Best Practice for Identification of Classical 21-Hydroxylase Deficiency Should Include 21 Deoxycortisol Analysis with Appropriate Isomeric Steroid Separation
Abstract
:1. Introduction

2. Materials and Methods
2.1. Victorian NBS Program Samples
2.2. Patients’ Samples
2.3. Mass Spectrometry Materials
2.4. Second-Tier Analytical Method
2.5. Method Validation Studies and Acceptance Criteria
- Precision—three experiments were performed to determine imprecision: (a) overall method within run imprecision using n = 20 replicates; (b) LC–MS/MS within run injection replicate imprecision using one vial injected n = 20 times; and (c) between run imprecision assessed with the bi-level IQC material. Acceptance criteria was set to be 50% of the biological variation, i.e., this represents the desirable imprecision for each analyte and ideally the CV should be <10%.
- Linearity—initial linearity was assessed with the calibration curve and then using the same sample matrix using a mixture of high (IQC) and low (pooled normal patient) and using non-matrix matched with high (standard) and low (pooled normal patient). Acceptance criteria were set as follows: Linearity—the calibration slope should be r = 0.99. Linearity outside the upper limit requires formal linearity studies to determine the upper reportable range. The Linchecker version 1.1.2.0 by Philippe Marquis (Metz, France) was used to assess this and if “linearity” is determined, then it is acceptable.
- Bias—assessed through recovery study and the external quality assurance sample comparison study. Note: Some, but not all steroid standards, are traceable to high order reference materials or methods listed in the JCTLM database. Acceptance criteria were set as follows: Bias–recovery studies with results between 85 and115% were acceptable. RCPAQAP and CDC EQA were the main determinants of actual bias with acceptable EQA performance.
- Method comparison—EQA samples and immunoassay GSP 17OHP results. Acceptance criteria were set. Method comparison—95% CI for Passing Bablok slope to include 1, 95% CI of Bland Altman plot to include zero. For analytes where the CI decision is not met, an evaluation of the clinical significance of deviation is made to determine acceptance. This is, however, limited as this is a new method and not a change. EQA for all steroids and first- and second-tier 17OHP will be compared but noting they are different measurands.
- Uncertainty of measurement—this was based on a coverage factor of 2 for between-run imprecision of IQC.
- Limit of blank/detection/quantification—assessed low (pooled normal patient) at different dilutions with (n = 10) of imprecision (standard deviation) for each calculation. Acceptance criteria were set as follows: Limit of quantitation (LOQ) of 20% or S/N of 10 and limit of detection (LOD) with a S/N of 2, which is relevant for analytes that are usually not present in patient samples, e.g., 21 DF. The LOQ should meet clinical relevance and fall at least into or below the cut-offs (analyte-specific).
- Carry-over—assessed by quantification of a blank injection after a high concentration sample (standard LV 5). Carry-over—less than 2%.
- Interference—known isomeric steroids to 17OHP (11-alpha and 11-beta hydroxy progesterone, 16-hydroxyprogesterone, and 21-hydroxyprogesterone) and 21DF (11-deoxycortisol and corticosterone) were tested to ensure resolution from their respective measurands. Acceptance criteria were set as follows: Interference—0% bias.
- Decision limits—cut-offs were assessed by running (1) normal neonates, (2) retrieval of known patients’ DBS samples, and (3) comparison with Australasian cut-offs already in place. Decision limits were determined in-house by visual assessment, centiles, and receiver operator sensitivity and specificity characteristics.
- Assessment of fitness for clinical purpose—the overall decision to implement the quantitation of each steroid was based on the method validation results and the overall performance against clinical need. Fitness for purpose—reviewed against the clinical utility of each analyte.
2.6. Statistical Analysis
3. Results
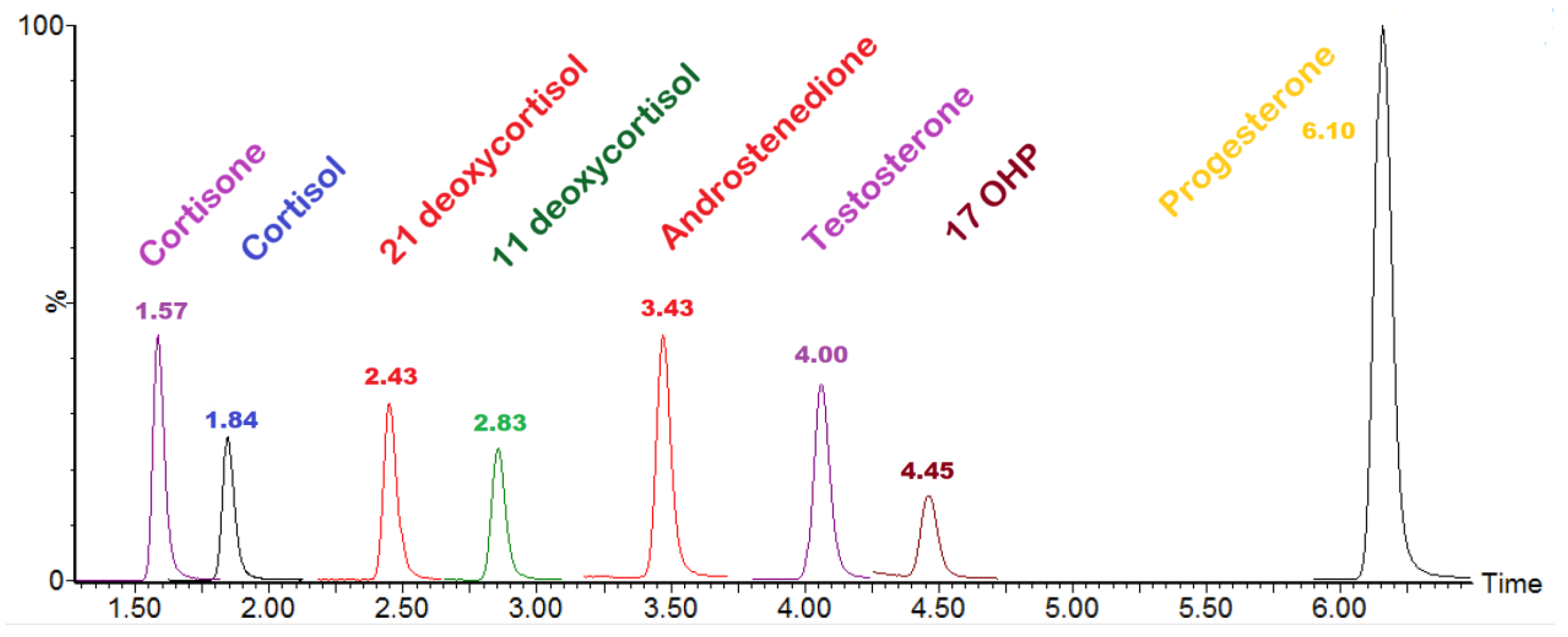
3.1. Method Validation and Steroid Isomer Separation
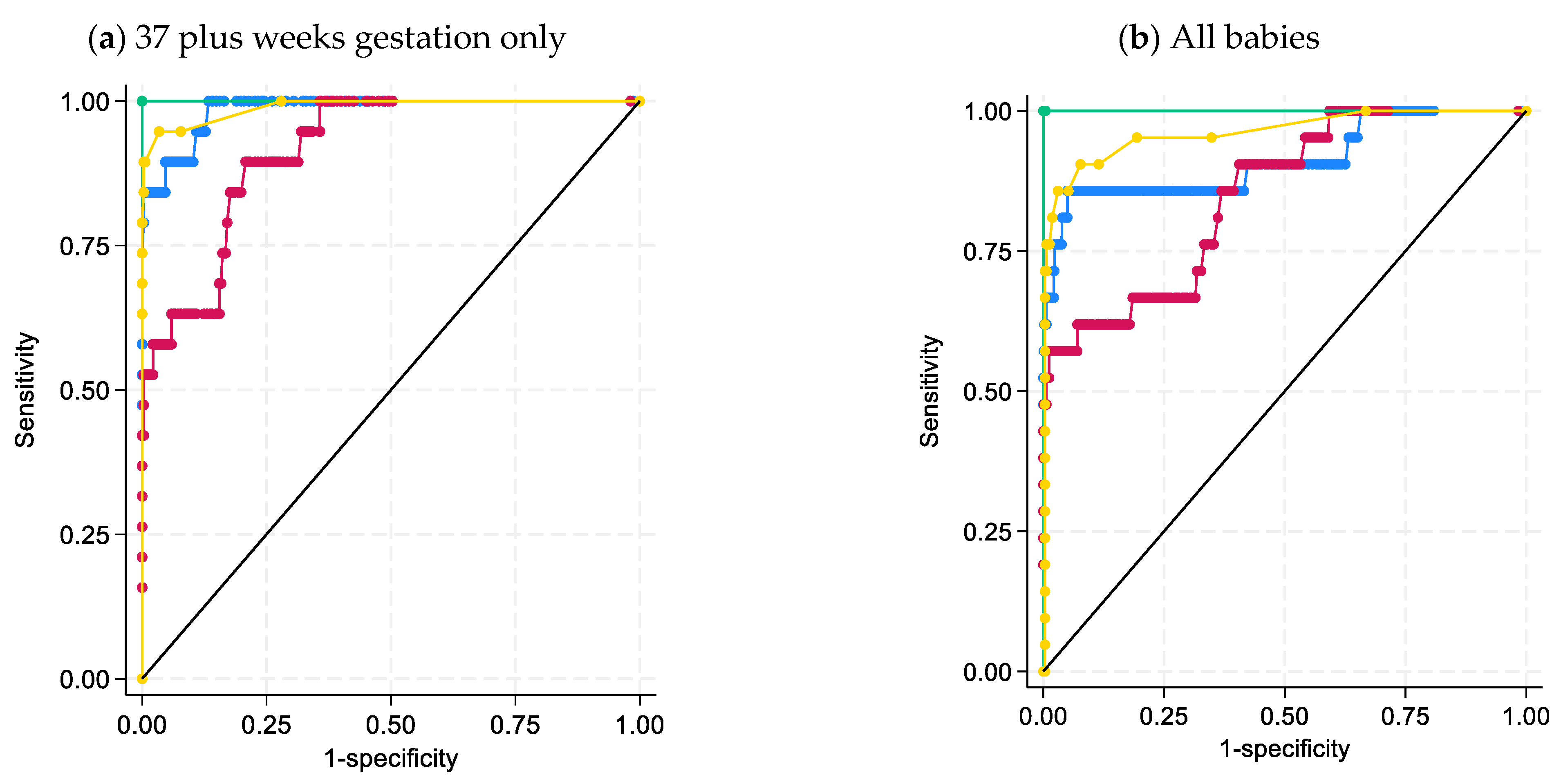
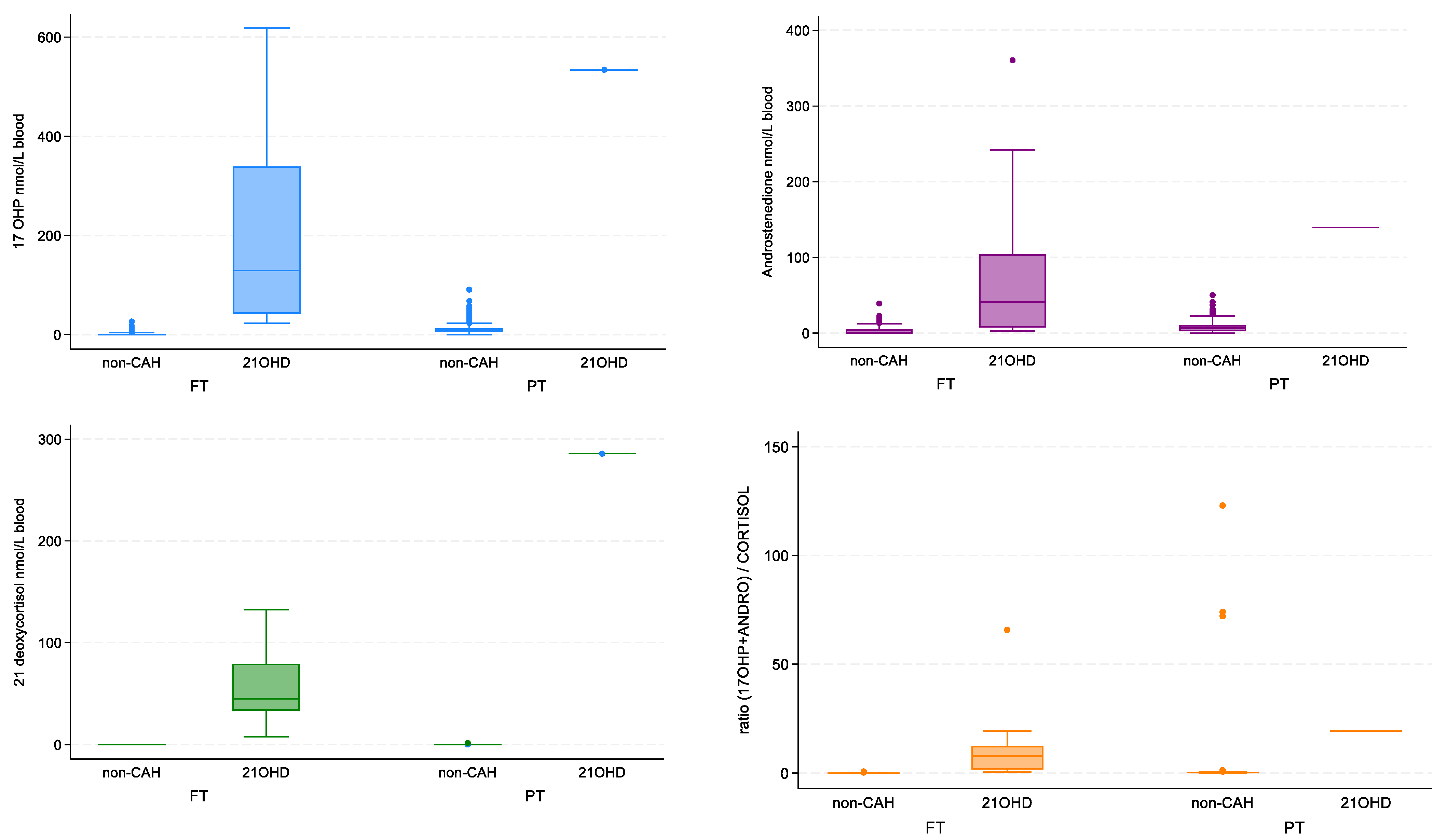
3.2. Statistical Analysis of Steroid Data
- 35% (n = 322) of non-CAH babies that were full term.
- 28% (n = 260) of non-CAH babies that were late preterm, i.e., 32 to <37 weeks.
- 34% (n = 313) of non-CAH babies had a recorded gestational age of <32 weeks.
- 3% (n = 29) of non-CAH babies did not have a gestational age recorded and were excluded from analysis if there was a division between preterm and full-term birth.
- Two female babies were identified prospectively to have CAH due to 21-hydroxylase deficiency within this data analysis period (both were full-term neonates).
- A further 14 archival DBS samples were retrieved from children with known 21-hydroxylase deficiency (diagnosed prior to initiation of CAH testing in the NBS program).
- One additional archival card was retrieved as part of the diagnostic work up of a child who presented at eight months with virilization and confirmed by our method to have 21OHase deficiency.
3.3. Classical CAH Due to 21-Hydroxylase Deficiency-Patient Review
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Consumable | Catalogue Number |
|---|---|
| Steroids Standards—PM Separations and NMIA | |
| 17OHP-NMI | H5752 |
| 11-Deoxycortisol 1 mg/mL acetonitrile 1 mL | COR-1627-1LA LIP |
| 21-Desoxycortisol 100 ug/mL 1 mL | S13407-0.1 ISO |
| Androstenedione 1 mg/mL methanol 1 mL | AND-1489-1LM LIP |
| Cortisol 1 mg/mL methanol 1 mL | COR-1630-1LM LIP |
| Cortisone 1 mg/mL methanol 1 mL | COR-1633-1LM LIP |
| Progesterone 1 mg/mL acetonitrile 1 mL | PRO-1683-1LA LIP |
| Testosterone 1 mg/mL methanol 1 mL | TES-842-1LM LIP |
| Internal Standard—PM Separations | |
| 17a-Hydroxyprogesterone-[2,3,4-13C3] 100 ug/mL 1 mL | S10333-0.1 ISO |
| Androst-4-ene-3,17-dione-[13C3] 100 ug/mL 1 mL | S9044-0.1 ISO |
| 21-Deoxycortisol-D8 (2,2,4,6,6,21,21,21-D8) 100 ug/mL 1 mL | D-076-1ML CER |
| 11-Deoxycortisol-[D5] 100 ug/mL 1 mL | S9012-0.1 ISO |
| Cortisol-[13C3] 100 ug/mL 1mL | S14465-0.1 ISO |
| Cortisone-[13C3] 100 ug/mL 1mL | S14466-0.1 ISO |
| Testosterone-D3 1 mg/mL methanol 1 mL | TES-1149-1LM LIP |
| Progesterone-[2,3,4-13C3] 100 ug/mL 1 mL | S10314-0.1 ISO |
| Internal Quality Controls—Australian Scientific Enterprise and Inhouse | |
| ASE level 2 and 5 | 920.2.03 & 920.5.03 |
| Mobile Phases—Thermofisher Scientific | |
| Methanol Optima LC-MS grade | FSBA456-4 |
| Formic acid Optima LC-MS grade | A117-50 |
| Analyte | Recovery (%) | Linearity (R2) | Sample Prep Imprecision (%) | Injections Imprecision (%) | Between Run Imprecision ASE LV 2 (CV %) | Between Run Imprecision ASE LV 5 (CV %) | Carryover (%) | Interference | S/N: Peak to Peak | Uncertainty of Measurement (%) | Cut-Off (nmol/L) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 17 OHP | 100.7 | 0.99 | 8.4 | 2.0 | 7.1 | 6.8 | 0 | Not detected | 5.46 | 16.8 | <20 |
| 11-Deoxycortisol | 102.7 | 0.99 | 8.9 | 2.9 | 7.3 | 7.5 | 0 | Not detected | 31.91 | 17.8 | <10 |
| 21-Deoxycortisol | 100.9 | 0.98 | 12.5 | 6.2 | 8.3 | 9.7 | 0 | Not detected | 45.16 | 25.0 | <1 * |
| Androstenedione | 101.9 | 0.99 | 6.5 | 0.8 | 7.2 | 9.6 | 0 | Not detected | 3.49 | 12.9 | <20 |
| Cortisol | 104.1 | 0.99 | 9.3 | 2.4 | 6.8 | 6.9 | 0 | Not detected | 3.33 | 18.6 | 20–800 |
| Cortisone | 103.5 | 0.99 | 6.6 | 1.3 | N/A | N/A | 0 | Not detected | 13.2 | 40–400 | |
| Testosterone | 105.6 | 0.99 | 10.2 | 2.6 | N/A | N/A | 0 | Not detected | 20.3 | <5 | |
| Progesterone | 104.2 | 0.99 | 8.1 | 1.8 | N/A | N/A | 0 | Not detected | 16.1 | <50 |
| Name | Material | Value | |
|---|---|---|---|
| Blank 1 | Cal 0 | 1.627 × 104 | |
| Blank 2 | Cal 0 | 1.678 × 104 | |
| Blank 3 | Cal 0 | 1.714 × 104 | |
| Blank 4 | Cal 0 | 1.634 × 104 | |
| Blank 5 | Cal 0 | 1.597 × 104 | |
| Blank 6 | Cal 0 | 1.777 × 104 | |
| Blank 7 | Cal 0 | 1.678 × 104 | |
| Blank 8 | Cal 0 | 1.705 × 104 | |
| Blank 9 | Cal 0 | 1.653 × 104 | |
| Blank 10 | Cal 0 | 1.724 × 104 | |
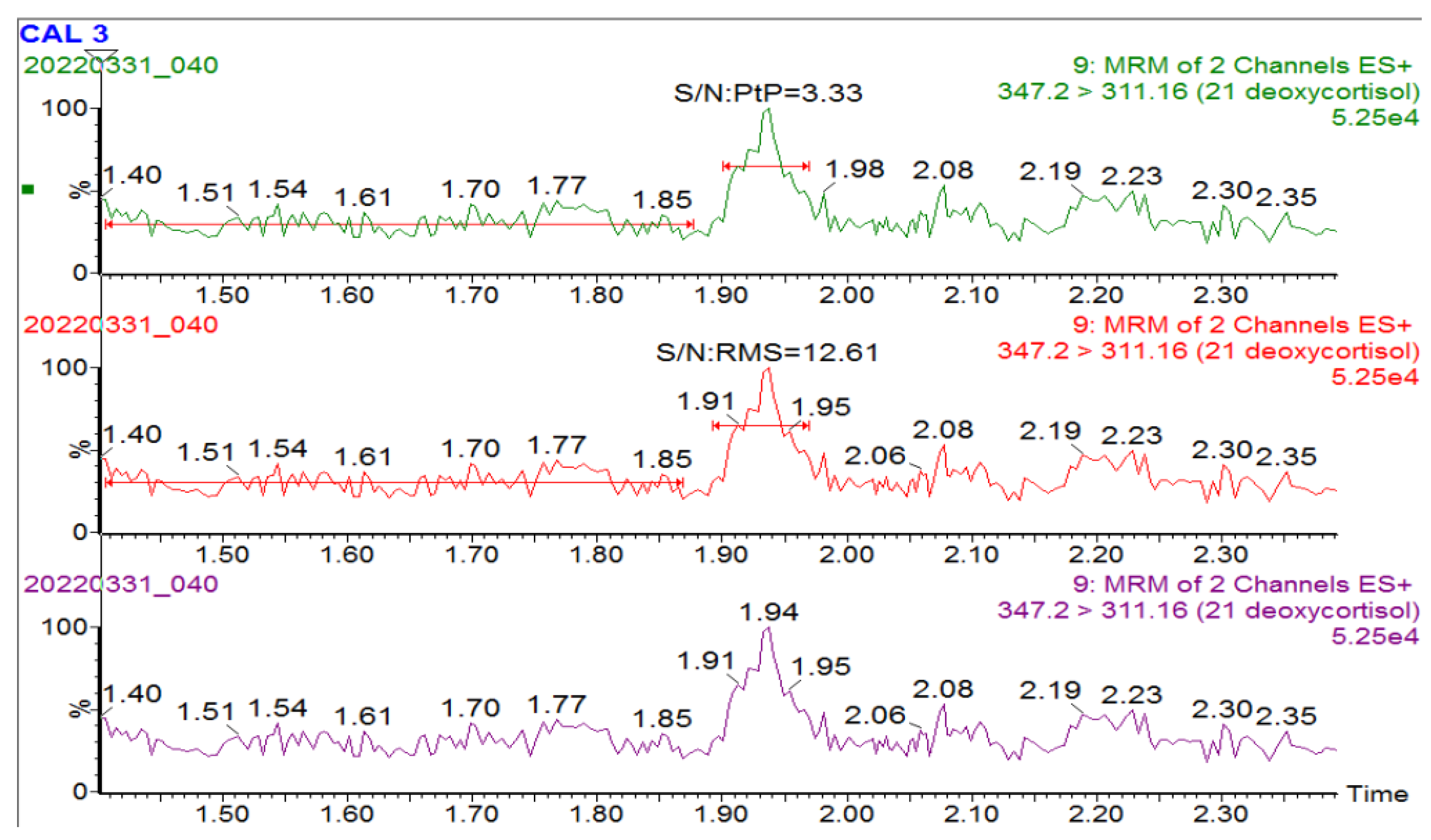
| Signal-to-Noise Ratio | |||
| Steroids | Conc (nmol/L) used to determine S/N | S/N: RMS | S/N: Peak to peak |
| 17OHP | 10.25 | 25.13 | 5.46 |
| Androstenedione | 46.17 | 139.9 | 31.91 |
| Cortisol | 45.5 + endogenous | 214.72 | 45.16 |
| 11 deoxycortisol | 4.95 | 17.19 | 3.49 |
| 21 deoxycortisol | 20.28 | 12.61 | 3.33 |
References
- Choi, R.; Park, H.-D.; Oh, H.J.; Lee, K.; Song, J.; Lee, S.-Y. Dried Blood Spot Multiplexed Steroid Profiling Using Liquid Chromatography Tandem Mass Spectrometry in Korean Neonates. Ann. Lab. Med. 2019, 39, 263–270. [Google Scholar] [CrossRef]
- De Hora, M.R.; Heather, N.L.; Patel, T.; Bresnahan, L.G.; Webster, D.; Hofman, P.L. Measurement of 17-Hydroxyprogesterone by LCMSMS Improves Newborn Screening for CAH Due to 21-Hydroxylase Deficiency in New Zealand. Int. J. Neonatal Screen. 2020, 6, 6. [Google Scholar] [CrossRef] [PubMed]
- Steigert, M.; Schoenle, E.J.; Biason-Lauber, A.; Torresani, T. High reliability of neonatal screening for congenital adrenal hyperplasia in Switzerland. J. Clin. Endocrinol. Metab. 2002, 87, 4106–4110. [Google Scholar] [CrossRef]
- Hammersen, J.; Bettendorf, M.; Bonfig, W.; Schönau, E.; Warncke, K.; Eckert, A.J.; Fricke-Otto, S.; Palm, K.; Holl, R.W.; Woelfle, J. Twenty years of newborn screening for congenital adrenal hyperplasia and congenital primary hypothyroidism—Experiences from the DGKED/AQUAPE study group for quality improvement in Germany. Med. Genet. 2022, 34, 29–40. [Google Scholar] [CrossRef]
- Navardauskaitė, R.; Banevičiūtė, K.; Songailienė, J.; Grigalionienė, K.; Čereškevičius, D.; Šukys, M.; Mockevicienė, G.; Smirnova, M.; Utkus, A.; Verkauskienė, R. Impact of Newborn Screening on Clinical Presentation of Congenital Adrenal Hyperplasia. Medicina 2021, 57, 1035. [Google Scholar] [CrossRef] [PubMed]
- Tsuji-Hosokawa, A.; Kashimada, K. Thirty-Year Lessons from the Newborn Screening for Congenital Adrenal Hyperplasia (CAH) in Japan. Int. J. Neonatal Screen. 2021, 7, 36. [Google Scholar] [CrossRef]
- Stroek, K.; Ruiter, A.; van der Linde, A.; Ackermans, M.; Bouva, M.J.; Engel, H.; Jakobs, B.; Kemper, E.A.; van den Akker, E.L.T.; van Albada, M.E.; et al. Second-tier Testing for 21-Hydroxylase Deficiency in the Netherlands: A Newborn Screening Pilot Study. J. Clin. Endocrinol. Metab. 2021, 106, e4487–e4496. [Google Scholar] [CrossRef]
- Edelman, S.; Desai, H.; Pigg, T.; Yusuf, C.; Ojodu, J. Landscape of Congenital Adrenal Hyperplasia Newborn Screening in the United States. Int. J. Neonatal Screen. 2020, 6, 64. [Google Scholar] [CrossRef]
- Zetterstrom, R.H.; Karlsson, L.; Falhammar, H.; Lajic, S.; Nordenstrom, A. Update on the Swedish Newborn Screening for Congenital Adrenal Hyperplasia Due to 21-Hydroxylase Deficiency. Int. J. Neonatal Screen. 2020, 6, 71. [Google Scholar] [CrossRef] [PubMed]
- Lai, F.; Srinivasan, S.; Wiley, V. Evaluation of a Two-Tier Screening Pathway for Congenital Adrenal Hyperplasia in the New South Wales Newborn Screening Programme. Int. J. Neonatal Screen. 2020, 6, 63. [Google Scholar] [CrossRef]
- Janzen, N.; Sander, S.; Terhardt, M.; Steuerwald, U.; Peter, M.; Das, A.M.; Sander, J. Rapid steroid hormone quantification for congenital adrenal hyperplasia (CAH) in dried blood spots using UPLC liquid chromatography–tandem mass spectrometry. Steroids 2011, 76, 1437–1442. [Google Scholar] [CrossRef] [PubMed]
- Monostori, P.; Szabó, P.; Marginean, O.; Bereczki, C.; Karg, E. Concurrent Confirmation and Differential Diagnosis of Congenital Adrenal Hyperplasia from Dried Blood Spots: Application of a Second-Tier LC-MS/MS Assay in a Cross-Border Cooperation for Newborn Screening. Horm. Res. Paediatr. 2015, 84, 311–318. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.; Lee, M.N.; Park, H.D.; Kim, J.W.; Chang, Y.S.; Park, W.S.; Lee, S.Y. Dried blood spot testing for seven steroids using liquid chromatography-tandem mass spectrometry with reference interval determination in the Korean population. Ann. Lab. Med. 2015, 35, 578–585. [Google Scholar] [CrossRef] [PubMed]
- Miller, W.L. Congenital Adrenal Hyperplasia: Time to Replace 17OHP with 21-Deoxycortisol. Horm. Res. Paediatr. 2019, 91, 416–420. [Google Scholar] [CrossRef]
- Held, P.K.; Bialk, E.R.; Lasarev, M.R.; Allen, D.B. 21-Deoxycortisol is a Key Screening Marker for 21-Hydroxylase Deficiency. J. Pediatr. 2022, 242, 213–219.e1. [Google Scholar] [CrossRef]
- Watanabe, K.; Tsuji-Hosokawa, A.; Hashimoto, A.; Konishi, K.; Ishige, N.; Yajima, H.; Sutani, A.; Nakatani, H.; Gau, M.; Takasawa, K.; et al. The High Relevance of 21-Deoxycortisol, (Androstenedione + 17α-Hydroxyprogesterone)/Cortisol, and 11-Deoxycortisol/17α-Hydroxyprogesterone for Newborn Screening of 21-Hydroxylase Deficiency. J. Clin. Endocrinol. Metab. 2022, 107, 3341–3352. [Google Scholar] [CrossRef] [PubMed]
- Wudy, S.A.; Schuler, G.; Sánchez-Guijo, A.; Hartmann, M.F. The art of measuring steroids: Principles and practice of current hormonal steroid analysis. J. Steroid Biochem. Mol. Biol. 2018, 179, 88–103. [Google Scholar] [CrossRef]
- Greaves, R.F.; Jevalikar, G.; Hewitt, J.K.; Zacharin, M.R. A guide to understanding the steroid pathway: New insights and diagnostic implications. Clin. Biochem. 2014, 47, 5–15. [Google Scholar] [CrossRef]
- Greaves, R.; Poomthavorn, P.; Zacharin, M. 11β-hydroxylase deficiency masked by alternative medicines. J. Paediatr. Child Health 2006, 42, 652–654. [Google Scholar] [CrossRef]
- Homma, K.; Hasegawa, T.; Takeshita, E.; Watanabe, K.; Anzo, M.; Toyoura, T.; Jinno, K.; Ohashi, T.; Hamajima, T.; Takahashi, Y.; et al. Elevated urine pregnanetriolone definitively establishes the diagnosis of classical 21-hydroxylase deficiency in term and preterm neonates. J. Clin. Endocrinol. Metab. 2004, 89, 6087–6091. [Google Scholar] [CrossRef]
- Greaves, R.F.; Ho Chung, S.; Loh Tze, P.; Chai Jia, H.; Jolly, L.; Graham, P.; Hartmann Michaela, F.; de Rijke Yolanda, B.; Wudy Stefan, A. Current state and recommendations for harmonization of serum/plasma 17-hydroxyprogesterone mass spectrometry methods. Clin. Chem. Lab. Med. 2018, 56, 1685–1697. [Google Scholar] [CrossRef]
- Lo, C.W.S.; Hoad, K.; Loh, T.P.; van den Berg, S.; Cooke, B.R.; Greaves, R.F.; Hartmann, M.F.; Wudy, S.A.; Ho, C.S. Endogenous isobaric interference on serum 17 hydroxyprogesterone by liquid chromatography-tandem mass spectrometry methods. Clin. Chem. Lab. Med. 2023, 61, e64–e66. [Google Scholar] [CrossRef] [PubMed]
- Miller, W.L. P450 oxidoreductase deficiency: A new disorder of steroidogenesis with multiple clinical manifestations. Trends Endocrinol. Metab. 2004, 15, 311–315. [Google Scholar] [CrossRef] [PubMed]
- Scott, R.R.; Miller, W.L. Genetic and clinical features of p450 oxidoreductase deficiency. Horm. Res. 2008, 69, 266–275. [Google Scholar] [CrossRef] [PubMed]
- Greaves, R.F.; Wudy, S.A.; Badoer, E.; Zacharin, M.; Hirst, J.J.; Quinn, T.; Walker, D.W. A tale of two steroids: The importance of the androgens DHEA and DHEAS for early neurodevelopment. J. Steroid Biochem. Mol. Bio. 2019, 188, 77–85. [Google Scholar] [CrossRef]
- Greaves, R.F.; Pitt, J.; McGregor, C.; Wall, M.; Christodoulou, J. Newborn bloodspot screening in the time of COVID-19. Genet. Med. 2021, 23, 1143–1150. [Google Scholar] [CrossRef] [PubMed]
- CLSI. Blood collection on filter paper for newborn screening programs; Approved Standard—Sixth Edition. In CLSI Document NBS01-A6; Clinical Laboratory Standards Institute: Wayne, PA, USA, 2013. [Google Scholar]
- Mei, J.V.; Zobel, S.D.; Hall, E.M.; De Jesús, V.R.; Adam, B.W.; Hannon, W.H. Performance properties of filter paper devices for whole blood collection. Bioanalysis 2010, 2, 1397–1403. [Google Scholar] [CrossRef]
- Gozzi, T.G.; Harris, N.P.; McGown, I.N.; Cowley, D.M.; Cotterill, A.M.; Campbell, P.E.; Anderson, P.K.; Warne, G.L. Autopsy diagnosis of 21-hydroxylase deficiency CAH in a case of apparent SIDS. Pediatr. Dev. Pathol. 2005, 8, 397–401. [Google Scholar] [CrossRef]
- Diver, M.; Hughes, J.; Hutton, J.; West, C.; Hipkin, L. The Long-Term Stability in Whole Blood of 14 Commonly-Requested Hormone Analytes. Ann. Clin. Biochem. 1994, 31 Pt 6, 561–565. [Google Scholar] [CrossRef]
- Gatelais, F.; Berthelot, J.; Beringue, F.; Descamps, P.; Bonneau, D.; Limal, J.M.; Coutant, R. Effect of single and multiple courses of prenatal corticosteroids on 17-hydroxyprogesterone levels: Implication for neonatal screening of congenital adrenal hyperplasia. Pediatr. Res. 2004, 56, 701–705. [Google Scholar] [CrossRef]
- CLSI. Newborn Screening for Preterm, Low Birth Weight, and Sick Newborns, 2nd ed.; CLSI Guideline NBS03; CLSI: Wayne, PA, USA, 2019; p. 82. [Google Scholar]
- Fiet, J.; Le Bouc, Y.; Guéchot, J.; Hélin, N.; Maubert, M.A.; Farabos, D.; Lamazière, A. A Liquid Chromatography/Tandem Mass Spectometry Profile of 16 Serum Steroids, Including 21-Deoxycortisol and 21-Deoxycorticosterone, for Management of Congenital Adrenal Hyperplasia. J. Endocr. Soc. 2017, 1, 186–201. [Google Scholar] [CrossRef] [PubMed]
- Lasarev, M.R.; Bialk, E.R.; Allen, D.B.; Held, P.K. Application of Principal Component Analysis to Newborn Screening for Congenital Adrenal Hyperplasia. J. Clin. Endocrinol. Metab. 2020, 105, e2930–e2940. [Google Scholar] [CrossRef] [PubMed]

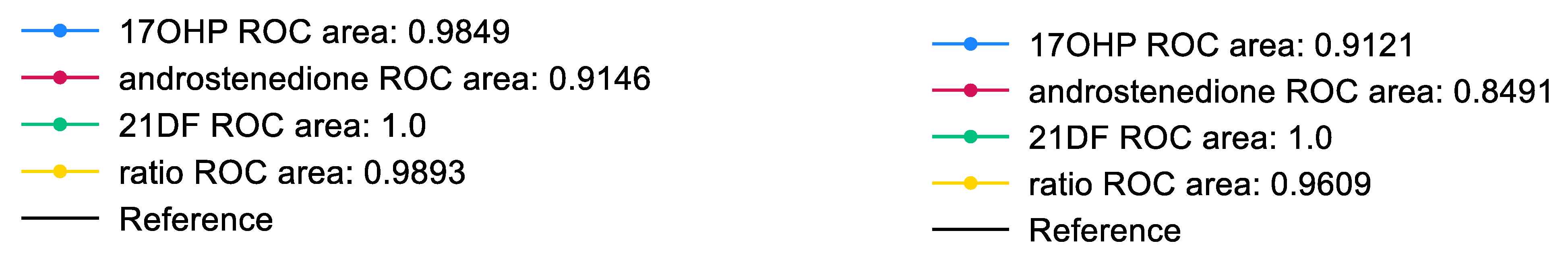

| Group | Steroid | MW (g/mol) | Parent Ion | Quantifier | Qualifier | Cone Voltage | RT | ||
|---|---|---|---|---|---|---|---|---|---|
| Ion | CE | Ion | CE | ||||||
| 1 | Cortisol | 362.47 | 363.1 | 121.0 | 20 | 327.2 | 14 | 20 | 1.83 |
| 21-Deoxycortisol | 346.46 | 347.2 | 311.2 | 16 | 121.0 | 32 | 25 | 2.43 | |
| 11-Deoxycortisol ** | 346.46 | 347.2 | 97.1 | 25 | 109.0 | 25 | 20 | 2.83 | |
| Androstenedione | 286.41 | 287.2 | 97.1 | 20 | 109.0 | 20 | 20 | 3.43 | |
| 17OHP | 330.46 | 331.3 | 97.1 | 20 | 109.2 | 26 | 20 | 4.44 | |
| Cortisone | 360.45 | 361.2 | 163.1 | 22 | 121.1 | 30 | 20 | 1.57 | |
| Testosterone | 288.42 | 289.2 | 97.1 | 20 | 109.0 | 24 | 30 | 4 | |
| Progesterone | 314.47 | 315.2 | 97.1 | 20 | 109.0 | 22 | 25 | 6.1 | |
| 2 | 16-OHP * | 330.46 | 331.2 | 97.0 | 22 | 109.0 | 24 | 20 | 2.86 |
| 11-Deoxycorticosterone * | 330.46 | 331.2 | 97.1 | 24 | 109.0 | 32 | 20 | 3.9 | |
| 21-Deoxycorticosterone * | 330.46 | 331.2 | 295.2 | 14 | 121.1 | 16 | 20 | 3.95 | |
| Corticosterone ** | 346.46 | 347.2 | 120.7 | 20 | 293.5 | 15 | 25 | 2.50 | |
| Unidentified steroid ** | 346.46 | 347.2 | 311.2 | 16 | 121.0 | 32 | 25 | 2.35 | |
| 3 | Cortisol [13C3] | 365.44 | 366.2 | 124.0 | 20 | 20 | 1.83 | ||
| 21-Deoxycortisol [D8] | 354.51 | 355.0 | 319.0 | 16 | 25 | 2.43 | |||
| 11-Deoxycortisol [2H5] | 351.49 | 352.2 | 100.0 | 25 | 20 | 2.83 | |||
| Androstenedione [13C3] | 289.39 | 290.2 | 100.0 | 20 | 20 | 3.43 | |||
| 17OHP [13C3] | 333.44 | 334.2 | 100.0 | 20 | 20 | 4.44 | |||
| Cortisone [13C3] | 363.42 | 364.2 | 166.1 | 22 | 20 | 1.57 | |||
| Testosterone [D3] | 291.44 | 292.2 | 97.1 | 20 | 30 | 4 | |||
| Progesterone [13C3] | 317.44 | 318.1 | 100.0 | 20 | 25 | 6.1 | |||
| Parameter | First Sample | Second Sample | Third Sample | Cut-Off |
|---|---|---|---|---|
| Patient’s age at sample collection (days) | 2 days (50 h of age) | 5 days | 6 days | |
| 17OHP–IA (nmol/L) | 52 | 122 | 125 | 1–20 |
| 17OHP–MS (nmol/L) | 16 | 68 | 100 | <20 |
| Androstenedione (Δ4A) (nmol/L) | 10 | 11 | 18 | <20 |
| 21-deoxycortisol (21DF) (nmol/L) | 10 | 7 | 8 | <1 |
| 11-deoxycortisol (S) (nmol/L) | 1 | 4 | 6.8 | <10 |
| Cortisol (F) (nmol/L) | 47 | 60 | 53 | 20–800 |
| Ratio (17OHP + Δ4A)/F | 0.6 | 1.3 | 2.2 | <1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Greaves, R.F.; Kumar, M.; Mawad, N.; Francescon, A.; Le, C.; O’Connell, M.; Chi, J.; Pitt, J. Best Practice for Identification of Classical 21-Hydroxylase Deficiency Should Include 21 Deoxycortisol Analysis with Appropriate Isomeric Steroid Separation. Int. J. Neonatal Screen. 2023, 9, 58. https://doi.org/10.3390/ijns9040058
Greaves RF, Kumar M, Mawad N, Francescon A, Le C, O’Connell M, Chi J, Pitt J. Best Practice for Identification of Classical 21-Hydroxylase Deficiency Should Include 21 Deoxycortisol Analysis with Appropriate Isomeric Steroid Separation. International Journal of Neonatal Screening. 2023; 9(4):58. https://doi.org/10.3390/ijns9040058
Chicago/Turabian StyleGreaves, Ronda F., Monish Kumar, Nazha Mawad, Alberto Francescon, Chris Le, Michele O’Connell, James Chi, and James Pitt. 2023. "Best Practice for Identification of Classical 21-Hydroxylase Deficiency Should Include 21 Deoxycortisol Analysis with Appropriate Isomeric Steroid Separation" International Journal of Neonatal Screening 9, no. 4: 58. https://doi.org/10.3390/ijns9040058






