Test–Retest Reproducibility of Reduced-Field-of-View Density-Weighted CRT MRSI at 3T
Abstract
:1. Introduction
2. Materials and Methods
2.1. Volunteers and Hardware
2.2. Data Acquisition
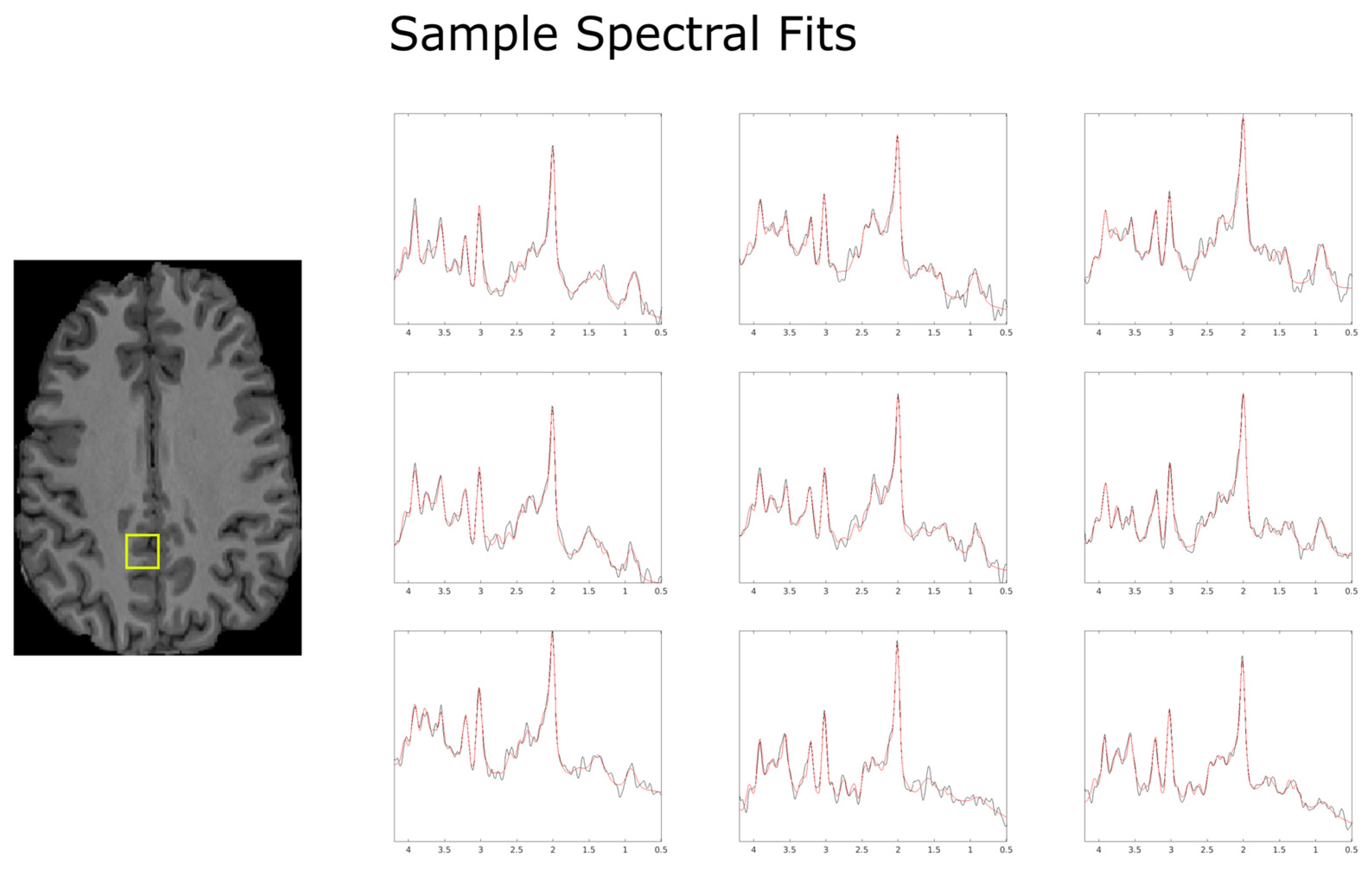
2.3. Data Processing
2.4. Statistical Analysis of Reproducibility
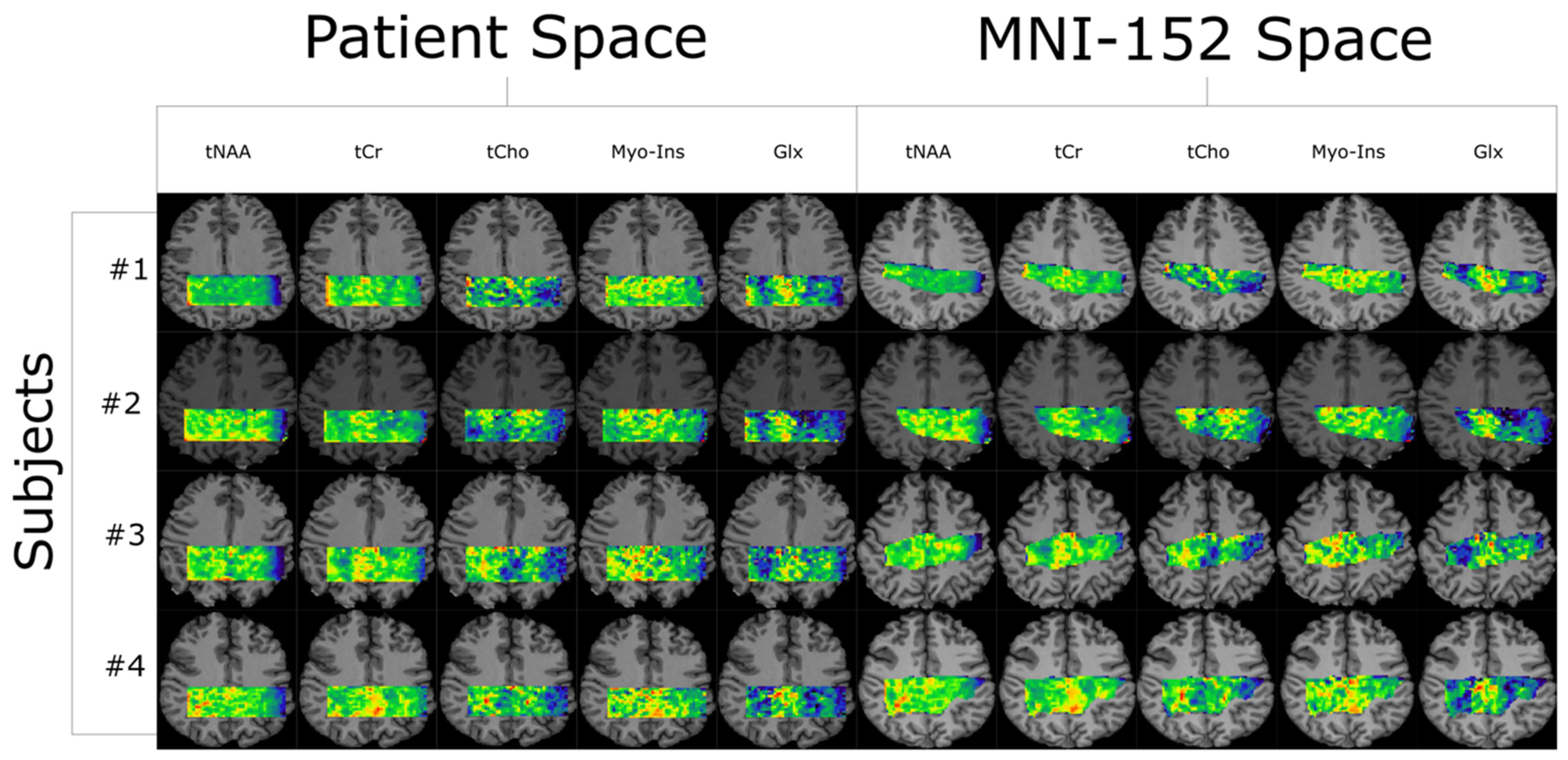
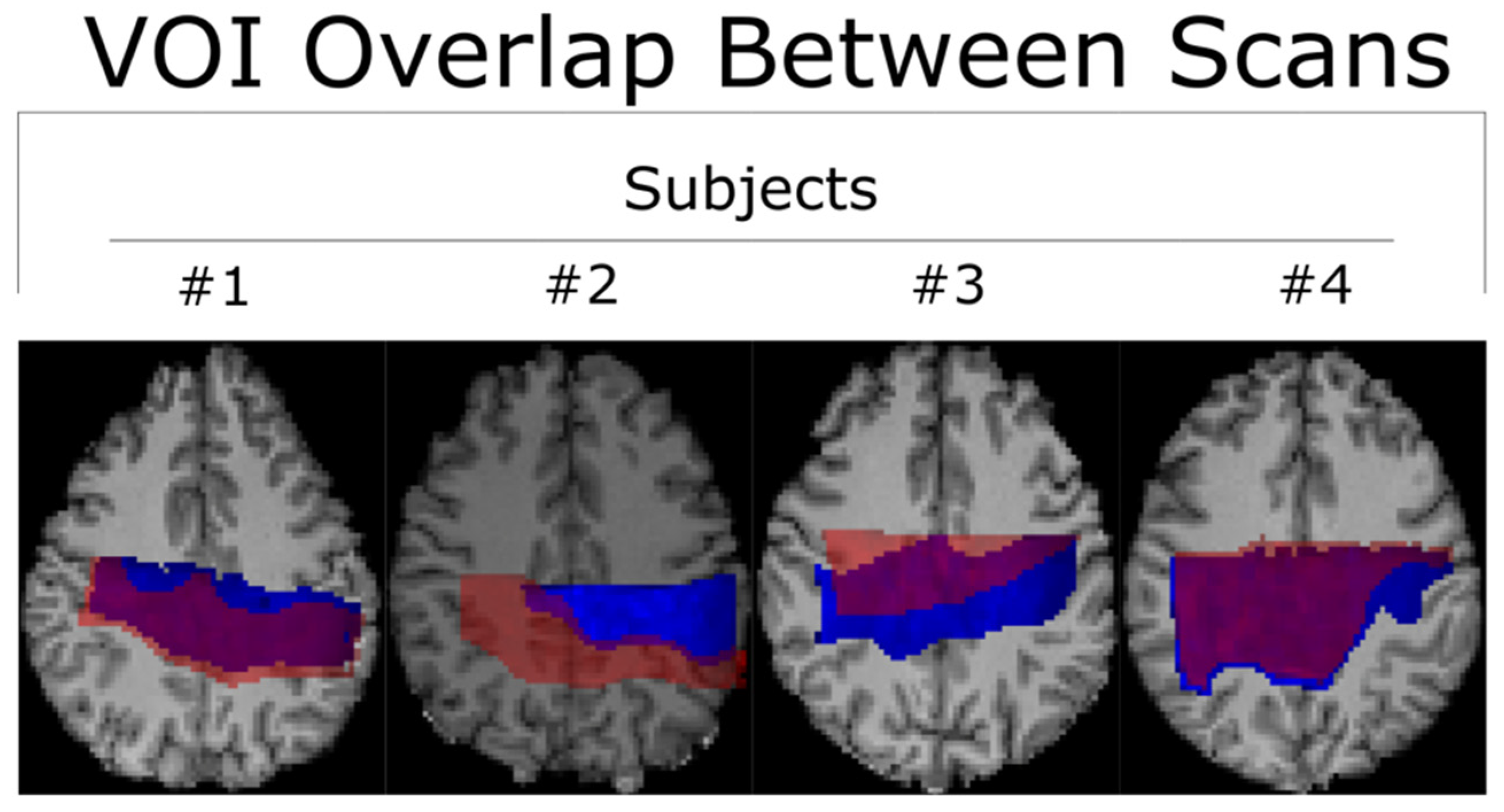
3. Results
Within-Subject and Between-Subject Reproducibility
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Moser, P.; Eckstein, K.; Hingerl, L.; Weber, M.; Motyka, S.; Strasser, B.; van der Kouwe, A.; Robinson, S.; Trattnig, S.; Bogner, W. Intra-Session and Inter-Subject Variability of 3D-FID-MRSI Using Single-Echo Volumetric EPI Navigators at 3T. Magn. Reson. Med. 2020, 83, 1920–1929. [Google Scholar] [CrossRef]
- Öz, G.; Alger, J.R.; Barker, P.B.; Bartha, R.; Bizzi, A.; Boesch, C.; Bolan, P.J.; Brindle, K.M.; Cudalbu, C.; Dinçer, A.; et al. Clinical Proton MR Spectroscopy in Central Nervous System Disorders. Radiology 2014, 270, 658–679. [Google Scholar] [CrossRef]
- Bogner, W.; Otazo, R.; Henning, A. Accelerated MR Spectroscopic Imaging—A Review of Current and Emerging Techniques. NMR Biomed. 2021, 34, e4314. [Google Scholar] [CrossRef] [PubMed]
- Furuyama, J.K.; Wilson, N.E.; Thomas, M.A. Spectroscopic Imaging Using Concentrically Circular Echo-Planar Trajectories In Vivo. Magn. Reson. Med. 2012, 67, 1515–1522. [Google Scholar] [CrossRef]
- Jiang, W.; Lustig, M.; Larson, P.E.Z. Concentric Rings K-Space Trajectory for Hyperpolarized 13C MR Spectroscopic Imaging. Magn. Reson. Med. 2016, 75, 19–31. [Google Scholar] [CrossRef] [PubMed]
- Chiew, M.; Jiang, W.; Burns, B.; Larson, P.; Steel, A.; Jezzard, P.; Albert Thomas, M.; Emir, U.E. Density-Weighted Concentric Rings k-Space Trajectory for 1H Magnetic Resonance Spectroscopic Imaging at 7 T. NMR Biomed. 2018, 31, e3838. [Google Scholar] [CrossRef]
- Hingerl, L.; Strasser, B.; Moser, P.; Hangel, G.; Motyka, S.; Heckova, E.; Gruber, S.; Trattnig, S.; Bogner, W. Clinical High-Resolution 3D-MR Spectroscopic Imaging of the Human Brain at 7 T. Investig. Radiol. 2020, 55, 239. [Google Scholar] [CrossRef]
- Nassirpour, S.; Chang, P.; Henning, A. High and Ultra-High Resolution Metabolite Mapping of the Human Brain Using 1H FID MRSI at 9.4T. NeuroImage 2018, 168, 211–221. [Google Scholar] [CrossRef] [PubMed]
- Emir, U.E.; Sood, J.; Chiew, M.; Thomas, M.A.; Lane, S.P. High-Resolution Metabolic Mapping of the Cerebellum Using 2D Zoom Magnetic Resonance Spectroscopic Imaging. Magn. Reson. Med. 2021, 85, 2349–2358. [Google Scholar] [CrossRef]
- Emir, U.E.; Burns, B.; Chiew, M.; Jezzard, P.; Thomas, M.A. Non-Water-Suppressed Short-Echo-Time Magnetic Resonance Spectroscopic Imaging Using a Concentric Ring k-Space Trajectory. NMR Biomed. 2017, 30, e3714. [Google Scholar] [CrossRef]
- Tkáč, I.; Deelchand, D.; Dreher, W.; Hetherington, H.; Kreis, R.; Kumaragamage, C.; Považan, M.; Spielman, D.M.; Strasser, B.; de Graaf, R.A. Water and Lipid Suppression Techniques for Advanced 1H MRS and MRSI of the Human Brain: Experts’ Consensus Recommendations. NMR Biomed. 2021, 34, e4459. [Google Scholar] [CrossRef]
- Fessler, J.A.; Sutton, B.P. Nonuniform Fast Fourier Transforms Using Min-Max Interpolation. IEEE Trans. Signal Process. 2003, 51, 560–574. Available online: https://ieeexplore.ieee.org/abstract/document/1166689 (accessed on 15 February 2024). [CrossRef]
- Fessler, J.A. On NUFFT-Based Gridding for Non-Cartesian MRI. J. Magn. Reson. 2007, 188, 191–195. [Google Scholar] [CrossRef] [PubMed]
- Steel, A.; Chiew, M.; Jezzard, P.; Voets, N.L.; Plaha, P.; Thomas, M.A.; Stagg, C.J.; Emir, U.E. Metabolite-Cycled Density-Weighted Concentric Rings k-Space Trajectory (DW-CRT) Enables High-Resolution 1 H Magnetic Resonance Spectroscopic Imaging at 3-Tesla. Sci. Rep. 2018, 8, 7792. [Google Scholar] [CrossRef]
- Provencher, S.W. Estimation of Metabolite Concentrations from Localized in Vivo Proton NMR Spectra. Magn. Reson. Med. 1993, 30, 672–679. [Google Scholar] [CrossRef]
- Provencher, S.W. Automatic Quantitation of Localized in Vivo 1H Spectra with LCModel. NMR Biomed. 2001, 14, 260–264. [Google Scholar] [CrossRef] [PubMed]
- Jenkinson, M.; Beckmann, C.F.; Behrens, T.E.J.; Woolrich, M.W.; Smith, S.M. FSL. NeuroImage 2012, 62, 782–790. [Google Scholar] [CrossRef] [PubMed]
- Geurts, J.J.G.; Barkhof, F.; Castelijns, J.A.; Uitdehaag, B.M.J.; Polman, C.H.; Pouwels, P.J.W. Quantitative 1H-MRS of Healthy Human Cortex, Hippocampus, and Thalamus: Metabolite Concentrations, Quantification Precision, and Reproducibility. J. Magn. Reson. Imaging 2004, 20, 366–371. [Google Scholar] [CrossRef]
- Wijtenburg, S.A.; Gaston, F.E.; Spieker, E.A.; Korenic, S.A.; Kochunov, P.; Hong, L.E.; Rowland, L.M. Reproducibility of Phase Rotation STEAM at 3T: Focus on Glutathione. Magn. Reson. Med. 2014, 72, 603–609. [Google Scholar] [CrossRef]
- Ziegs, T.; Wright, A.M.; Henning, A. Test–Retest Reproducibility of Human Brain Multi-Slice 1H FID-MRSI Data at 9.4T after Optimization of Lipid Regularization, Macromolecular Model, and Spline Baseline Stiffness. Magn. Reson. Med. 2023, 89, 11–28. [Google Scholar] [CrossRef]
- Ebel, A.; Maudsley, A.A. Improved Spectral Quality for 3D MR Spectroscopic Imaging Using a High Spatial Resolution Acquisition Strategy. Magn. Reson. Imaging 2003, 21, 113–120. [Google Scholar] [CrossRef] [PubMed]
- van de Bank, B.L.; Emir, U.E.; Boer, V.O.; van Asten, J.J.A.; Maas, M.C.; Wijnen, J.P.; Kan, H.E.; Oz, G.; Klomp, D.W.J.; Scheenen, T.W.J. Multi-Center Reproducibility of Neurochemical Profiles in the Human Brain at 7 T. NMR Biomed. 2015, 28, 306–316. [Google Scholar] [CrossRef]
- Terpstra, M.; Cheong, I.; Lyu, T.; Deelchand, D.K.; Emir, U.E.; Bednařík, P.; Eberly, L.E.; Öz, G. Test-Retest Reproducibility of Neurochemical Profiles with Short-Echo, Single-Voxel MR Spectroscopy at 3T and 7T. Magn. Reson. Med. 2016, 76, 1083–1091. [Google Scholar] [CrossRef] [PubMed]
- Träber, F.; Block, W.; Freymann, N.; Gür, O.; Kucinski, T.; Hammen, T.; Ende, G.; Pilatus, U.; Hampel, H.; Schild, H.H.; et al. A Multicenter Reproducibility Study of Single-Voxel 1H-MRS of the Medial Temporal Lobe. Eur. Radiol. 2006, 16, 1096–1103. [Google Scholar] [CrossRef]
- Wijnen, J.P.; van Asten, J.J.A.; Klomp, D.W.J.; Sjobakk, T.E.; Gribbestad, I.S.; Scheenen, T.W.J.; Heerschap, A. Short Echo Time 1H MRSI of the Human Brain at 3T with Adiabatic Slice-Selective Refocusing Pulses; Reproducibility and Variance in a Dual Center Setting. J. Magn. Reson. Imaging 2010, 31, 61–70. [Google Scholar] [CrossRef]
- Maudsley, A.A.; Domenig, C.; Sheriff, S. Reproducibility of Serial Whole-Brain MR Spectroscopic Imaging. NMR Biomed. 2010, 23, 251–256. [Google Scholar] [CrossRef] [PubMed]
- Veenith, T.V.; Mada, M.; Carter, E.; Grossac, J.; Newcombe, V.; Outtrim, J.; Lupson, V.; Nallapareddy, S.; Williams, G.B.; Sheriff, S.; et al. Comparison of Inter Subject Variability and Reproducibility of Whole Brain Proton Spectroscopy. PLoS ONE 2014, 9, e115304. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.W.; Deelchand, D.K.; Joers, J.M.; Hanna, B.; Berrington, A.; Gillen, J.S.; Kantarci, K.; Soher, B.J.; Barker, P.B.; Park, H.; et al. AutoVOI: Real-Time Automatic Prescription of Volume-of-Interest for Single Voxel Spectroscopy. Magn. Reson. Med. 2018, 80, 1787–1798. [Google Scholar] [CrossRef] [PubMed]
- Klauser, A.; Klauser, P.; Grouiller, F.; Courvoisier, S.; Lazeyras, F. Whole-Brain High-Resolution Metabolite Mapping with 3D Compressed-Sensing SENSE Low-Rank 1H FID-MRSI. NMR Biomed. 2022, 35, e4615. [Google Scholar] [CrossRef]
- Inglese, M.; Spindler, M.; Babb, J.S.; Sunenshine, P.; Law, M.; Gonen, O. Field, Coil, and Echo-Time Influence on Sensitivity and Reproducibility of Brain Proton MR Spectroscopy. Am. J. Neuroradiol. 2006, 27, 684–688. [Google Scholar]
- Bøgh, N.; Vaeggemose, M.; Schulte, R.F.; Hansen, E.S.S.; Laustsen, C. Repeatability of Deuterium Metabolic Imaging of Healthy Volunteers at 3 T. Eur. Radiol. Exp. 2024, 8, 44. [Google Scholar] [CrossRef]
- Bednarik, P.; Goranovic, D.; Svatkova, A.; Niess, F.; Hingerl, L.; Strasser, B.; Deelchand, D.; Spurny-Dworak, B.; Krssak, M.; Trattnig, S.; et al. 2H Labeling Enables Non-Invasive 3D Proton MR Imaging of Glucose and Neurotransmitter Metabolism at 7T in the Human Brain. Nat. Biomed. Eng. 2023, 7, 1001–1013. [Google Scholar] [CrossRef] [PubMed]
- Xia, P.; Chiew, M.; Zhou, X.; Thomas, A.; Dydak, U.; Emir, U.E. Density-Weighted Concentric Ring Trajectory Using Simultaneous Multi-Band Acceleration: 3D Metabolite-Cycled Magnetic Resonance Spectroscopy Imaging at 3 T. bioRxiv 2019. [Google Scholar] [CrossRef]


| Within-Subject CV (%) | Metabolites | ||||
|---|---|---|---|---|---|
| Versus Method | |||||
| Grey Matter | tCho | tCr | Glx | Ins | tNAA |
| Absolute | 11.0 ± 4.7 | 1.8 ± 0.76 | 10.0 ± 4.3 | 12.1 ± 5.2 | 2.0 ± 0.84 |
| Normalized | 10.4 ± 4.4 | 4.0 ± 1.7 | 9.7 ± 4.1 | 14.7 ± 6.3 | 0.9 ± 0.37 |
| tCr Ratio | 10.6 ± 4.5 | - | 11.7 ± 5.0 | 12.1 ± 5.2 | 2.7 ± 1.1 |
| TF Correction | 11.7 ± 5.0 | 1.7 ± 0.72 | 9.3 ± 3.9 | 13.4 ± 5.7 | 2.2 ± 0.93 |
| White Matter | tCho | tCr | Glx | Ins | tNAA |
| Absolute | 8.5 ± 3.6 | 4.2 ± 1.8 | 16.1 ± 6.9 | 8.8 ± 3.7 | 1.9 ± 0.80 |
| Normalized | 8.8 ± 3.7 | 3.2 ± 1.4 | 11.9 ± 5.1 | 13.3 ± 5.7 | 3.9 ± 1.6 |
| tCr Ratio | 9.7 ± 4.1 | - | 13.0 ± 5.6 | 14.1 ± 6.0 | 4.0 ± 1.7 |
| TF Correction | 8.8 ± 3.7 | 4.1 ± 1.7 | 16.1 ± 6.9 | 8.8 ± 3.7 | 1.9 ± 0.80 |
| Mixed Composition | tCho | tCr | Glx | Ins | tNAA |
| Absolute | 10.0 ± 4.2 | 3.4 ± 1.4 | 12.3 ± 5.2 | 3.8 ± 1.6 | 3.2 ± 1.4 |
| Normalized | 11.0 ± 4.7 | 3.2 ± 1.4 | 18.1 ± 7.8 | 4.4 ± 1.9 | 5.0 ± 2.1 |
| tCr Ratio | 8.6 ± 3.6 | - | 11.9 ± 5.1 | 7.2 ± 3.0 | 2.7 ± 1.1 |
| TF Correction | 10.1 ± 4.3 | 3.2 ± 1.4 | 12.0 ± 5.1 | 7.8 ± 3.3 | 3.0 ± 1.3 |
| Between-Subject CV (%) | Metabolites | ||||
| Versus Method | |||||
| Grey Matter | tCho | tCr | Glx | Ins | tNAA |
| Absolute | 12.0 ± 5.1 | 7.0 ± 3.0 | 13.4 ± 5.7 | 12.8 ± 5.5 | 6.7 ± 2.8 |
| Normalized | 10.7 ± 4.6 | 3.7 ± 1.6 | 8.3 ± 3.5 | 13.4 ± 5.7 | 3.7 ± 1.6 |
| tCr Ratio | 11.3 ± 4.8 | - | 10.2 ± 4.3 | 12.4 ± 5.3 | 4.0 ± 1.7 |
| TF Correction | 12.5 ± 5.3 | 7.2 ± 3.0 | 12.5 ± 5.3 | 14.4 ± 6.2 | 7.6 ± 3.2 |
| White Matter | tCho | tCr | Glx | Ins | tNAA |
| Absolute | 13.1 ± 5.6 | 8.2 ± 3.5 | 18.0 ± 7.8 | 11.4 ± 4.9 | 4.9 ± 2.1 |
| Normalized | 10.7 ± 4.6 | 3.4 ± 1.4 | 15.3 ± 6.6 | 12.6 ± 5.4 | 5.2 ± 2.2 |
| tCr Ratio | 10.2 ± 4.3 | - | 16.9 ± 7.3 | 13.1 ± 5.6 | 6.3 ± 2.7 |
| TF Correction | 12.4 ± 5.3 | 7.0 ± 3.0 | 17.5 ± 7.5 | 11.2 ± 4.8 | 4.9 ± 2.1 |
| Mixed Composition | tCho | tCr | Glx | Ins | tNAA |
| Absolute | 12.8 ± 5.5 | 5.9 ± 2.5 | 17.1 ± 7.4 | 6.3 ± 2.7 | 5.2 ± 2.2 |
| Normalized | 14.4 ± 6.2 | 6.0 ± 2.5 | 23.2 ± 10.2 | 8.6 ± 3.6 | 7.7 ± 3.3 |
| tCr Ratio | 10.6 ± 4.5 | - | 17.8 ± 7.7 | 13.0 ± 5.6 | 5.1 ± 2.2 |
| TF Correction | 11.9 ± 5.1 | 5.8 ± 2.5 | 21.4 ± 9.3 | 14.4 ± 6.2 | 5.0 ± 2.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Farley, N.; Susnjar, A.; Chiew, M.; Emir, U.E. Test–Retest Reproducibility of Reduced-Field-of-View Density-Weighted CRT MRSI at 3T. Tomography 2024, 10, 493-503. https://doi.org/10.3390/tomography10040038
Farley N, Susnjar A, Chiew M, Emir UE. Test–Retest Reproducibility of Reduced-Field-of-View Density-Weighted CRT MRSI at 3T. Tomography. 2024; 10(4):493-503. https://doi.org/10.3390/tomography10040038
Chicago/Turabian StyleFarley, Nicholas, Antonia Susnjar, Mark Chiew, and Uzay E. Emir. 2024. "Test–Retest Reproducibility of Reduced-Field-of-View Density-Weighted CRT MRSI at 3T" Tomography 10, no. 4: 493-503. https://doi.org/10.3390/tomography10040038






