Variation of Goliathus orientalis (Moser, 1909) Elytra Nanostructurations and Their Impact on Wettability
Abstract
:1. Introduction
2. Materials and Methods
2.1. Dried Insect Specimens
2.2. Sample Preparation
2.3. Surface Characterization
3. Results
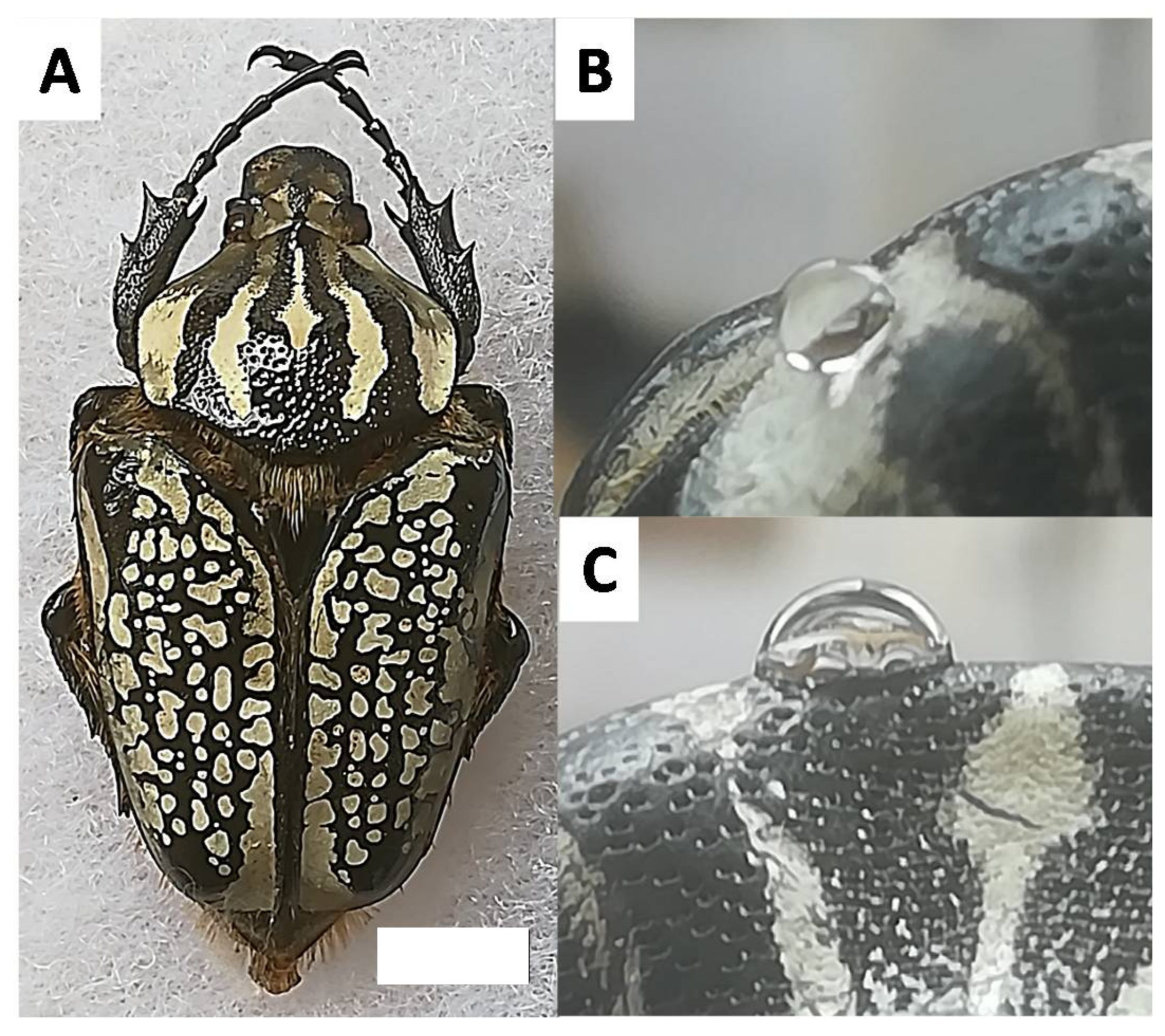
3.1. Materials
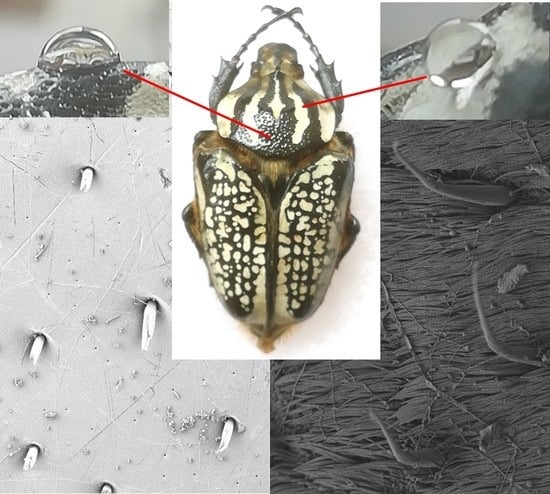
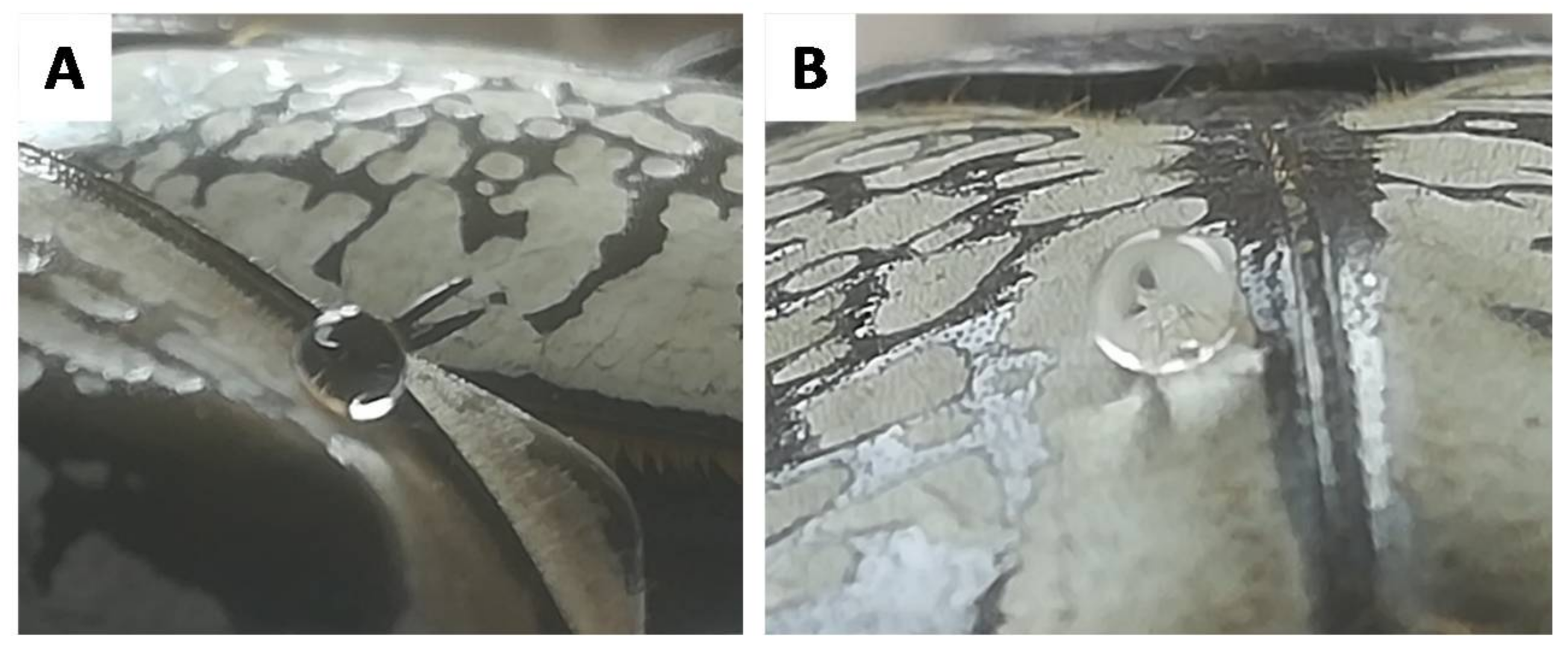
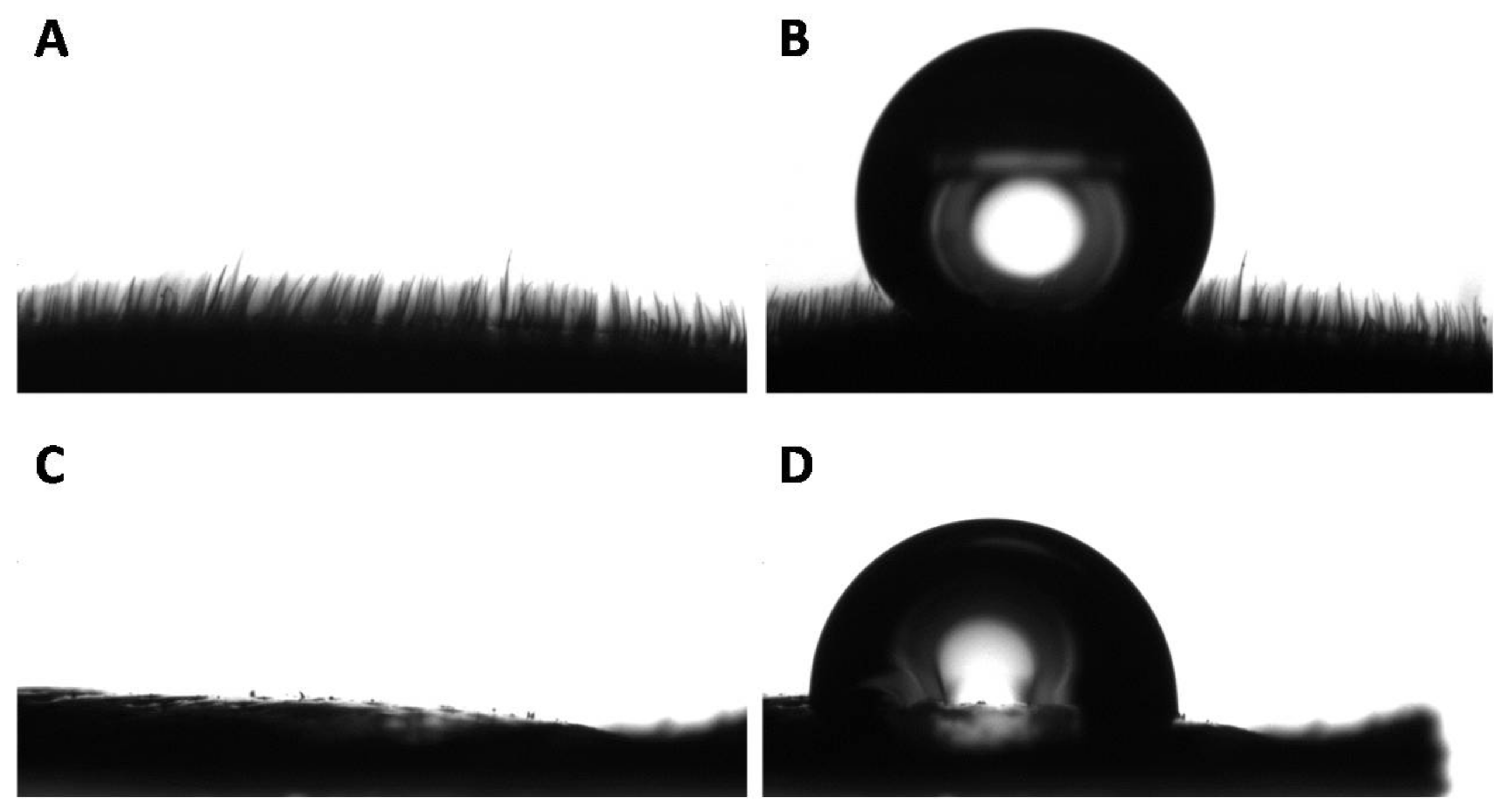
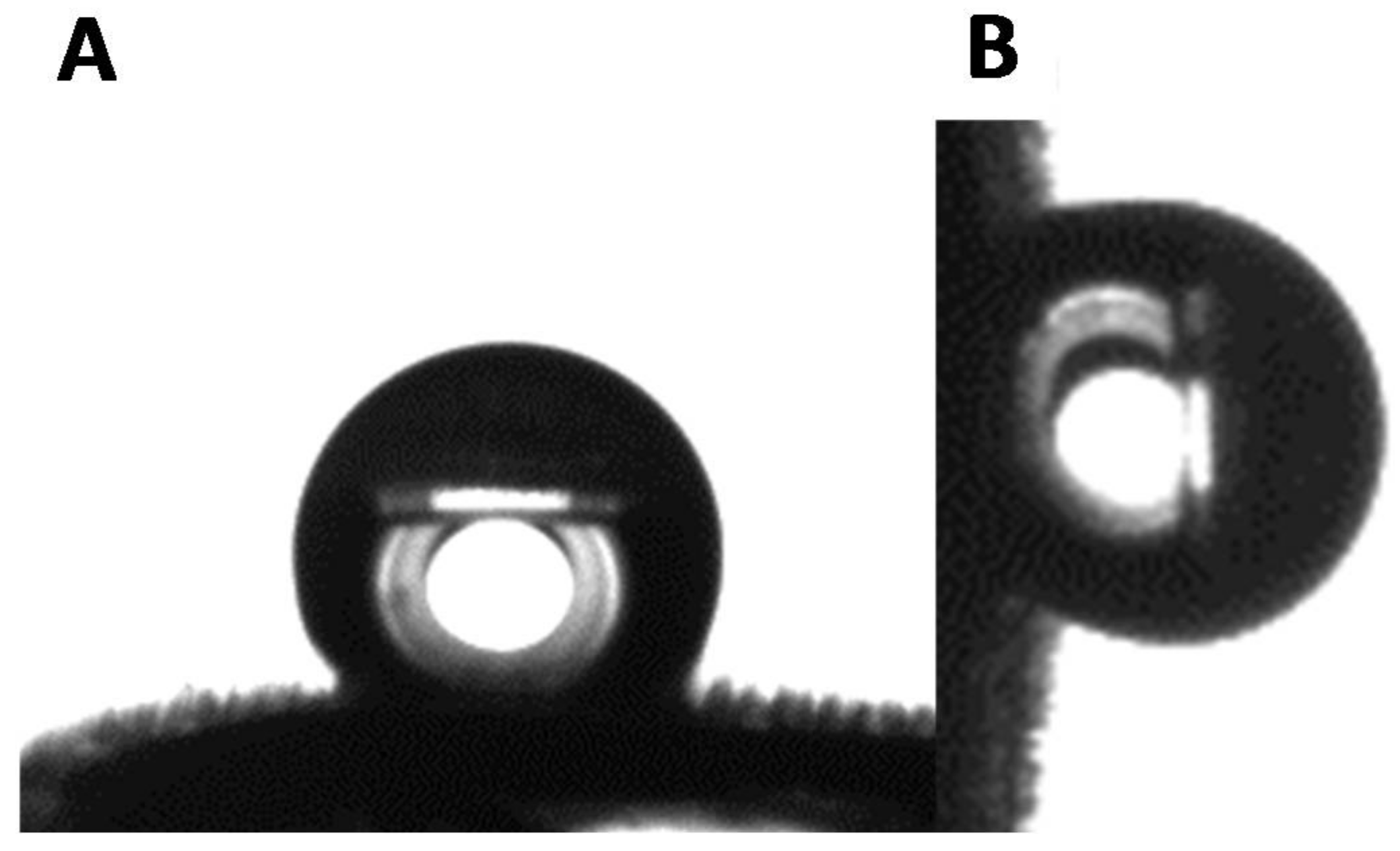
3.2. Wettability of the Goliathus Elytra
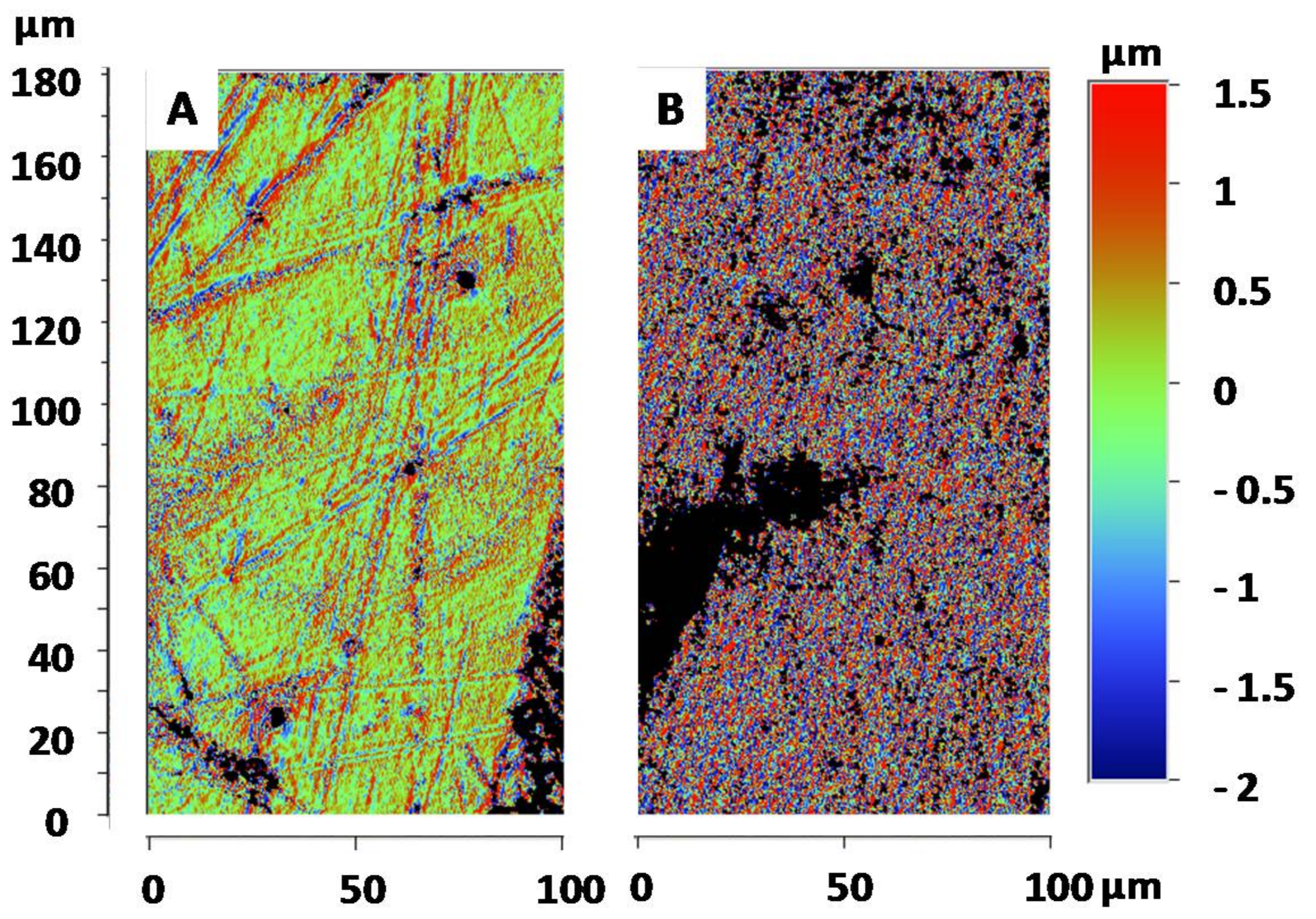
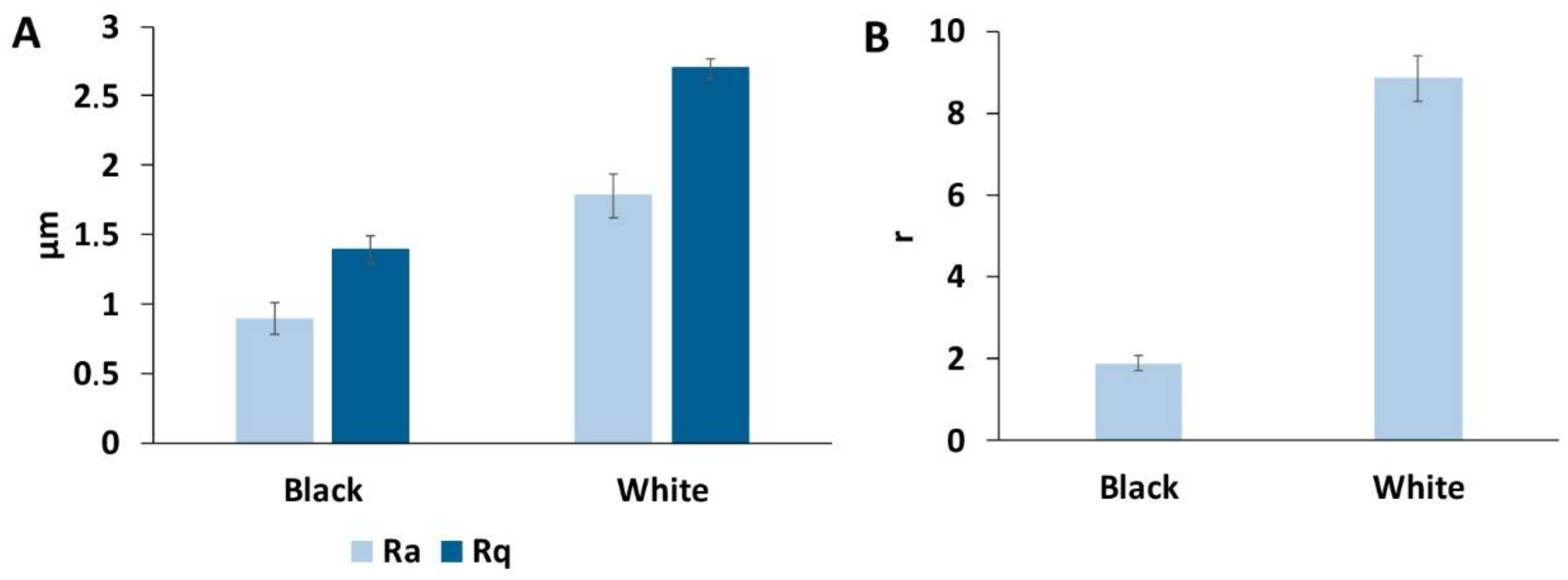
3.3. Surface Roughness of the Goliathus Elytra
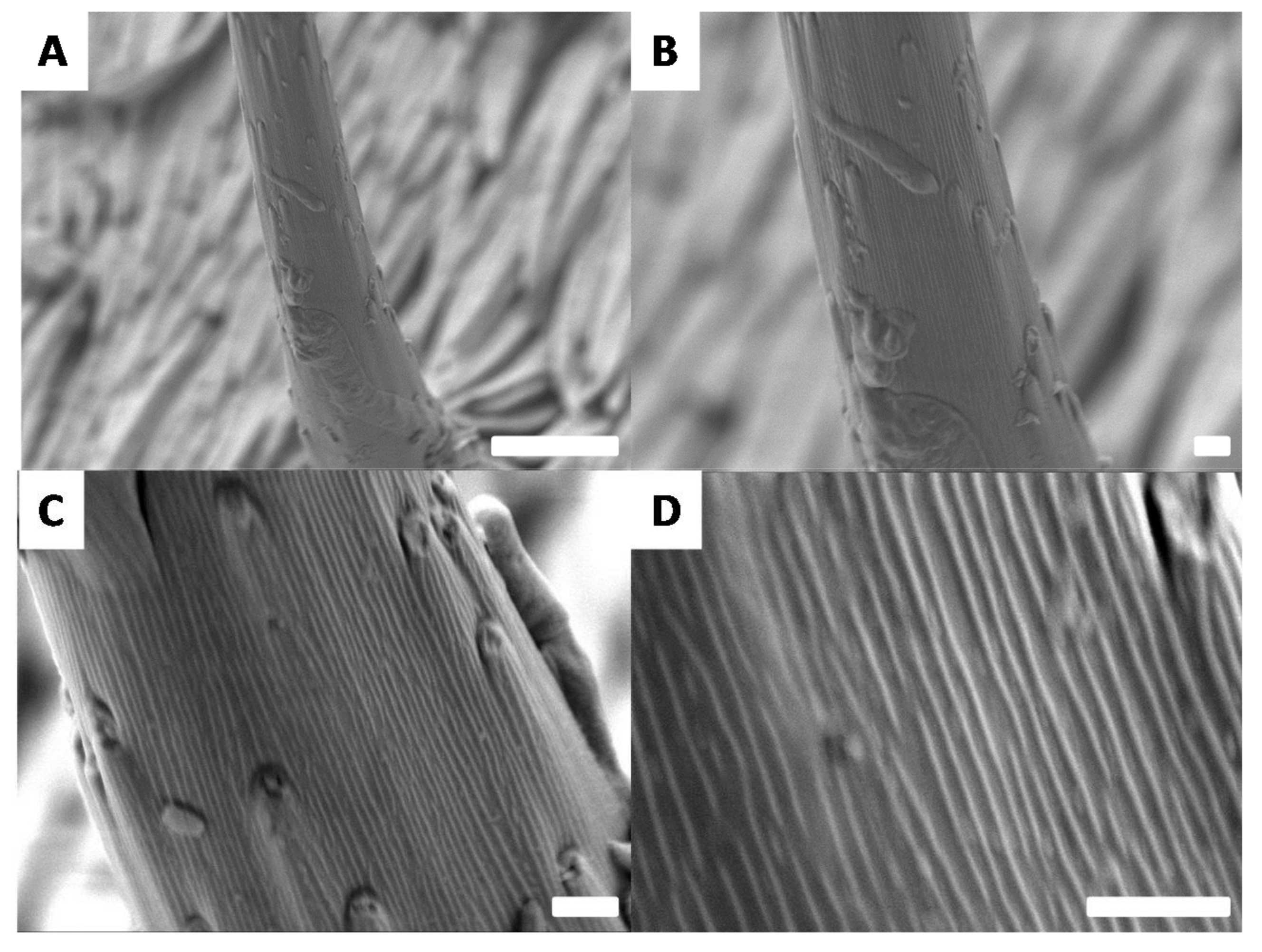
3.4. Surface Morphologies of the Goliathus Elytra
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Darmanin, T.; Guittard, F. Superhydrophobic and superoleophobic properties in nature. Mater. Today 2015, 18, 273–285. [Google Scholar] [CrossRef]
- Koch, K.; Bhushan, B.; Barthlott, W. Multifunctional surface structures of plants: An inspiration for biomimetics. Prog. Mater. Sci. 2009, 54, 137–178. [Google Scholar] [CrossRef]
- Bush, J.W.M.; Hu, D.L.; Prakash, M. The integument of water-walking arthropods: Form and function. Adv. Insect Physiol. 2007, 34, 117–192. [Google Scholar]
- Bixler, G.D.; Bhushan, B. Fluid drag reduction and efficient self-cleaning with rice leaf and butterfly wing bioinspired surfaces. Nanoscale 2013, 5, 7685–7710. [Google Scholar] [CrossRef] [PubMed]
- Hensel, R.; Helbig, R.; Aland, S.; Braun, H.-G.; Voigt, A.; Neinhuis, C.; Werner, C. Wetting resistance at its topographical limit: The benefit of mushroom and serif T structures. Langmuir 2013, 29, 1100–1112. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Liao, T.; Liu, K.; Jiang, L.; Kim, J.H.; Dou, S.X. Fly-eye inspired superhydrophobic anti-fogging inorganic nanostructures. Small 2014, 10, 3001–3006. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.; Watson, G.S.; Zheng, Y.; Watson, J.A.; Liang, A. Wetting properties on nanostructured surfaces of cicada wings. J. Exp. Biol. 2009, 212, 3148–3155. [Google Scholar] [CrossRef] [PubMed]
- Byun, D.; Hong, J.; Ko, J.H.; Lee, Y.J.; Park, H.C.; Byun, B.-K.; Lukes, J.R. Wetting characteristics of insect wing surfaces. J. Bionic Eng. 2009, 6, 63–70. [Google Scholar] [CrossRef]
- Dickerson, A.; Shankles, P.; Madhavan, N.; Hu, D. Mosquitoes survive raindrop collisions by virtue of their low mass. Proc. Natl. Acad. Sci. USA 2012, 109, 9822–9827. [Google Scholar] [CrossRef] [PubMed]
- Dickerson, A.; Liu, X.; Ting, Z.; Hu, D. Fog spontaneously folds mosquito wings. Phys. Fluids 2015, 27, 021901. [Google Scholar] [CrossRef]
- Dickerson, A.; Shankles, P.; Hu, D. Raindrops push and splash flying insects. Phys. Fluids 2014, 26, 027104. [Google Scholar] [CrossRef]
- Dickerson, A.; Hu, D. Mosquitoes actively remove drops deposited by fog and dew. Integr. Comp. Biol. 2014, 54, 1008–1013. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.; Guo, Z.; Liu, W. Biomimetic transparent and superhydrophobic coatings: From nature and beyond nature. Chem. Commun. 2015, 51, 1775–1794. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.; Chen, Y.; Zheng, Y.; Zhen, M.; Shu, C.; Dai, Z.; Liang, A.; Gorb, S.N. Wettability gradient on the elytra in the aquatic beetle Cybister chinensis and its role in angular position of the beetle at water-air interface. Acta Biomater. 2017, 51, 408–417. [Google Scholar] [CrossRef] [PubMed]
- Young, T., III. An essay on the cohesion of fluids. Philos. Trans. R. Soc. Lond. 1805, 95, 65–87. [Google Scholar] [CrossRef]
- Wenzel, R.N. Resistance of solid surfaces to wetting by water. Ind. Eng. Chem. 1936, 28, 988–994. [Google Scholar] [CrossRef]
- Cassie, A.B.D.; Baxter, S. Wettability of porous surfaces. Trans. Faraday Soc. 1944, 40, 546–551. [Google Scholar] [CrossRef]
- Feng, L.; Zhang, Y.; Xi, J.; Zhu, Y.; Wang, N.; Xia, F.; Jiang, L. Petal effect: A superhydrophobic state with high adhesive force. Langmuir 2008, 24, 4114–4119. [Google Scholar] [CrossRef] [PubMed]
- Bhushan, B.; Nosonovsky, M. The rose petal effect and the modes of superhydrophobicity. Phil. Trans. R. Soc. A 2010, 368, 4713–4728. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Du, J.; Wu, J.; Jiang, L. Superhydrophobic gecko feet with high adhesive forces towards water and their bio-inspired materials. Nanoscale 2012, 4, 768–772. [Google Scholar] [CrossRef] [PubMed]
- Marmur, A. Hydro-hygro-oleo-omni-phobic? Terminology of wettability classification. Soft Matter 2012, 2, 6867–6870. [Google Scholar] [CrossRef]
- Parker, A.R.; Lawrence, C.R. Water capture by a desert beetle. Nature 2001, 414, 33–34. [Google Scholar] [CrossRef] [PubMed]
- Ju, J.; Bai, H.; Zheng, Y.; Zhao, T.; Fang, R.; Jiang, L. A multi-structural and multi-functional integrated fog collection system in cactus. Nat. Commun. 2012, 3, 1247. [Google Scholar] [CrossRef] [PubMed]
- Cao, M.; Ju, J.; Li, K.; Dou, S.; Liu, K.; Jiang, L. Facile and large-scale fabrication of a cactus-inspired continuous fog collector. Adv. Funct. Mater. 2014, 24, 3235–3240. [Google Scholar] [CrossRef]
- Ju, J.; Xiao, K.; Yao, X.; Bai, H.; Jiang, L. Bioinspired conical copper wire with gradient wettability for continuous and efficient fog collection. Adv. Mater. 2013, 25, 5937–5942. [Google Scholar] [CrossRef] [PubMed]
- Heng, X.; Xiang, M.; Lu, Z.; Luo, C. Branched ZnO wire structures for water collection inspired by cacti. ACS Appl. Mater. Interfaces 2014, 6, 8032–8041. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.; Appel, E.; Kovalev, A.; Gorb, E.; Liang, A.; Gorb, S.N. The influence of the topography and physico-chemical properties of the cuticle surface on the wettability and adhesive properties of the elytra of the dung beetle Geotrupes stercorarius (Coleoptera, Scarabaeidae). Bioinspir. Biomim. 2017, 13, 016008. [Google Scholar] [CrossRef] [PubMed]
- Darmanin, T.; Guittard, F. Recent advances in the potential applications of bioinspired superhydrophobic materials. J. Mater. Chem. A 2014, 2, 16319–16359. [Google Scholar] [CrossRef]
- Su, B.; Tian, Y.; Jiang, L. Bioinspired interfaces with superwettability: From materials to chemistry. J. Am. Chem. Soc. 2016, 138, 1727–1748. [Google Scholar] [CrossRef] [PubMed]
- Rassart, M.; Colomer, J.-F.; Tabarrant, T.; Vigneron, J.-P. Diffractive hygrochromic effect in the cuticle of the hercules beetle Dynastes hercules. New J. Phys. 2008, 10, 033014. [Google Scholar] [CrossRef]
- Lachaume, G. Les Coléoptères du Monde 3. In Goliathini 1: Goliathus, Argyrophegges, Fornasinius, Hegemus; Sciences Nat: Venette, France, 1983; pp. 1–113. [Google Scholar]
- Jiang, L.; Dong, B.; Liu, X.; Liu, F.; Zi, J. Structural origin of sexual dichromatic coloration and luster in the beetle Goliathus cacicus. Chin. Sci. Bull. 2012, 57, 3211–3217. [Google Scholar] [CrossRef]
- Wiebes, J.T. Catalogue of the Coleoptera Cetoniinae in the Leiden Museum. 1. Goliathus Lamarck, sensu lato. Zool. Meded. 1968, 43, 19–40. [Google Scholar]
- Sakai, K.; Nagai, S. The Cetoniine Beetles of the World. In Mushi-Sha’s Iconographic Series of Insects 3; Fujita: Tokyo, Japan, 1998; pp. 1–565. [Google Scholar]
- Mawdsley, J.R. Taxonomy of the Goliath beetle Goliathus orientalis Moser, 1909 (Coleoptera: Scarabaeidae: Cetoniinae). J. Nat. Hist. 2013, 47, 1451–1461. [Google Scholar] [CrossRef]
- Zhai, L.; Berg, M.C.; Cebeci, F.Ç.; Kim, Y.; Milwid, J.M.; Rubner, M.F.; Cohen, R.E. Patterned superhydrophobic surfaces: Toward a synthetic mimic of the Namib Desert beetle. Nano Lett. 2006, 6, 1213–1217. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Bhushan, B.; Tong, J. Structural coloration in nature. RSC Adv. 2013, 3, 14862–14889. [Google Scholar] [CrossRef]



© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Godeau, G.; Godeau, R.-P.; Orange, F.; Szczepanski, C.R.; Guittard, F.; Darmanin, T. Variation of Goliathus orientalis (Moser, 1909) Elytra Nanostructurations and Their Impact on Wettability. Biomimetics 2018, 3, 6. https://doi.org/10.3390/biomimetics3020006
Godeau G, Godeau R-P, Orange F, Szczepanski CR, Guittard F, Darmanin T. Variation of Goliathus orientalis (Moser, 1909) Elytra Nanostructurations and Their Impact on Wettability. Biomimetics. 2018; 3(2):6. https://doi.org/10.3390/biomimetics3020006
Chicago/Turabian StyleGodeau, Guilhem, René-Paul Godeau, François Orange, Caroline R. Szczepanski, Frédéric Guittard, and Thierry Darmanin. 2018. "Variation of Goliathus orientalis (Moser, 1909) Elytra Nanostructurations and Their Impact on Wettability" Biomimetics 3, no. 2: 6. https://doi.org/10.3390/biomimetics3020006