichmann, J.L.; Hardie, A.D.; Schoepf, U.J.; Felmly, L.M.; Perry, J.D.; Varga-Szemes, A.; Mangold, S.; Caruso, D.; Canstein, C.; Vogl, T.J.; et al. Single-and dual-energy CT of the abdomen: Comparison of radiation dose and image quality of 2nd and 3rd generation dual-source CT. Eur. Radiol. 2017, 27, 642–650. [Google Scholar] [CrossRef] [PubMed]
- Schuhbaeck, A.; Achenbach, S.; Layritz, C.; Eisentopf, J.; Hecker, F.; Pflederer, T.; Gauss, S.; Rixe, J.; Kalender, W.; Daniel, W.G.; et al. Image quality of ultra-low radiation exposure coronary CT angiography with an effective dose< 0.1 mSv using high-pitch spiral acquisition and raw data-based iterative reconstruction. Eur. Radiol. 2013, 23, 597–606. [Google Scholar]
- Nakaura, T.; Kidoh, M.; Sakaino, N.; Utsunomiya, D.; Oda, S.; Kawahara, T.; Harada, K.; Yamashita, Y. Low contrast-and low radiation dose protocol for cardiac CT of thin adults at 256-row CT: Usefulness of low tube voltage scans and the hybrid iterative reconstruction algorithm. Int. J. Cardiovasc. Imaging 2013, 29, 913–923. [Google Scholar] [CrossRef] [PubMed]
- Nagayama, Y.; Iwashita, K.; Maruyama, N.; Uetani, H.; Goto, M.; Sakabe, D.; Emoto, T.; Nakato, K.; Shigematsu, S.; Kato, Y.; et al. Deep learning-based reconstruction can improve the image quality of low radiation dose head CT. Eur. Radiol. 2023, 33, 3253–3265. [Google Scholar] [CrossRef] [PubMed]
- Vassileva, J.; Rehani, M. Diagnostic reference levels. AJR Am. J. Roentgenol. 2015, 204, W1–W3. [Google Scholar] [CrossRef] [PubMed]
- Vañó, E.; Miller, D.; Martin, C.; Rehani, M.; Kang, K.; Rosenstein, M.; Ortiz-López, P.; Mattsson, S.; Padovani, R.; Rogers, A. ICRP publication 135: Diagnostic reference levels in medical imaging. Ann. ICRP 2017, 46, 1–144. [Google Scholar] [CrossRef]
- Seibert, J.A. Tradeoffs between image quality and dose. Pediatr. Radiol. 2004, 34, S183–S195. [Google Scholar] [CrossRef]
- Strauss, K.J.; Goske, M.J.; Kaste, S.C.; Bulas, D.; Frush, D.P.; Butler, P.F.; Morrison, G.; Callahan, M.J.; Applegate, K.E. Image gently: Ten steps you can take to optimize image quality and lower CT dose for pediatric patients. Am. J. Roentgenol. 2010, 194, 868–873. [Google Scholar] [CrossRef] [PubMed]
- Power, S.P.; Moloney, F.; Twomey, M.; James, K.; O’Connor, O.J.; Maher, M.M. Computed tomography and patient risk: Facts, perceptions and uncertainties. World J. Radiol. 2016, 8, 902. [Google Scholar] [CrossRef]
- Brody, A.S.; Frush, D.P.; Huda, W.; Brent, R.L.; Section on Radiology. Radiation risk to children from computed tomography. Pediatrics 2007, 120, 677–682. [Google Scholar] [CrossRef] [PubMed]
- Omer, H.; Alameen, S.; Mahmoud, W.E.; Sulieman, A.; Nasir, O.; Abolaban, F. Eye lens and thyroid gland radiation exposure for patients undergoing brain computed tomography examination. Saudi J. Biol. Sci. 2021, 28, 342–346. [Google Scholar] [CrossRef] [PubMed]
- Tarkiainen, J.; Nadhum, M.; Heikkilä, A.; Rinta-Kiikka, I.; Joutsen, A. Radiation dose of the eye lens in CT examinations of the brain in clinical practice—The effect of radiographer training to optimise gantry tilt and scan length. Radiat. Prot. Dosim. 2023, 199, 391–398. [Google Scholar] [CrossRef]
- Tien, H.C.; Tremblay, L.N.; Rizoli, S.B.; Gelberg, J.B.; Spencer, F.; Caldwell, C.; Brenneman, F.D. Radiation exposure from diagnostic imaging in severely injured trauma patients. J. Trauma Acute Care Surg. 2007, 62, 151–156. [Google Scholar] [CrossRef] [PubMed]
- Lin, E.C. Radiation risk from medical imaging. In Mayo Clinic Proceedings; Elsevier: Amsterdam, The Netherlands, 2010; pp. 1142–1146. [Google Scholar]
- Smith-Bindman, R.; Kwan, M.L.; Marlow, E.C.; Theis, M.K.; Bolch, W.; Cheng, S.Y.; Bowles, E.J.A.; Duncan, J.R.; Greenlee, R.T.; Kushi, L.H.; et al. Trends in use of medical imaging in US health care systems and in Ontario, Canada, 2000–2016. JAMA 2019, 322, 843–856. [Google Scholar] [CrossRef]
- Geise, R.A. Computed tomography: Physical principles, clinical applications, and quality control. Radiology 1995, 194, 782. [Google Scholar] [CrossRef]
- Mayo-Smith, W.W.; Hara, A.K.; Mahesh, M.; Sahani, D.V.; Pavlicek, W. How I do it: Managing radiation dose in CT. Radiology 2014, 273, 657–672. [Google Scholar] [CrossRef]
- McNitt-Gray, M.F. AAPM/RSNA physics tutorial for residents: Topics in CT: Radiation dose in CT. Radiographics 2002, 22, 1541–1553. [Google Scholar] [CrossRef]
- Raman, S.P.; Mahesh, M.; Blasko, R.V.; Fishman, E.K. CT scan parameters and radiation dose: Practical advice for radiologists. J. Am. Coll. Radiol. 2013, 10, 840–846. [Google Scholar] [CrossRef] [PubMed]
- Stiller, W. Basics of iterative reconstruction methods in computed tomography: A vendor-independent overview. Eur. J. Radiol. 2018, 109, 147–154. [Google Scholar] [CrossRef] [PubMed]
- Geyer, L.L.; Schoepf, U.J.; Meinel, F.G.; Nance, J.W., Jr.; Bastarrika, G.; Leipsic, J.A.; Paul, N.S.; Rengo, M.; Laghi, A.; De Cecco, C.N. State of the art: Iterative CT reconstruction techniques. Radiology 2015, 276, 339–357. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Yoon, H.J.; Lee, E.; Kim, I.; Cha, Y.K.; Bak, S.H. Validation of deep-learning image reconstruction for low-dose chest computed tomography scan: Emphasis on image quality and noise. Korean J. Radiol. 2021, 22, 131. [Google Scholar] [CrossRef]
- Padole, A.M.; Sagar, P.; Westra, S.J.; Lim, R.; Nimkin, K.; Kalra, M.K.; Gee, M.S.; Rehani, M.M. Development and validation of image quality scoring criteria (IQSC) for pediatric CT: A preliminary study. Insights Imaging 2019, 10, 1–11. [Google Scholar] [CrossRef]
- Scholtz, J.-E.; Kaup, M.; Kraft, J.; Nöske, E.-M.; Scheerer, F.; Schulz, B.; Burck, I.; Wagenblast, J.; Kerl, J.M.; Bauer, R.W.; et al. Objective and subjective image quality of primary and recurrent squamous cell carcinoma on head and neck low-tube-voltage 80-kVp computed tomography. Neuroradiology 2015, 57, 645–651. [Google Scholar] [CrossRef]
- Tamura, A.; Mukaida, E.; Ota, Y.; Kamata, M.; Abe, S.; Yoshioka, K. Superior objective and subjective image quality of deep learning reconstruction for low-dose abdominal CT imaging in comparison with model-based iterative reconstruction and filtered back projection. Br. J. Radiol. 2021, 94, 20201357. [Google Scholar] [CrossRef]
- Pahn, G.; Skornitzke, S.; Schlemmer, H.P.; Kauczor, H.U.; Stiller, W. Toward standardized quantitative image quality (IQ) assessment in computed tomography (CT): A comprehensive framework for automated and comparative IQ analysis based on ICRU Report 87. Phys. Med. 2016, 32, 104–115. [Google Scholar] [CrossRef]
- Hussain, F.A.; Mail, N.; Shamy, A.M.; Alghamdi, S.; Saoudi, A. A qualitative and quantitative analysis of radiation dose and image quality of computed tomography images using adaptive statistical iterative reconstruction. J. Appl. Clin. Med. Phys. 2016, 17, 419–432. [Google Scholar] [CrossRef]
- Mail, T.B. Catphan® 700 Manual. 2013. Available online: https://static1.squarespace.com/static/5367b059e4b05a1adcd295c2/t/58b5c5ff2994ca89008893d9/1488307742189/Catphan700Manual.pdf (accessed on 1 August 2023).
- Goodenough, D.; Levy, J.; Kristinsson, S.; Fredriksson, J.; Olafsdottir, H.; Healy, A. Method and phantom to study combined effects of in-plane (x, y) and z-axis resolution for 3D CT imaging. J. Appl. Clin. Med. Phys. 2016, 17, 440–452. [Google Scholar] [CrossRef]
- Neusoft Medical Systems Co., Ltd. Nuesoft NeuViz 128 Computed Tomography [Internet]. 2016. Available online: https://intermed1.com/wp-content/uploads/2019/03/NeuViz128_brochure_0716.pdf (accessed on 15 September 2023).
- Dillon, C.; Davidson, C.; Hernandez, D. 2017 Computed Tomography Quality Control Manual. American College of Radiology. 2017, pp. 73–74. Available online: https://www.acr.org/-/media/ACR/Files/Clinical-Resources/QC-Manuals/CT_QCManual.pdf (accessed on 15 September 2023).
- Anam, C.; Amilia, R.; Naufal, A.; Budi, W.S.; Maya, A.T.; Dougherty, G. The automated measurement of CT number linearity using an ACR accreditation phantom. Biomed. Phys. Eng. Express 2022, 9, 017002. [Google Scholar] [CrossRef] [PubMed]
- McCollough, C.H.; Bruesewitz, M.R.; McNitt-Gray, M.F.; Bush, K.; Ruckdeschel, T.; Payne, J.T.; Brink, J.A.; Zeman, R.K. The phantom portion of the American College of Radiology (ACR) Computed Tomography (CT) accreditation program: Practical tips, artifact examples, and pitfalls to avoid. Med. Phys. 2004, 31, 2423–2442. [Google Scholar] [CrossRef] [PubMed]
- Friedman, S.N.; Fung, G.S.; Siewerdsen, J.H.; Tsui, B.M. A simple approach to measure computed tomography (CT) modulation transfer function (MTF) and noise-power spectrum (NPS) using the American College of Radiology (ACR) accreditation phantom. Med. Phys. 2013, 40, 051907. [Google Scholar] [CrossRef] [PubMed]
- Takenaga, T.; Katsuragawa, S.; Goto, M.; Hatemura, M.; Uchiyama, Y.; Shiraishi, J. Modulation transfer function measurement of CT images by use of a circular edge method with a logistic curve-fitting technique. Radiol. Phys. Technol. 2015, 8, 53–59. [Google Scholar] [CrossRef] [PubMed]
- Judy, P.F. The line spread function and modulation transfer function of a computed tomographic scanner. Med. Phys. 1976, 3, 233–236. [Google Scholar] [CrossRef] [PubMed]
- Bissonnette, J.P.; Moseley, D.J.; Jaffray, D.A. A quality assurance program for image quality of cone-beam CT guidance in radiation therapy. Med. Phys. 2008, 35, 1807–1815. [Google Scholar] [CrossRef]
- STRÅLSKYDDSFRÅGOR NRO. A Quality Control Programme for Radiodiagnostic Equipment: Acceptance Tests. 1999. Available online: https://citeseerx.ist.psu.edu/document?repid=rep1&type=pdf&doi=bf7cceea8dabd373fd33df43393d1c569f70be03 (accessed on 15 September 2023).
- Li, Y.; Jiang, Y.; Liu, H.; Yu, X.; Chen, S.; Ma, D.; Gao, J.; Wu, Y. A phantom study comparing low-dose CT physical image quality from five different CT scanners. Quant. Imaging Med. Surg. 2022, 12, 766. [Google Scholar] [CrossRef]
- Bellesi, L.; Wyttenbach, R.; Gaudino, D.; Colleoni, P.; Pupillo, F.; Carrara, M.; Braghetti, A.; Puligheddu, C.; Presilla, S. A simple method for low-contrast detectability, image quality and dose optimisation with CT iterative reconstruction algorithms and model observers. Eur. Radiol. Exp. 2017, 1, 1–10. [Google Scholar] [CrossRef]
- Anam, C.; Amilia, R.; Naufal, A.; Sutanto, H.; Dwihapsari, Y.; Fujibuchi, T.; Dougherty, G. Impact of Noise Level on the Accuracy of Automated Measurement of CT Number Linearity on ACR CT and Computational Phantoms. J. Biomed. Phys. Eng. 2023, 13, 353. [Google Scholar]
- Romans, L.E. Computed Tomography for Technologist, A Comprehensive Text; Wolter Kluwer Health; Lippincott Williams and Wilkins: Philadelphia, PA, USA, 2011. [Google Scholar]
- Baxter, B.S.; Sorenson, J.A. Factors affecting the measurement of size and CT number in computed tomography. Investig. Radiol. 1981, 16, 337–341. [Google Scholar] [CrossRef]
- Cann, C.E. Quantitative CT for determination of bone mineral density: A review. Radiology 1988, 166, 509–522. [Google Scholar] [CrossRef]
- Rueckel, J.; Stockmar, M.; Pfeiffer, F.; Herzen, J. Spatial resolution characterization of a X-ray microCT system. Appl. Radiat. Isot. 2014, 94, 230–234. [Google Scholar] [CrossRef] [PubMed]
- Grimmer, R.; Krause, J.; Karolczak, M.; Lapp, R.; Kachelriess, M. Assessment of spatial resolution in CT. In 2008 IEEE Nuclear Science Symposium Conference Record; IEEE: New York, NY, USA, 2008; pp. 5562–5566. [Google Scholar]
- Roa, A.M.A.; Andersen, H.K.; Martinsen, A.C.T. CT image quality over time: Comparison of image quality for six different CT scanners over a six-year period. J. Appl. Clin. Med. Phys. 2015, 16, 350–365. [Google Scholar] [CrossRef] [PubMed]
- Gulliksrud, K.; Stokke, C.; Martinsen, A.C.T. How to measure CT image quality: Variations in CT-numbers, uniformity and low contrast resolution for a CT quality assurance phantom. Phys. Medica 2014, 30, 521–526. [Google Scholar] [CrossRef] [PubMed]
- Manson, E.N.; Fletcher, J.J.; Della Atuwo-Ampoh, V.; Addison, E.K.; Schandorf, C.; Bambara, L. Assessment of some image quality tests on a 128 slice computed tomography scanner using a Catphan700 phantom. J. Med. Phys./Assoc. Med. Phys. India 2016, 41, 153. [Google Scholar] [CrossRef] [PubMed]
- Ghetti, C.; Ortenzia, O.; Serreli, G. CT iterative reconstruction in image space: A phantom study. Phys. Medica 2012, 28, 161–165. [Google Scholar] [CrossRef] [PubMed]
- Löve, A.; Siemund, R.; Höglund, P.; Van Westen, D.; Stenberg, L.; Petersen, C.; Björkman-Burtscher, I.M. Hybrid iterative reconstruction algorithm in brain CT: A radiation dose reduction and image quality assessment study. Acta Radiol. 2014, 55, 208–217. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; He, W.; Chen, H.; Hu, Z.; Li, J.; Zhang, T. Impact of the adaptive statistical iterative reconstruction technique on image quality in ultra-low-dose CT. Clin. Radiol. 2013, 68, 902–908. [Google Scholar] [CrossRef]
- Hojreh, A.; Weber, M.; Homolka, P. Effect of staff training on radiation dose in pediatric CT. Eur. J. Radiol. 2015, 84, 1574–1578. [Google Scholar] [CrossRef]
- Trattner, S.; Pearson, G.D.; Chin, C.; Cody, D.D.; Gupta, R.; Hess, C.P.; Kalra, M.K.; Kofler, J.M.; Krishnam, M.S.; Einstein, A.J. Standardization and optimization of CT protocols to achieve low dose. J. Am. Coll. Radiol. 2014, 11, 271–278. [Google Scholar] [CrossRef]







| Parameters | Default (Optimized Protocol) |
|---|---|
| kVp | 120 (80, 100, 140) |
| mAs | 300 (100, 200, 400) |
| Rotation time | 1.0 s |
| Pitch | 0.5 |
| Slice thickness | 5 mm |
| SNR level | 1.0 (0.3, 0.7, 1.3, 1.7) |
| FOV | 250 mm |
| Kernel | F20 |
| IR | 50% (20%, 30%, 40%, 60%) Clearview |
| Matrix | 512 × 12 |
| Materials | 80 kVp 300 mAs | 100 kVp 300 mAs | 120 kVp 300 mAs (Default) | 140 kVp 300 mAs | 120 kVp 100 mAs | 120 kVp 200 mAs | 120 kVp 400 mAs | |
|---|---|---|---|---|---|---|---|---|
| Air | −973.8 | −971.9 | −967.8 | −968 | −968 | −968 | −969.1 | |
| Lung | −806.5 | −805.1 | −798.5 | −800.3 | −798.4 | −800 | −800 | |
| PMP | −211.2 | −190.8 | −177.1 | −171.1 | −176.5 | −176.6 | −176.5 | |
| LDPE | −123.9 | −103.7 | −90 | −82.1 | −89.1 | −88.8 | −89 | |
| Polystyrene | −67.5 | −46.9 | −33.6 | −27.7 | −34.7 | −33.3 | −33.8 | |
| Water | −0.9 | 1.3 | 2.8 | 0.7 | 3.5 | 3.5 | 3.3 | |
| Acrylic | 98.5 | 112.6 | 122.6 | 127.1 | 123.5 | 124.6 | 123.3 | |
| Bone20 | 302 | 168.5 | 186.9 | 223.6 | 186.2 | 187.1 | 187.5 | |
| Delrin | 330.4 | 244.7 | 301.1 | 360.9 | 300.3 | 301.3 | 301.1 | |
| Bone50 | 908.3 | 643.1 | 629.9 | 636 | 628.2 | 631 | 629 | |
| Teflon | 969 | 813.5 | 882 | 931.1 | 882.4 | 882.1 | 883.1 | |
| r | 0.998 | 0.998 | 0.999 | 0.999 | 0.999 | 0.999 | 0.999 | |
| Materials | SNR 0.3 | SNR 0.7 | SNR 1.3 | SNR 1.7 | 20% Clearview | 30% Clearview | 40% Clearview | 60% Clearview |
| Air | −969.6 | −968.6 | −966.7 | −967.6 | −969.9 | −969.6 | −970.2 | −965.3 |
| Lung | −800.5 | −799.4 | −798.3 | −797.8 | −800.7 | −799.7 | −801.5 | −797.8 |
| PMP | −177.5 | −176.9 | −177.4 | −176.5 | −176.6 | −176.9 | −177.5 | −175.9 |
| LDPE | −90.8 | −89.3 | −88.8 | −89.2 | −89.3 | −89.9 | −89.7 | −89.6 |
| Polystyrene | −35.5 | −34.7 | −33.9 | −34.3 | −34.6 | −34.5 | −35 | −33.6 |
| Water | 2.2 | 2.5 | 1.8 | 2.6 | 2.7 | 3.7 | 1.4 | 3.2 |
| Acrylic | 122.6 | 124.1 | 123.3 | 123.8 | 123.6 | 123.2 | 123 | 123.3 |
| Bone20 | 188.8 | 186.4 | 187.7 | 188.3 | 188.8 | 189.1 | 187.8 | 184 |
| Delrin | 300.4 | 299.8 | 300.1 | 300 | 299.3 | 300.7 | 299.7 | 300.8 |
| Bone50 | 628.6 | 629.3 | 628.1 | 629.1 | 628.6 | 628.8 | 628.4 | 628.3 |
| Teflon | 881.6 | 881.7 | 879.7 | 880.4 | 882.4 | 881.1 | 879.9 | 882.7 |
| r | 0.999 | 0.999 | 0.999 | 0.999 | 0.999 | 0.999 | 0.999 | 0.999 |
| MTF (%) | 80 kVp 300 mAs | 100 kVp 300 mAs | 120 kVp 300 mAs (Default) | 140 kVp 300 mAs | 120 kVp 100 mAs | 120 kVp 200 mAs | 120 kVp 400 mAs | |
|---|---|---|---|---|---|---|---|---|
| 50 | 3.74 | 4.37 | 4.18 | 3.86 | 4.03 | 4.28 | 4.5 | |
| 10 | 6.85 | 7.07 | 7.1 | 6.69 | 6.97 | 6.97 | 6.97 | |
| 2 | 8.33 | 8.34 | 8.82 | 8.41 | 8.09 | 8.09 | 8.31 | |
| MTF (%) | SNR 0.3 | SNR 0.7 | SNR 1.3 | SNR 1.7 | 20% Clearview | 30% Clearview | 40% Clearview | 60% Clearview |
| 50 | 4.41 | 4.41 | 4.41 | 4.41 | 3.95 | 4.12 | 4.12 | 4.29 |
| 10 | 6.87 | 6.93 | 6.94 | 7.02 | 6.83 | 6.95 | 7.12 | 7.12 |
| 2 | 8.45 | 8.5 | 8.52 | 8.62 | 8.54 | 8.55 | 8.59 | 8.59 |
| 80 kVp 300 mAs | 100 kVp 300 mAs | 120 kVp 300 mAs (Default) | 140 kVp 300 mAs | 120 kVp 100 mAs | 120 kVp 200 mAs | 120 kVp 400 mAs | ||
|---|---|---|---|---|---|---|---|---|
| % Diff of CTDIvol | −69.9 | −39.0 | 0.0 | 45.2 | −66.7 | −33.3 | 33.6 | |
| % Diff of DLP | −70.7 | −40.4 | 0.0 | 41.8 | −67.4 | −34.8 | 30.3 | |
| SNR 0.3 | SNR 0.7 | SNR 1.3 | SNR 1.7 | 20% Clearview | 30% Clearview | 40% Clearview | 60% Clearview | |
| % Diff of CTDIvol | −80.1 | −57.3 | 45.4 | 147.6 | 0.0 | 0.0 | 0.0 | 0.0 |
| % Diff of DLP | −80.4 | −58.3 | 45.3 | 141.8 | 0.0 | 0.0 | 0.0 | 0.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Prabsattroo, T.; Wachirasirikul, K.; Tansangworn, P.; Punikhom, P.; Sudchai, W. The Dose Optimization and Evaluation of Image Quality in the Adult Brain Protocols of Multi-Slice Computed Tomography: A Phantom Study. J. Imaging 2023, 9, 264. https://doi.org/10.3390/jimaging9120264
Prabsattroo T, Wachirasirikul K, Tansangworn P, Punikhom P, Sudchai W. The Dose Optimization and Evaluation of Image Quality in the Adult Brain Protocols of Multi-Slice Computed Tomography: A Phantom Study. Journal of Imaging. 2023; 9(12):264. https://doi.org/10.3390/jimaging9120264
Chicago/Turabian StylePrabsattroo, Thawatchai, Kanokpat Wachirasirikul, Prasit Tansangworn, Puengjai Punikhom, and Waraporn Sudchai. 2023. "The Dose Optimization and Evaluation of Image Quality in the Adult Brain Protocols of Multi-Slice Computed Tomography: A Phantom Study" Journal of Imaging 9, no. 12: 264. https://doi.org/10.3390/jimaging9120264





