Assessing the Content Quality of Online Parental Resources about Newborn Metabolic Disease Screening: A Content Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Content Abstraction—Data Coding
3. Results
3.1. Information on the Programme
3.1.1. Public Sectors
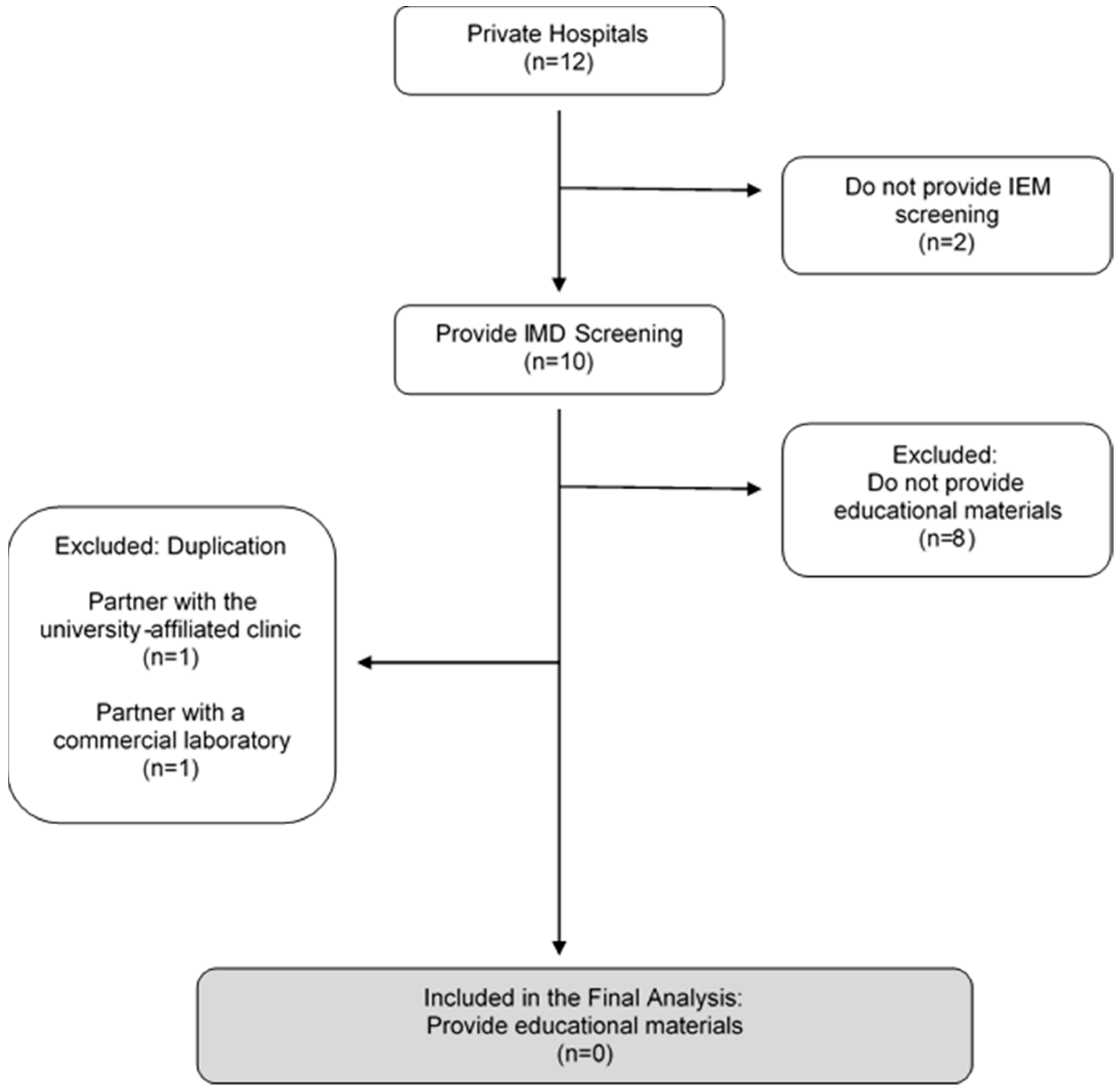
3.1.2. Private Sectors
Commercial Laboratories
University-Affiliated Clinic
Private Hospitals
3.2. Information on the Programme
3.2.1. Fundamental Message—Blood Tests
3.2.2. Fundamental Message—Urine Tests
4. Discussion
5. Study Limitations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| # | Inborn Errors of Metabolism | Code | ACMG Classification * | L1 | L2 | U1 | G1 |
|---|---|---|---|---|---|---|---|
| Amino Acid Disorders | |||||||
| 1 | Argininosuccinic Aciduria | ASA | Core | Yes | Yes | Yes | Yes |
| 2 | Citrullinaemia Type 1 | CITI | Core | Yes | Yes | Yes | Yes |
| 3 | Homocystinuria/Homocystinemia | HCU | Core | Yes | Yes | Yes | Yes |
| 4 | Maple Syrup Urine Disease | MSUD | Core | Yes | Yes | Yes | Yes |
| 5 | Phenylketonuria | PKU | Core | Yes | Yes | Yes | Yes |
| 6 | Tyrosinaemia Type 1 | TYR I | Core | Yes | Yes | Yes | Yes |
| 7 | Citrullinaemia Type 2 | CIT II | 2 | Yes | Yes | Yes | Yes |
| 8 | Arginase Deficiency | ARG | 2 | Yes | Yes | Yes | Yes |
| 9 | Defects of Biopterin Cofactor Biosynthesis and Regeneration | BIOPT (BS & REG) | 2 | - | - | Yes | - |
| 10 | Tyrosinaemia II | TYR II | 2 | Yes | Yes | - | - |
| 11 | Tyrosinaemia III | TYR III | 2 | Yes | Yes | - | - |
| 12 | 6-Pyru’voyl-Tetrahydropterin Synthase Deficiency | PTPS | - | - | - | - | Yes |
| 13 | Carbamyl Phosphate Synthase Deficiency | CPS | - | Yes | Yes | - | - |
| 14 | Histidinemia | - | - | Yes | Yes | - | - |
| 15 | Hyperammonemia | - | - | - | Yes | - | - |
| 16 | Hypermethioninemia Hyperornithinemia Homocitrullinuria Syndrome | HHH | - | Yes | Yes | - | - |
| 17 | Hyperornithinemia | - | - | Yes | Yes | - | - |
| 18 | Hyperornithinemia with Gyralatrophy | HOGA | - | Yes | - | - | |
| 19 | Hypervlinemia | - | - | Yes | Yes | - | - |
| 20 | Hypermethioninemia | - | - | Yes | - | - | - |
| 21 | Hyperphenylalaninemia | - | - | Yes | - | - | - |
| 22 | Tetrahydrobiopterin Deficiency | BH4D | - | Yes | Yes | - | - |
| 23 | N-Acetyglutamate Synthase Deficiency | NAGS | - | Yes | Yes | - | - |
| 24 | Non Ketotic Hyperglycinemia | NKH | - | Yes | Yes | - | - |
| 25 | Ornithine Transcarbamylase Deficiency | OTO | - | Yes | Yes | - | - |
| Fatty Acid Oxidation Disorders | |||||||
| 26 | Carnitine Update Defect | CUD | Core | Yes | Yes | Yes | Yes |
| 27 | Long-Chain 3-Hydroxyl-Acyl-CoA Dehydrogenase Deficiency | LCHAD | Core | Yes | Yes | Yes | - |
| 28 | Medium-Chain Acyl-CoA Dehydrogenase Deficiency | MCAD | Core | Yes | Yes | Yes | Yes |
| 29 | Trifunctional Protein Deficiency | TFP | Core | Yes | Yes | Yes | - |
| 30 | Very Long-Chain Acyl-CoA Dehydrogenase Deficiency | VLCAD | Core | Yes | Yes | Yes | Yes |
| 31 | 2,4- Dienoyl-CoA Reductase Deficiency | DE RED | 2 | Yes | Yes | - | - |
| 32 | Carnitine Palmitoyltransferase I Deficiency | CPTI | 2 | Yes | Yes | Yes | - |
| 33 | Carnitine Palmitoyltransferase II Deficiency | CPTII | 2 | Yes | Yes | Yes | Yes |
| 34 | Carnitine-Acylcarnitine Translocase Deficiency | CACT | 2 | Yes | Yes | Yes | Yes |
| 35 | Glutaric Acidaemia Type II | GAII | 2 | Yes | Yes | - | Yes |
| 36 | Medium/Short-Chain Hydroxyl-Acyl-CoA Dehydrogenase | M/SCHAD | 2 | Yes | Yes | Yes | - |
| 37 | Medium-Chain Ketoacyl-CoA Thiolase Deficiency | MCKAT | 2 | Yes | Yes | - | - |
| 38 | Short-Chain Acyl-CoA Dehydrogenase Deficiency | SCAD | 2 | Yes | Yes | Yes | - |
| 39 | Ethylmalonic Encephalopathy | EE | - | Yes | Yes | - | - |
| 40 | Malonyl-CoA Decarboxylase Deficiency | MCD | - | Yes | Yes | - | - |
| Organic Acid Disorders | |||||||
| 41 | 3-Hydroxy-3-Methylglutaryl-CoA Lyase Deficiency | HMG | Core | Yes | Yes | Yes | Yes |
| 42 | 3-Methylcrotonyl-CoA Carboxylase Deficiency | 3MCC | Core | Yes | Yes | Yes | - |
| 43 | Glutaric Aciduria Type 1 | GAI | Core | Yes | Yes | Yes | Yes |
| 44 | Isovaleric Aciduria | IVA | Core | Yes | Yes | Yes | Yes |
| 45 | Methylmalonic Aciduria | Cbl A,B | Core | ? | - | Yes | - |
| 46 | Methylmalonic Aciduria | MUT | Core | ? | Yes | Yes | Yes |
| 47 | Multiple Carboxylase Deficiency | MCD | Core | Yes | Yes | Yes | Yes |
| 48 | Propionic Acidaemia | PA | Core | Yes | Yes | Yes | Yes |
| 49 | ß-Ketothiolase Deficiency | BKT | Core | Yes | Yes | Yes | Yes |
| 50 | 3-Methylglutaconic Aciduria Type I | 3MGA | 2 | Yes | Yes | Yes | - |
| 51 | Methylmalonic Aciduria | Cbl C,D | 2 | ? | - | Yes | Yes |
| 52 | Malonic Aciduria | MAL | 2 | - | - | Yes | - |
| 53 | 2-Methyl-3-Hydroxybutyryl-CoA Aciduria | 2M3HBA | 2 | Yes | Yes | - | - |
| 54 | 2-Methylbutyryl-CoA Dehydrogenase Deficiency | 2MBG | 2 | Yes | Yes | - | - |
| 55 | Isobutyryl-CoA Dehydrogenase Deficiency | IBD | 2 | Yes | Yes | - | - |
| Others | |||||||
| 55 | Biotinidase Deficiency | BIOT | Core | - | - | - | Yes |
| 56 | Congenital Adrenal Hyperplasia | CAH | Core | - | - | Yes | Yes |
| 57 | Cystic Fibrosis | CF | Core | - | - | Yes | - |
| 58 | X-linked adrenoleukodystrophy | ALD | Core | - | - | Yes ^ | - |
| 59 | Classic Galactosaemia | GALT | Core | - | - | - | Yes |
| 60 | Galactose Epimerase Deficiency | GALE | 2 | - | - | - | - |
| 61 | Galactokinase deficiency | GALK | 2 | - | - | - | - |
| 62 | Severe Combined Immunodeficiency | SCID | - | - | - | Yes ^ | Yes # |
| 63 | Spinal Muscular Atrophy | SMA | - | - | - | Yes ^ | - |
Appendix B
| 1. 2-Oxoadipic Aciduria 2. 3-Hydroxy 3-Methyl Glutaric Aciduria 3. 3-Hydroxyisobutyryl-CoA Deacylase Deficiency 4. 3-Methylcrotonyl-CoA Carboxylase Deficiency 5. 3-Methylglutaconic Aciduria (Type I) 6. 3-Methylglutaconic Aciduria (Type II) 7. 3-Methylglutaconic Aciduria (Type III) 8. 3-Methylglutaconic Aciduria (Type IV) 9. 3-Methylglutaconic Aciduria (Type V) 10. 5-Oxoprolinuria 11. 5,10-Methylenetetrahydrofolate Reductase (MTHFR) Deficiency 12. ß-Ureidopropionase Deficiency 13. Adenine Phosphoribosyltransferase Deficiency 14. Adenosine Deaminase Deficiency 15. Adenylosuccinate Lyase Deficiency (ASLD) 16. Adult-onset Type II Citrullinemia (CTLN2) 17. Alkaptonuria 18. Aminoadipic Aciduria 19. Argininemia 20. Argininosuccinase Deficiency 21. Argininosuccinate Synthase Deficiency 22. Aspartylglucosaminuria (AGU) 23. Aspirin Poisoning 24. Biotinidase Deficiency 25. Canavan Disease 26. Carbamoyl Phosphate Synthetase Deficiency 27. Carnitine Palmitoyl Synthase Deficiency (CPSD) 28. Carnitine Palmitoyl Synthase I Deficiency (CPSID) 29. Carnitine Palmitoyl Synthase II Deficiency (CPSIID) 30. Carnitine Transport Defect 31. Citrullinemia 32. Cystathioninuria 33. Cystinuria 34. Diabetes Mellitus Type I 35. Diabetes Mellitus Type II 36. Dicarboxylic Aciduria 37. Dihydrolipoyl Dehydrogenase (E3) Deficiency 38. Dihydropyridinase Deficiency 39. Dihydropyrimidine Dehydrogenase Deficiency 40. Endogenous Sucrosuria 41. Ethanolaminosis 42. Ethylene Glycol Poisoning 43. Ethylhydracrylic Aciduria 44. Fanconi Syndrome 45. Formiminoglutamic Aciduria 46. Fructosuria 47. Fructose-1, 6-Diphosphatase Deficiency 48. Fumaric Aciduria 49. Galactosemia I 50. Galactosemia II 51. Galactosemia III 52. Gestational Diabetes Mellitus 53. Gluconeogenesis Disorder 54. Glutaric Aciduria Type I 55. Glutathione Synthetase Deficiency 56. Glyceric Aciduria 57. Glyceroluria 58. Hartnup Syndrome 59. Hawkinsinuria 60. Hepatic Failure 61. Hepatic Tyrosinemia 62. Hereditary Fructose Intolerance 63. Hereditary Xanthinuria 64. Histidinemia 65. Holocarboxylase Synthetase Deficiency 66. Homocystinuria 67. Hemosiderinuria 68. Hydroxyprolinemia 69. Hypernoxaluria Type I 70. Hyper- β -Alaninemia 71. Hyperoxaluria Type Il 72. Hyperdibasicaminoaciduria 73. Hyperglycinuria 74. Hyperleucinemia 75. Hyperleucinuria 76. Hyperlysinemia 77. Hypermethioninemia 78. Hyperornithinemia-hyperammonemia-homocitrullinuria (HHH) syndrome 79. Hyperornithinemia 80. Hyperphenylalaninemia 81. Hyperprolinemia Type I, Prolidase Deficiency 82. Hypersarcosinemia 83. Hypervalinemia 84. Hypervalinemia 85. Hypophosphatasia 86. Hypoxanthine Adenine Phosphoribosyltransferase Deficiency 87. Infantile Refsum Disease 88. Intestinal Bacterial Overgrowth 89. Isovaleric Acidemia 90. Ketoadipic Aciduria 91. Lactic Acidemia 92. Lactose Intolerance 93. Lesch-Nyhan Syndrome 94. Long-chain acyl-CoA Dehydrogenase Deficiency 95. Long-chain 3-hydroxyacyl-CoA Dehydrogenase (LCHAD) Deficiency 96. Lysine Malabsorption 97. Lysinuric Protein Intolerance 98. Malabsorption Syndromes 99. Malonyl-CoA Decarboxylase Deficiency 100. Maple Syrup Urine Disease 101. MCT Oil Fed Dicarboxylic Aciduria 102. Medium Chain Acyl-CoA Dehydrogenase Deficiency 103. Menkes Disease 104. Mercaptolactate-Cysteine Disulfiduria 105. Methylmalonic Acidemia (Cbi D-HC) 106. Methylmalonic Acidemia (Cbi D-MMA/HC) 107. Methylmalonic Acidemia (Cbi E) 108. Methylmalonic Acidemia (Cbi F) 109. Methylmalonic Acidemia (Cbi G) 110. Methylmalonic Acidemia (Cbl A) 111. Methylmalonic Acidemia (Cbl B) 112. Methylmalonic Acidemia (Cbl C) 113. Methylmalonic Acidemia (MUT) 114. Methylmalonic Acidemia (Muto) 115. Methylmalonic Acidemia (Vitamin B 12 Deficiency) 116. Mevalonate Kinase Deficiency 117. Mitochondrial Trifunctional Proscin Deficiency (MTPD) 118. Molybdenum Cofactor Deficiency/Sulfite Oxidase Deficiency 119. Multiple acyl-CoA Dehydrogenase Deficiency 120. N-Acetylglutamate Synthase Deficiency 121. Neonatal Adrenoleukodystrophy 122. Neonatal Intrahepatic Cholestasis (NICCD) 123. Neuroblastoma 124. Normal Fed Medium Chain Triglyceride Formulas Dicarboxylic Aciduria 125. Ornithine Transcarbamylase Deficiency 126. Orotic Aciduria 127. Pentosuria 128. Phenylketonuria 129. Pheochromocytoma 130. Primary Hyperoxaluria 131. Propionic Acidemia 132. Pyridoxamine 5-Phosphate Oxidase Deficiency 133. Pyruvate Carboxylase Deficiency 134. Pyruvate Dehydrogenase Deficiency 135. Renal Dysfunction 136. Renal Glycosuria 137. Saccharopinuria 138. Short Chain Acyl-CoA Dehydrogenase Deficiency 139. Short-Chain 3-Hydroxyacyl-CoA Dehydrogenase Deficiency (SCHAD) 140. Sialic Acid Storage Disease 141. Succinic Semialdehyde Dehydrogenase Deficiency (4-Hydrozybutyric Aciduria) 142. Succinyl-CoA:3-ketoacid CoA Transferase Deficiency 143. Tegretol Poisoning 144. Tetrahydrobiopterin (BH4) Deficiency 145. Transient Galactosemia 146. Transient Neonatal Tyrosinemia 147. Trimethylaminuria 148. Tryptophanuria 149. Tyrosinemia Type 1 150. Tyrosinemia Type III 151. Tyrosinemia Type Il 152. Valproate Toxicity 153. Very Long-Chain Acyl-CoA Dehydrogenase Deficiency (VLCAD) 154. Vitamin B12 Deficiency or Malabsorption 155. Zellweger Syndrome 156. Zellweger-Like Syndrome 157. ß-Ketothiolase (T2) Deficiency 158. ß-Aminoisobutyric Aciduria (BAIB) |
References
- Dietzen, D.J.; Rinaldo, P.; Whitley, R.J.; Rhead, W.J.; Hannon, W.H.; Garg, U.C.; Lo, S.F.; Bennett, M.J. National academy of clinical biochemistry laboratory medicine practice guidelines: Follow-up testing for metabolic disease identified by expanded newborn screening using tandem mass spectrometry; executive summary. Clin. Chem. 2009, 55, 1615–1626. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mak, C.M.; Lee, H.C.H.; Chan, A.Y.W.; Lam, C.W. Inborn errors of metabolism and expanded newborn screening: Review and update. Critical reviews in clinical laboratory sciences. Crit. Rev. Clin. Lab. Sci. 2013, 50, 142–162. [Google Scholar] [CrossRef] [PubMed]
- Therrell, B.L.; Padilla, C.D.; Loeber, J.G.; Kneisser, I.; Saadallah, A.; Borrajo, G.J.; Adams, J. Current status of newborn screening worldwide: 2015. Semin. Perinatol. 2015, 39, 171–187. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smith, R.A. Can we improve on Wilson and Jungner’s principles of screening for disease? Can. Med Assoc. J. 2018, 190, E414–E415. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martínez-Morillo, E.; Prieto García, B.; Álvarez Menéndez, F.V. Challenges for worldwide harmonisation of newborn screening programs. Clin. Chem. 2016, 62, 689–698. [Google Scholar] [CrossRef] [Green Version]
- Forman, J.; Coyle, F.; Levy-Fisch, J.; Roberts, P.; Terry, S.; Legge, M. Screening criteria: The need to deal with new developments and ethical issues in newborn metabolic screening. J. Community Genet. 2013, 4, 59–67. [Google Scholar] [CrossRef] [Green Version]
- Yi, H.; Hallowell, N.; Griffiths, S.; Leung, T.Y. Motivations for undertaking DNA sequencing-based non-invasive prenatal testing for fetal aneuploidy: A qualitative study with early adopter patients in Hong Kong. PLoS ONE 2013, 8, e81794. [Google Scholar] [CrossRef] [Green Version]
- DeLuca, J.M.; Kearney, M.H.; Norton, S.A.; Arnold, G.L. Parents’ experiences of expanded newborn screening evaluations. Pediatrics 2011, 128, 53–61. [Google Scholar] [CrossRef]
- Chudleigh, J.; Buckingham, S.; Dignan, J.; O’Driscoll, S.; Johnson, K.; Rees, D.; Wyatt, H.; Metcalfe, A. Parents’ experiences of receiving the initial positive newborn screening (NBS) result for cystic fibrosis and sickle cell disease. J. Genet. Couns. 2016, 25, 1215–1226. [Google Scholar] [CrossRef] [Green Version]
- DeLuca, J.M.; Kearney, M.H.; Norton, S.A.; Arnold, G.L. Internet use by parents of infants with positive newborn screens. J. Inherit. Metab. Dis. 2012, 35, 879–884. [Google Scholar] [CrossRef]
- Araia, M.H.; Potter, B.K. Newborn screening education on the internet: A content analysis of North American newborn screening program websites. J. Community Genet. 2011, 2, 127–134. [Google Scholar] [CrossRef] [Green Version]
- The Task Force on the Pilot Study of Newborn Screening for Inborn Errors of Metabolism. Evaluation of the 18-month “Pilot Study of Newborn Screening for Inborn Errors of Metabolism” in Hong Kong. HK J. Paediatr. (New Ser.) 2020, 25, 16–22. [Google Scholar]
- Pitt, J.J. Newborn screening. Clin. Biochem. Rev. 2010, 31, 57. [Google Scholar]
- Ozben, T. Expanded newborn screening and confirmatory follow-up testing for inborn errors of metabolism detected by tandem mass spectrometry. Clin. Chem. Lab. Med. 2013, 51, 157–176. [Google Scholar] [CrossRef]
- Berelson, B. Content Analysis in Communication Research; Haffner Press: New York, NY, USA, 1971. [Google Scholar]
- Hsieh, H.F.; Shannon, S.E. Three approaches to qualitative content analysis. Qual. Health Res. 2005, 15, 1277–1288. [Google Scholar] [CrossRef]
- Mercer, M.; Agatisa, P.; Farrell, R. What patients are reading about non-invasive prenatal testing: An evaluation of Internet content and implications for patient-centered care. Prenat. Diagn. 2014, 34, 986–993. [Google Scholar] [CrossRef]
- American Academy of Pediatrics, Newborn Screening Task Force. Serving the family from birth to the medical home. Newborn screening: A blueprint for the future. A call for a national agenda on state newborn screening programs. Pediatrics 2000, 106, 389–427. [Google Scholar]
- Davis, T.C.; Humiston, S.G.; Arnold, C.L.; Bocchini, J.A.; Bass, P.F.; Kennen, E.M.; Bocchini, A.; Williams, D.; Kyler, P.; Lloyd-Puryear, M. Recommendations for effective newborn screening communication: Results of focus groups with parents, providers, and experts. Pediatrics 2006, 117 (Suppl. 3), S326–S340. [Google Scholar] [CrossRef] [Green Version]
- Chong, S.C.; Law, L.K.; Hui, J.; Lai, C.Y.; Leung, T.Y.; Yuen, Y.P. Expanded newborn metabolic screening programme in Hong Kong: A three-year journey. Hong Kong Med. J. 2017, 23, 489–496. [Google Scholar] [CrossRef]
- Tang, N.L.S.; Hui, J. 20 Years After Discovery of the Causative Gene of Primary Carnitine Deficiency, How Much More Have We Known About the Disease? HK J. Paediatr. (New Ser.) 2020, 25, 23–29. [Google Scholar]
- Watson, M.S.; Mann, M.Y.; Lloyd-Puryear, M.A.; Rinaldo, P.; Howell, R.R.; American College of Medical Genetics Newborn Screening Expert Group. Newborn screening: Toward a uniform screening panel and system. Genet. Med. 2006, 8, S296–S307, Erratum in Pediatrics 2006, 117, S296–S307. [Google Scholar]
- Wolfe, L.; Jethva, R.; Oglesbee, D.; Vockley, J. Short-Chain Acyl-CoA Dehydrogenase Deficiency; Adam, M.P., Everman, D.B., Mirzaa, G.M., Pagon, R.A., Wallace, S.E., Eds.; GeneReviews® [Internet]; University of Washington: Seattle, WA, USA, 1993–2022. Available online: https://www.ncbi.nlm.nih.gov/books/NBK63582/ (accessed on 29 November 2022).
- Pandor, A.; Eastham, J.; Beverley, C.; Chilcott, J.; Paisley, S. Clinical effectiveness and cost-effectiveness of neonatal screening for inborn errors of metabolism using tandem mass spectrometry: A systematic review. Health Technol. Assess. 2004, 8, 1–121. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Embade, N.; Cannet, C.; Diercks, T.; Gil-Redondo, R.; Bruzzone, C.; Ansó, S.; Echevarría, L.R.; Ayucar, M.; Collazos, L.; Lodoso, B.; et al. NMR-based newborn urine screening for optimized detection of inherited errors of metabolism. Sci. Rep. 2019, 9, 13067. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hong Kong Annual Digest of Statistics. Census and Statistics Department, Hong Kong Special Administrative Region. 2021. Available online: https://www.censtatd.gov.hk/en/data/stat_report/product/B1010003/att/B10100032022AN22B0100.pdf (accessed on 4 November 2022).
- Ngan, O.M.Y.; Yi, H.; Bryant, L.; Sahota, D.S.; Chan, O.Y.M.; Ahmed, S. Parental expectations of raising a child with disability in decision-making for prenatal testing and termination of pregnancy: A mixed methods study. Patient Educ. Couns. 2020, 103, 2373–2383. [Google Scholar] [CrossRef]
- Zayts, O.; Luo, Z. Commodification and marketisation of genetic testing through online direct-to-consumer platforms in Hong Kong. Discourse Commun. 2017, 11, 630–647. [Google Scholar] [CrossRef]
- Hui, V.C.; Li, H.C.; Chow, J.H.; Ng, C.S.; Lui, C.Y.; Fung, J.L.; Mak, C.C.; Chung, B.H.; Lau, K.K. Understanding and perception of direct-to-consumer genetic testing in Hong Kong. J. Genet. Couns. 2021, 30, 1640–1648. [Google Scholar] [CrossRef]
- Hock, K.T.; Christensen, K.D.; Yashar, B.M.; Roberts, J.S.; Gollust, S.E.; Uhlmann, W.R. Direct-to-consumer genetic testing: An assessment of genetic counselors’ knowledge and beliefs. Genet. Med. 2011, 13, 325–332. [Google Scholar] [CrossRef] [Green Version]
- Strategic Development of Genomic Medicine in Hong Kong (Full Report), Food and Health Bureau, Hong Kong Special Administrative Region. 2020. Available online: https://www.healthbureau.gov.hk/download/press_and_publications/otherinfo/200300_genomic/SCGM_report_en.pdf (accessed on 4 November 2022).
- Cutiongco-de la Paz, E.M.; Chung, B.H.Y.; Faradz, S.M.; Thong, M.K.; David-Padilla, C.; Lai, P.S.; Lin, S.P.; Chen, Y.H.; Sura, T.; Laurino, M. Training in clinical genetics and genetic counseling in Asia. Am. J. Med. Genet. Part C: Semin. Med. Genet. 2019, 181, 177–186. [Google Scholar] [CrossRef]
- Kalokairinou, L.; Howard, H.C.; Slokenberga, S.; Fisher, E.; Flatscher-Thöni, M.; Hartlev, M.; van Hellemondt, R.; Juškevičius, J.; Kapelenska-Pregowska, J.; Kováč, P.; et al. Legislation of direct-to-consumer genetic testing in Europe: A fragmented regulatory landscape. J. Community Genet. 2018, 9, 117–132. [Google Scholar] [CrossRef]

| Theme | Definition |
|---|---|
| Purpose | The test detects health problems that would not or might not be apparent without testing |
| Benefits | The test may prevent serious health problems |
| Sampling Procedure | How testing is performed |
| Results | How will you receive the results |
| Healthcare Provider Involvement | You may ask your healthcare providers for the results |
| Purpose of Re-test | There is a possibility of re-testing or follow-up |
| Importance of Re-test | The importance of responding quickly to a request for follow-up testing |
| Contact | How to contact the test programme |
| False-positive | Possibility of receiving a false-positive result |
| False-negative | Possibility of receiving a false-negative result |
| Risks | Risk of pain or infection |
| List of Conditions | A list of the conditions screened |
| Storage Policy | Information about policies and practices related to the storage and use of the bio-sample |
| Number of Conditions | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Code | Sectors | Include in Analysis | Type of Test | Online Sources | Total | Organic Acid | Amino Acid | Fatty Acid | Other |
| Public Sectors | |||||||||
| G1 | Public Hospital | Yes | Blood (MS/MS) | Yes | 26 | 8 | 9 | 6 | 3 |
| Private Sectors | |||||||||
| L1 | Commercial Laboratory | Yes | Blood (MS/MS) | Yes | 48 | 12 | 21 | 15 | 0 |
| L2 | Commercial Laboratory | Yes | Blood (MS/MS) | Yes | 48 | 12 | 21 | 15 | 0 |
| L3 | Commercial Laboratory | Yes | Urine (GC/MS) | Yes | 106 # | - | - | - | - |
| L4 | Commercial Laboratory | Yes | Urine (GC/MS) | Yes | 106 | 59 | 6 | 41 | |
| L5 | Commercial Laboratory | Yes | Urine (GC/MS) | Yes | 106 | 59 | 6 | 41 | |
| L6 | Commercial Laboratory | Yes | Urine (GC/MS) | Yes | 158 | - | - | - | - |
| L7 | Commercial Laboratory | Yes | Urine (GC/MS) | Yes | 158 | - | - | - | - |
| U1 | University-affiliated Clinic | Yes | Blood (MS/MS) | Yes | 33 | 12 | 9 | 10 | 2 |
| P1 | Maternity Hospital | No ^ | Blood (MS/MS) | No | - # | - | - | - | - |
| P2 | Maternity Hospital | No ^ | Blood (MS/MS) | No | 26 | 8 | 9 | 6 | 3 |
| P3 | Maternity Hospital | No ^ | Blood (MS/MS) | No | 33 | 12 | 9 | 10 | 2 |
| P4 | Maternity Hospital | No ^ | Blood (MS/MS) | No | 33 | 12 | 9 | 10 | 2 |
| P5 | Maternity Hospital | No ^ | Blood (MS/MS) | No | 33 | 12 | 9 | 10 | 2 |
| P6 | Maternity Hospital | No ^ | Blood (MS/MS) | No | 33 | 12 | 9 | 10 | 2 |
| P7 | Maternity Hospital | No ^ | Blood (MS/MS) | Yes | 33 | 12 | 9 | 10 | 2 |
| P8 | Maternity Hospital | No ^ | Blood (MS/MS) | No | 31 | 11 | 10 | 10 | 0 |
| P9 | Maternity Hospital | No ^ | Blood (MS/MS) | Yes | 48 | 12 | 21 | 15 | 0 |
| P10 | Maternity Hospital | No ^ | Blood (MS/MS) | No | 49 | 11 | 17 | 15 | 6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ngan, O.M.Y.; Wong, W.K.; Tam, J.C.; Li, C.K. Assessing the Content Quality of Online Parental Resources about Newborn Metabolic Disease Screening: A Content Analysis. Int. J. Neonatal Screen. 2022, 8, 63. https://doi.org/10.3390/ijns8040063
Ngan OMY, Wong WK, Tam JC, Li CK. Assessing the Content Quality of Online Parental Resources about Newborn Metabolic Disease Screening: A Content Analysis. International Journal of Neonatal Screening. 2022; 8(4):63. https://doi.org/10.3390/ijns8040063
Chicago/Turabian StyleNgan, Olivia M. Y., Wing Ki Wong, Janice Ching Tam, and Chi Kong Li. 2022. "Assessing the Content Quality of Online Parental Resources about Newborn Metabolic Disease Screening: A Content Analysis" International Journal of Neonatal Screening 8, no. 4: 63. https://doi.org/10.3390/ijns8040063





