A Cost-Effectiveness Analysis of Newborn Screening for Severe Combined Immunodeficiency in the UK
Abstract
:1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Recommended Uniform Screening Panel. U.S. Department of Health and Human Services, 2018. Available online: https://www.hrsa.gov/advisory-committees/heritable-disorders/rusp/index.html (accessed on 11 September 2018).
- Gaspar, H. A Practical Guide to Implementing Population Newborn Screening (NBS) for Severe Combined Immunodeficiency (SCID). Int. J. Neonatal Screen. 2017, 3, 29. [Google Scholar] [CrossRef]
- Blom, M.; Bredius, R.G.M.; Weijman, G.; Dekkers, E.H.B.M.; Kemper, E.A.; Van den Akker-van Marle, M.E.; van der Ploeg, C.P.B.; van der Burg, M.; Schielen, P.C.J.I. Introducing Newborn Screening for Severe Combined Immunodeficiency (SCID) in the Dutch Neonatal Screening Program. Int. J. Neonatal Screen. 2018, 4, 40. [Google Scholar] [CrossRef]
- Public Health England. Criteria for Appraising the Viability, Effectiveness and Appropriateness of a Screening Programme. 2015. Available online: https://www.gov.uk/government/publications/evidence-review-criteria-national-screening-programmes/criteria-for-appraising-the-viability-effectiveness-and-appropriateness-of-a-screening-programme (accessed on 27 October 2016).
- Buckley, R.H.; Schiff, S.E.; Schiff, R.I.; Markert, L.; Williams, L.W.; Roberts, J.L.; Myers, L.A.; Ward, F.E. Hematopoietic stem-cell transplantation for the treatment of severe combined immunodeficiency. N. Engl. J. Med. 1999, 340, 508–516. [Google Scholar] [CrossRef] [PubMed]
- Brown, L.; Xu-Bayford, J.; Allwood, Z.; Slatter, M.; Cant, A.; Davies, E.G.; Veys, P.; Gennery, A.R.; Gaspar, H.B. Neonatal diagnosis of severe combined immunodeficiency leads to significantly improved survival outcome: The case for newborn screening. Blood 2011, 117, 3243–3246. [Google Scholar] [CrossRef] [PubMed]
- Railey, M.D.; Lokhnygina, Y.; Buckley, R.H. Long-term clinical outcome of patients with severe combined immunodeficiency who received related donor bone marrow transplants without pretransplant chemotherapy or post-transplant GVHD prophylaxis. J. Pediatr. 2009, 155, 834–840. [Google Scholar] [CrossRef] [PubMed]
- Pai, S.Y.; Logan, B.R.; Griffith, L.M.; Buckley, R.H.; Parrott, R.E.; Dvorak, C.C.; Kapoor, N.; Hanson, I.C.; Filipovich, A.H.; Jyonouchi, S.; et al. Transplantation outcomes for severe combined immunodeficiency, 2000–2009. N. Engl. J. Med. 2014, 371, 434–446. [Google Scholar] [CrossRef] [PubMed]
- Myers, L.A.; Patel, D.D.; Puck, J.M.; Buckley, R.H. Hematopoietic stem cell transplantation for severe combined immunodeficiency in the neonatal period leads to superior thymic output and improved survival. Blood 2002, 99, 872–878. [Google Scholar] [CrossRef] [Green Version]
- Clement, M.C.; Mahlaoui, N.; Mignot, C.; Le Bihan, C.; Rabetrano, H.; Hoang, L.; Neven, B.; Moshous, D.; Cavazzana, M.; Blanche, S.; et al. Systematic neonatal screening for severe combined immunodeficiency and severe T-cell lymphopenia: Analysis of cost-effectiveness based on French real field data. J. Allergy Clin. Immunol. 2015, 135, 1589–1593. [Google Scholar] [CrossRef]
- Kubiak, C.; Jyonouchi, S.; Kuo, C.; Garcia-Lloret, M.; Dorsey, M.J.; Sleasman, J.; Zbrozek, A.S.; Perez, E.E. Fiscal Implications of Newborn Screening in the Diagnosis of Severe Combined Immunodeficiency. J. Allergy Clin. Immunol. Pract. 2014, 2, 697–702. [Google Scholar] [CrossRef] [Green Version]
- McGhee, S.A.; Stiehm, E.R.; McCabe, E.R.B. Potential costs and benefits of newborn screening for severe combined immunodeficiency. J. Pediatr. 2005, 147, 603–608. [Google Scholar] [CrossRef]
- Chan, K.; Davis, J.; Pai, S.Y.; Bonilla, F. A Markov model to analyze cost-effectiveness of screening for severe combined immunodeficiency (SCID). Mol. Genet. Metab. 2011, 104, 383–389. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ding, Y.T.J.; Kobrynski, L.; Ojodu, J.; Zarbalian, G.; Grosse, S. Cost-effectiveness/Cost-benefit analysis of newborn screening for severe combined immune deficiency in Washington state. J. Pediatr. 2016, 172, 127–135. [Google Scholar] [CrossRef] [PubMed]
- Cost-Effectiveness of Newborn Screening for Severe Combined Immune Deficiency. A Report Prepared for the National Screening Unit; Health Partners Consulting Group, 2014. Available online: https://www.nsu.govt.nz/system/files/resources/cost-effectiveness-newborn-screening-severe-combined-immune-deficiency.pdf (accessed on 15 January 2019).
- Van der Ploeg, C.P.B.; Blom, M.; Bredius, R.G.M.; van der Burg, M.; Schielen, P.C.J.I.; Verkerk, P.H.; Van den Akker-van Marle, M.E. Cost-effectiveness of newborn screening for severe combined immunodeficiency. Eur. J. Pediatr. 2019, 178, 721–729. [Google Scholar] [CrossRef] [PubMed]
- Northern Ireland Statistics and Research Agency. Live Births, 1887 to 2014. 2015. Available online: https://www.nisra.gov.uk/publications/birth-statistics (accessed on 15 November 2016).
- National Records of Scotland. Vital Events-Births. 2015. Available online: https://www.nrscotland.gov.uk/statistics-and-data/statistics/statistics-by-theme/vital-events/general-publications/vital-events-reference-tables (accessed on 15 November 2016).
- Office for National Statistics. Birth Summary Tables-England and Wales 2014. 2015. Available online: https://www.ons.gov.uk/peoplepopulationandcommunity/birthsdeathsandmarriages/livebirths/bulletins/birthsummarytablesenglandandwales/2017 (accessed on 15 November 2016).
- Gaspar, B.; University College London-Great Ormond Street Institute of Child Health, London, UK. Personal communication–UK incidence estimates, 2015.
- Amatuni, G.S.; Currier, R.J.; Church, J.A.; Bishop, T.; Grimbacher, E.; Nguyen, A.A.C.; Agarwal-Hashmi, R.; Aznar, C.P.; Butte, M.J.; Cowan, M.J.; et al. Newborn Screening for Severe Combined Immunodeficiency and T-cell Lymphopenia in California, 2010–2017. Pediatrics 2019, 143, e20182300. [Google Scholar] [CrossRef] [PubMed]
- Adams, S.P.; Rashid, S.; Premachandra, T.; Harvey, K.; Ifederu, A.; Wilson, M.C.; Gaspar, H.B. Screening of neonatal UK dried blood spots using a duplex TREC screening assay. J. Clin. Immunol. 2014, 34, 323–330. [Google Scholar] [CrossRef] [PubMed]
- Van der Spek, J.; Groenwold, R.H.; van der Burg, M.; van Montfrans, J.M. TREC Based Newborn Screening for Severe Combined Immunodeficiency Disease: A Systematic Review. J. Clin. Immunol. 2015, 35, 416–430. [Google Scholar] [CrossRef] [Green Version]
- Gaspar, B.; University College London-Great Ormond Street Institute of Child Health, London, UK. Personal communication–Length of stay and other costs estimates, 2016.
- Gaspar, B.; Ladomenou, F.; University College London-Great Ormond Street Institute of Child Health, London, UK. Personal communication–EQ-5D-3L esimates, 2016.
- Department of Health and Social Care. NHS Reference Costs 2014 to 2015. 2015. Available online: https://www.gov.uk/government/publications/nhs-reference-costs-2014-to-2015 (accessed on 17 February 2016).
- National Health Service UK Genetic Testing Network. Find A Test. 2016. Available online: http://ukgtn.nhs.uk/find-a-test/ (accessed on 17 February 2016).
- Liu, Z.; Albon, E.; Hyde, C. The Effectiveness and Cost-Effectiveness of Immunoglobulin Replacement Thearpy for Primary Immunodeficiency and Chronic Lymphocytic Leukaemia: A Systmatic Review and Economic Evaluation; West Midlands Health Technology Assessment Collaboration, Department of Public Health and Epidemiology, The University of Birmingham: Birmingham, UK, 2005; Available online: http://www.birmingham.ac.uk/Documents/college-mds/haps/projects/WMHTAC/REPreports/2005/IgRT.pdf (accessed on 17 February 2016).
- Paediatric Formulary Committee. BNF for Children; BMJ Group; Pharmaceutical Press; RCPCH Publications: London, UK; Available online: https://about.medicinescomplete.com/publication/british-national-formulary-for-children/ (accessed on 17 February 2016).
- The Health and Social Care Information Centre. Health Survey for England 2011 Children Trend Tables. 2012. Available online: https://digital.nhs.uk/data-and-information/publications/statistical/health-survey-for-england (accessed on 17 February 2016).
- Leaviss, J.; Bessey, A.; de la Cruz, C.; Wong, R.; Chilcott, J. Systematic Reviews of Screening for Severe Combined Immunodeficiency (SCID) in the NHS Newborn Blood Spot Screening Programme: Incidence, Screening Test Characteristics and the Effectiveness of Treatments; School of Health and Related Research (ScHARR), University of Sheffield: Sheffield, UK, 2017; Available online: https://legacyscreening.phe.org.uk/scid (accessed on 20 November 2018).
- Chan, A.; Scalchunes, C.; Boyle, M.; Puck, J.M. Early vs. delayed diagnosis of severe combined immunodeficiency: A family perspective survey. Clin. Immunol. 2011, 138, 3–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gaspar, H.B.; Qasim, W.; Davies, E.G.; Rao, K.; Amrolia, P.J.; Veys, P. How I treat severe combined immunodeficiency. Blood 2013, 122, 3749–3758. [Google Scholar] [CrossRef]
- Kwan, A.; Church, J.A.; Cowan, M.J.; Agarwal, R.; Kapoor, N.; Kohn, D.B.; Lewis, D.B.; McGhee, S.A.; Moore, T.B.; Stiehm, E.R.; et al. Newborn screening for severe combined immunodeficiency and T-cell lymphopenia in California: Results of the first 2 years. J. Allergy Clin. Immunol. 2013, 132, 140–150. [Google Scholar] [CrossRef]
- Curtis, L.B.; Burns, A. Unit Costs of Health and Social Care 2015; Personal Social Services Research Unit, University of Kent: Canterbury, UK, 2015; Available online: https://www.pssru.ac.uk/project-pages/unit-costs/ (accessed on 17 February 2016).
- Kwan, A.; Abraham, R.S.; Currier, R.; Brower, A.; Andruszewski, K.; Abbott, J.K.; Baker, M.; Ballow, M.; Bartoshesky, L.E.; Bonilla, F.A.; et al. Newborn screening for severe combined immunodeficiency in 11 screening programs in the United States. JAMA 2014, 312, 729–738. [Google Scholar] [CrossRef]
- Dolan, P. Modeling Valuations for EuroQol Health States. Med. Care 1997, 35, 1095–1108. [Google Scholar] [CrossRef] [PubMed]
- EuroQol Group. EuroQol—A new facility for the measurement of health-related quality of life. Health Policy 1990, 16, 199–208. [Google Scholar] [CrossRef]
- Neven, B.; Leroy, S.; Decaluwe, H.; Le Deist, F.; Picard, C.; Moshous, D.; Mahlaoui, N.; Debré, M.; Casanova, J.L.; Dal Cortivo, L.; et al. Long-term outcome after hematopoietic stem cell transplantation of a single-center cohort of 90 patients with severe combined immunodeficiency. Blood 2009, 113, 4114–4124. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patel, N.C.; Chinen, J.; Rosenblatt, H.M.; Hanson, I.C.; Brown, B.S.; Paul, M.E.; Abramson, S.L.; Ritz, J.; Shearer, W.T. Long-term outcomes of nonconditioned patients with severe combined immunodeficiency transplanted with HLA-identical or haploidentical bone marrow depleted of T cells with anti-CD6 mAb. J. Allergy Clin. Immunol. 2008, 122, 1185–1193. [Google Scholar] [CrossRef] [PubMed]
- Patel, N.C.; Chinen, J.; Rosenblatt, H.M.; Hanson, I.C.; Krance, R.A.; Paul, M.E.; Abramson, S.L.; Noroski, L.M.; Davis, C.M.; Seeborg, F.O.; et al. Outcomes of patients with severe combined immunodeficiency treated with hematopoietic stem cell transplantation with and without preconditioning. J. Allergy Clin. Immunol. 2009, 124, 1062–1069. [Google Scholar] [CrossRef] [PubMed]
- Slatter, M.A.; Brigham, K.; Dickinson, A.M.; Harvey, H.L.; Barge, D.; Jackson, A.; Bown, N.; Flood, T.J.; Cant, A.J.; Abinun, M.; et al. Long-term immune reconstitution after anti-CD52-treated or anti-CD34-treated hematopoietic stem cell transplantation for severe T-lymphocyte immunodeficiency. J. Allergy Clin. Immunol. 2008, 121, 361–367. [Google Scholar] [CrossRef] [PubMed]
- Mazzolari, E.; Forino, C.; Guerci, S.; Imberti, L.; Lanfranchi, A.; Porta, F.; Notarangelo, L.D. Long-term immune reconstitution and clinical outcome after stem cell transplantation for severe T-cell immunodeficiency. J. Allergy Clin. Immunol. 2007, 120, 892–899. [Google Scholar] [CrossRef]
- National Institute of Health and Care Excellence. Methods for the Development of NICE Public Health Guidance, 3rd ed.; National Institute of Health and Care Excellence: London, UK, 2012; Available online: https://www.nice.org.uk/process/pmg4/chapter/introduction (accessed on 27 October 2018).
- National Institute of Health and Care Excellence. Guide to the Methods of Technology Appraisal 2013. 2013. Available online: https://www.nice.org.uk/process/pmg9/chapter/foreword (accessed on 27 October 2018).
- Strong, M.; Oakley, J.E.; Brennan, A. Estimating Multiparameter Partial Expected Value of Perfect Information from a Probabilistic Sensitivity Analysis Sample: A Nonparametric Regression Approach. Med. Decis. Mak. 2014, 34, 311–326. [Google Scholar] [CrossRef]
- Heimall, J.; Logan, B.R.; Cowan, M.J.; Notarangelo, L.D.; Griffith, L.M.; Puck, J.M.; Kohn, D.B.; Pulsipher, M.A.; Parikh, S.; Martinez, C.; et al. Immune reconstitution and survival of 100 SCID patients post–hematopoietic cell transplant: A PIDTC natural history study. Blood 2017, 130, 2718–2727. [Google Scholar] [CrossRef]
- Morillo-Gutierrez, B.; Worth, A.; Valappil, M.; Gaspar, H.B.; Gennery, A.R. Chronic Infection with Rotavirus Vaccine Strains in UK Children with Severe Combined Immunodeficiency. Pediatr. Infect. Dis. J. 2015, 34, 1040–1041. [Google Scholar] [CrossRef]
- Lingen, M.; Albers, L.; Borchers, M.; Haass, S.; Gärtner, J.; Schröder, S.; Goldbeck, L.; von Kries, R.; Brockmann, K.; Zirn, B. Obtaining a genetic diagnosis in a child with disability: Impact on parental quality of life. Clin. Genet. 2016, 89, 258–266. [Google Scholar] [CrossRef] [PubMed]
- Wong, S.H.; McClaren, B.J.; Archibald, A.D.; Weeks, A.; Langmaid, T.; Ryan, M.M.; Kornberg, A.; Metcalfe, S.A. A mixed methods study of age at diagnosis and diagnostic odyssey for Duchenne muscular dystrophy. Eur. J. Hum. Genet. 2015, 23, 1294–1300. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lawton, S.; Hickerton, C.; Archibald, A.D.; McClaren, B.J.; Metcalfe, S.A. A mixed methods exploration of families’ experiences of the diagnosis of childhood spinal muscular atrophy. Eur. J. Hum. Genet. 2015, 23, 575–580. [Google Scholar] [CrossRef] [PubMed]
- Goldenberg, A.J.; Comeau, A.M.; Grosse, S.D.; Tanksley, S.; Prosser, L.A.; Ojodu, J.; Botkin, J.R.; Kemper, A.R.; Green, N.S. Evaluating Harms in the Assessment of Net Benefit: A Framework for Newborn Screening Condition Review. Matern. Child Health J. 2016, 20, 693–700. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barry, J.C.; Crowley, T.B.; Jyonouchi, S.; Heimall, J.; Zackai, E.H.; Sullivan, K.E.; McDonald-McGinn, D.M. Identification of 22q11.2 Deletion Syndrome via Newborn Screening for Severe Combined Immunodeficiency. J. Clin. Immunol. 2017, 37, 476. [Google Scholar] [CrossRef] [PubMed]
- Dorsey, M.J.; Dvorak, C.C.; Cowan, M.J.; Puck, J.M. Treatment of infants identified as having severe combined immunodeficiency by means of newborn screening. J. Allergy Clin. Immunol. 2017, 139, 733–742. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ungar, W. Economic Evaluation in Child Health; Oxford University Press: Oxford, UK, 2009. [Google Scholar]
- Steven, K. Valuation of the Child Health Utility 9D Index. Pharmacoeconomics 2012, 30, 729–747. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marciano, B.E.; Huang, C.Y.; Joshi, G.; Rezaei, N.; Carvalho, B.C.; Allwood, Z.; Ikinciogullari, A.; Reda, S.M.; Gennery, A.; Thon, V.; et al. BCG vaccination in patients with severe combined immunodeficiency: Complications, risks, and vaccination policies. J. Allergy Clin. Immunol. 2014, 133, 1134–1141. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Public Health England. Tuberculosis: The Green Book, Chapter 32. In The Green Book; Public Health England: London, UK, 2018. Available online: https://www.gov.uk/government/publications/tuberculosis-the-green-book-chapter-32 (accessed on 27 October 2018).
- Public Health England. The UK NSC Recommendation on Severe Combined Immunodeficiency. 2017. Available online: https://legacyscreening.phe.org.uk/scid (accessed on 27 October 2018).


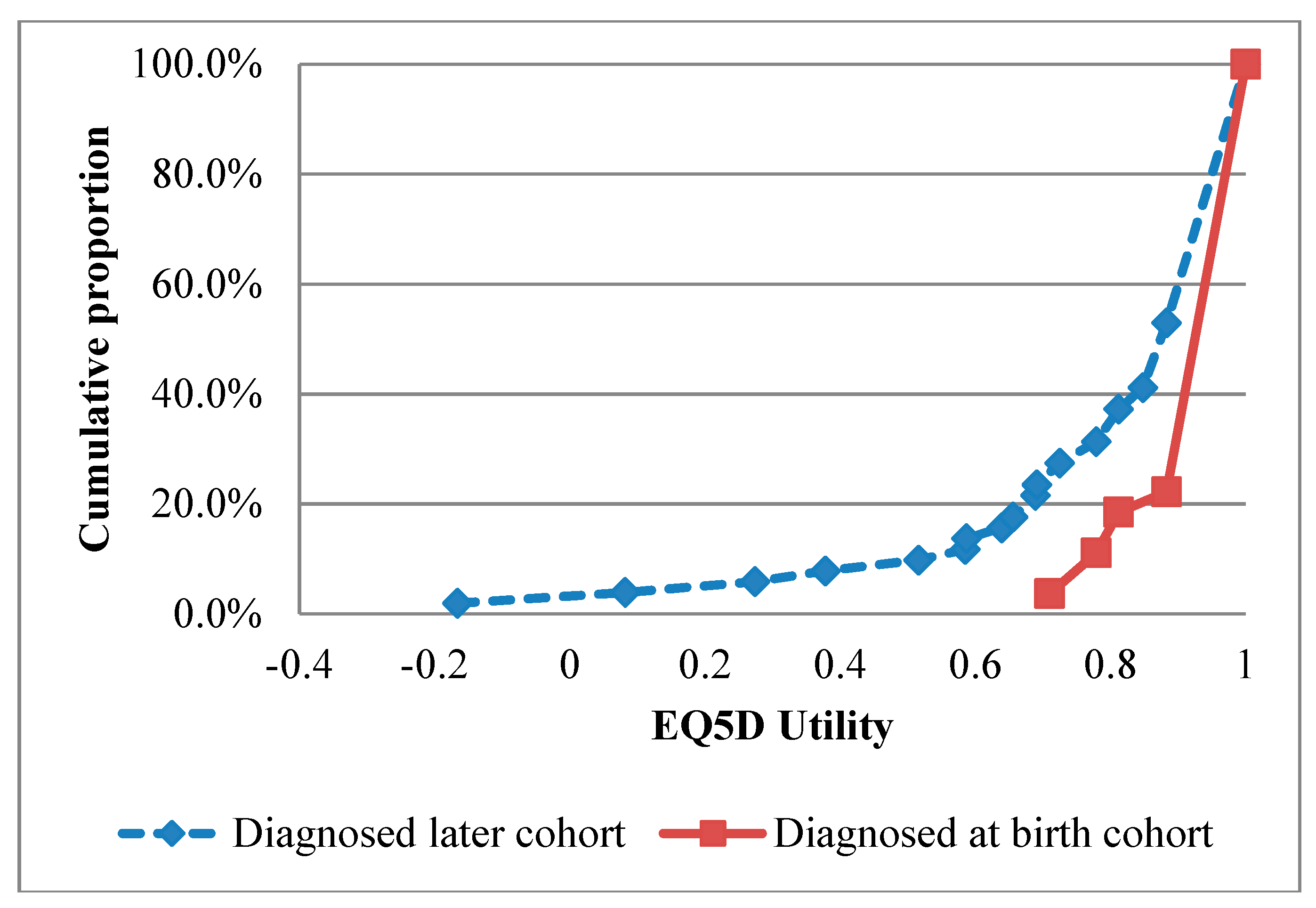

| Parameter | Mean (95% Confidence Interval) | Reference |
|---|---|---|
| Number of births (UK) | 780,835 | [17,18,19] |
| Incidence of SCID | 1:49,000 (1:39,857, 1:61,527) | [20] |
| Incidence of undiagnosed SCID | 1:521,000 (1:167,052, 1:7,236,800) | [20] |
| Incidence of syndromes | 1:45,000 (1:24,390, 1:110,606) | [21] |
| Incidence of secondary conditions | 1:130,000 (1:50,686, 1:782,506) | [21] |
| Incidence of idiopathic TCL | 1:99,000 (1:42,255, 1:432,482) | [21] |
| Incidence of positive TREC in pre-terms | 1:99,000 (1:42,255, 1:432,482) | [21] |
| Presumptive positives (20 copies/µL) | 0.041% (0.0035%, 0.1018%) | [22] |
| Sensitivity for SCID | 0.99 (0.985, 0.998) | [23] |
| Proportion of SCID patients with a family history | 0.30 (0.21, 0.41) | [20] |
| Proportion of SCID that is ADA-SCID | 0.17 (0.1, 0.26) | [20] |
| Proportion of SCID patients with a matched family donor available | 0.25 (0.07, 0.5) | [20] |
| Pre HSCT mortality (late diagnosed) | 35.3% (22.8%, 49.3%) | [6] |
| Pre HSCT mortality (early diagnosed) | 1.68% (0.11%, 7.63%) | [6] |
| HSCT mortality (late diagnosed) | 38.7% (22.4% 56.3%) | [6] |
| HSCT mortality (early diagnosed) | 8.48% (1.79%, 23.4%) | [6] |
| Number of days HSCT | 54.0 | [24] |
| Early diagnosis—Total days non-critical care | 82.6 (50.3, 122.8) | [24] |
| Early diagnosis—Total days critical care | 3.96 (0.15, 8.41) | [24] |
| Late diagnosis—Total days non-critical care | 144 (108.6, 184.3) | [24] |
| Late diagnosis—Total days critical care | 8.19 (3.72, 14.4) | [24] |
| QALYs—early diagnosis (1979–2015 cohort, base case values) | 0.95 | [25] |
| QALYs—late diagnosis (1979–2015 cohort, base case values) | 0.82 | [25] |
| QALYs—early diagnosis (2000–2015 cohort) | 0.96 | [25] |
| QALYs—late diagnosis (2000–2015 cohort) | 0.87 | [25] |
| Cost HSCT (early diagnosed) | £128,363 | [24,26] |
| Cost HSCT (late diagnosed) | £231,186 | [24,26] |
| Cost death prior to HSCT | £43,368 | [24,26] |
| Presumptive positive cost | £276 | [24,26] |
| Diagnosis SCID | £711 | [26,27] |
| Diagnosis idiopathic SCID and syndromes | £1,551 | [26,27] |
| Idiopathic SCID follow up (5 years discounted) | £20,142 | [26,28,29,30] |
| Syndromes 4 year follow up | £4,872 | [24,26] |
| Follow up preterm & secondary to other conditions | £533 | [24,26] |
| Screening | 95% CI | No Screening | 95% CI | Incremental | 95% CI | ||
|---|---|---|---|---|---|---|---|
| Outcomes | SCID cases diagnosed symptomatically | 0.2 | (0.1, 0.3) | 11.0 | (8.4, 14.1) | −10.9 | (−13.9, −8.2) |
| SCID cases diagnosed via a family history | 0 | (0, 0) | 4.9 | (3.1, 7.0) | −4.9 | (−3.1, −7.0) | |
| SCID cases not diagnosed | 0 | (0, 0) | 1.5 | (0.1, 4.8) | −1.5 | (−0.1, −4.8) | |
| SCID cases screen detected | 17.2 | (13.5, 21.7) | 0 | (0, 0) | 17.2 | (13.5, 21.7) | |
| ADA SCID | 2.9 | (1.6, 4.7) | 2.9 | (1.6, 4.7) | 0 | (0.0, 0.0) | |
| SCID mortality | 1.7 | (0.6, 4.0) | 8.1 | (5.3, 12.0) | −6.3 | (−9.7, −4) | |
| Screening outcomes | Non SCID TCL | 31.1 | (16.3, 50.1) | 0 | (0, 0) | 31 | (16.3, 50.1) |
| Pre-term | 8.0 | (1.9, 18.7) | 0 | (0, 0) | 8 | (1.9, 18.7) | |
| Total presumptive positives | 322.1 | (79.5, 852.9) | 0 | (0, 0) | 0 | (79.5, 852.9) | |
| Costs | Direct screening costs | £3.04m | (£2.97m, £3.19m) | £0.00m | (£0.00m, £0.00m) | £3.04m | (£2.97m, £3.19m) |
| Diagnosis and follow up pre-terms | £4,394 | (£1,029.36, £10,311) | £0 | (£0.00m, £0.00m) | £4,394 | (£1,029.36, £10,311) | |
| Diagnosis and follow up non SCID TCL | £0.27m | (£0.12m, £0.51m) | £0.00m | (£0.00m, £0.00m) | £0.27m | (£0.12m, £0.51m) | |
| Diagnosis costs SCID | £14,235 | (£11,112, £17,967) | £13,023 | (£13,023, £15,954) | £1,212 | (£61.7, £3,919) | |
| SCID treatment up and including HSCT | £3.35m | (£2.20m, £4.83m) | £3.63m | (£3.63m, £2.65m) | −£0.28m | (−£1.10m, £0.62m) | |
| SCID long-term costs | £2.03m | (£1.20m, £3.12m) | £1.30m | (£1.30m, £0.78m) | £0.72m | (£0.23m, £1.37m) | |
| Totals | Total costs | £7.30m | (£5.96m, £9.06m) | £3.96m | (£3.96m, £2.92m) | £3.34m | (£2.36m, £4.47m) |
| Total QALYs | 410.1 | (308.3, 527.3) | 226.9 | (226.9, 164.9) | 183.17 | (109.3, 267.2) | |
| ICER | £18,222 | (£12,013, £27,763) |
| Sensitivity Analysis | Screening | No Screening | Incremental | Probability Cost-Effective at Threshold | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Costs | QALYs | Costs | QALYs | Costs | QALYs | ICER | £20k | £30k | ||
| Base case | £7.30m | 410 | £3.96m | 227 | £3.34m | 183 | £18,222 | 65% | 99% | |
| Incidence | Halved | £5.49m | 223 | £2.01m | 113 | £3.48m | 110 | £31,647 | 2% | 35% |
| Doubled | £10.97m | 785 | £7.89m | 453 | £3.07m | 332 | £9,266 | 100% | 100% | |
| TREC cut off | Additional false positives | £7.53m | 412 | £3.99m | 227 | £3.54m | 185 | £19,137 | 56% | 98% |
| Increase proportionately | £7.44m | 409 | £3.98m | 227 | £3.46m | 182 | £18,983 | 56% | 98% | |
| Family History | 10% family history | £7.32m | 411 | £4.06m | 183 | £3.26m | 229 | £14,237 | 95% | 100% |
| 50% family history | £7.30m | 410 | £3.89m | 269 | £3.42m | 141 | £24,208 | 14% | 84% | |
| Test cost | £1.50 | £5.75m | 410 | £3.98m | 227 | £1.77m | 183 | £9,674 | 100% | 100% |
| £2.50 | £6.55m | 413 | £3.99m | 228 | £2.56m | 184 | £13,876 | 95% | 100% | |
| £4.50 | £8.10m | 410 | £3.98m | 227 | £4.11m | 183 | £22,471 | 25% | 92% | |
| Discount rate | 1.5% both costs and health benefits | £8.82m | 702 | £5.02m | 391 | £3.80m | 311 | £12,219 | 99% | 100% |
| 1.5% health benefits & 3.5% costs | £7.32m | 703 | £4.00m | 392 | £3.32m | 311 | £10,680 | 100% | 100% | |
| QALYs | 2000–2015 cohort QALYs used | £7.32m | 415 | £3.98m | 235 | £3.34m | 180 | £18,588 | 61% | 98% |
| Pre-transplant mortality | Early diagnosed mortality (OR) | |||||||||
| 8.15%(0.23) | £7.12m | 381 | £3.67m | 206 | £3.45m | 175 | £19,691 | 51% | 97% | |
| 29.40%(0.83) | £6.44m | 294 | £3.48m | 182 | £2.95m | 113 | £26,237 | 12% | 68% | |
| Post-transplant mortality | Early diagnosed mortality (OR) | |||||||||
| 17.42%(0.45) | £7.16m | 369 | £3.68m | 203 | £3.48m | 166 | £20,915 | 39% | 96% | |
| 36.77%(0.95) | £6.79m | 283 | £3.58m | 178 | £3.21m | 105 | £30,746 | 3% | 43% | |
| Healthy at Birth Disbenefit (QALY) | Cost per TREC Test £3.50 | |
|---|---|---|
| False-Positive Disbenefit Threshold (Quality-Adjusted Days) | ||
| Discounting 3.5% | Discounting 1.5% | |
| Mean (95% CI) | Mean (95% CI) | |
| 0 | 17 (0, 22) | 171 (1,113, 91) |
| 1 | 6 (0, 14) | 159 (1,069,83) |
| 2 | 0 (0, 6) | 148 (1,025, 75) |
| 3 | 0 (0, 0) | 136 (980, 67) |
| 4 | 0 (0, 0) | 125 (936, 59) |
| 10 | 0 (0, 0) | 55 (672, 11) |
| 20 | 0 (0, 0) | 0 (230, 0) |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bessey, A.; Chilcott, J.; Leaviss, J.; de la Cruz, C.; Wong, R. A Cost-Effectiveness Analysis of Newborn Screening for Severe Combined Immunodeficiency in the UK. Int. J. Neonatal Screen. 2019, 5, 28. https://doi.org/10.3390/ijns5030028
Bessey A, Chilcott J, Leaviss J, de la Cruz C, Wong R. A Cost-Effectiveness Analysis of Newborn Screening for Severe Combined Immunodeficiency in the UK. International Journal of Neonatal Screening. 2019; 5(3):28. https://doi.org/10.3390/ijns5030028
Chicago/Turabian StyleBessey, Alice, James Chilcott, Joanna Leaviss, Carmen de la Cruz, and Ruth Wong. 2019. "A Cost-Effectiveness Analysis of Newborn Screening for Severe Combined Immunodeficiency in the UK" International Journal of Neonatal Screening 5, no. 3: 28. https://doi.org/10.3390/ijns5030028





