Phase Change Materials—Applications and Systems Designs: A Literature Review
Abstract
:1. Introduction
- -
- PCMs applications with a focus on areas where PCMs entered recently as well as on areas where PCMs utilization has been long established but the literature reports are scarce. Among most recent PCMs applications reported in the literature the most important can be mentioned: temperature control in DCs, fresh e-commerce, thermal management of electric vehicles battery systems and thermal comfort in the vehicle cabins. Other important PCMs applications that have been long implemented but not extensively presented in the literature are aerospace applications. This review attempts to synthesize main applications in the aerospace sector;
- -
- PCM systems design: challenges to overcome, design strategies and solutions.
2. Phase Change Materials applications
2.1. Solar Energy Storage
2.2. Space Applications
- Lafdi et al. [13] carried out a numerical study to predict the thermal performance of graphite foams infiltrated with PCMs for terrestrial and space applications. For space applications, the average value of the output power of the new energy storage system has been increased by more than eight times. In the case of terrestrial applications, the average output power using carbon foam of porosity 97% was about five times greater than that for using pure PCM. Other studies discussed the appropriateness of various PCMs for the particular conditions of the space environment.
- Cui et al. [14] studied numerically and experimentally the eutectic mixture. It was found that the output temperature of the working fluid tubes met the expected demand during the sunlight and eclipse period and the maximum temperature.
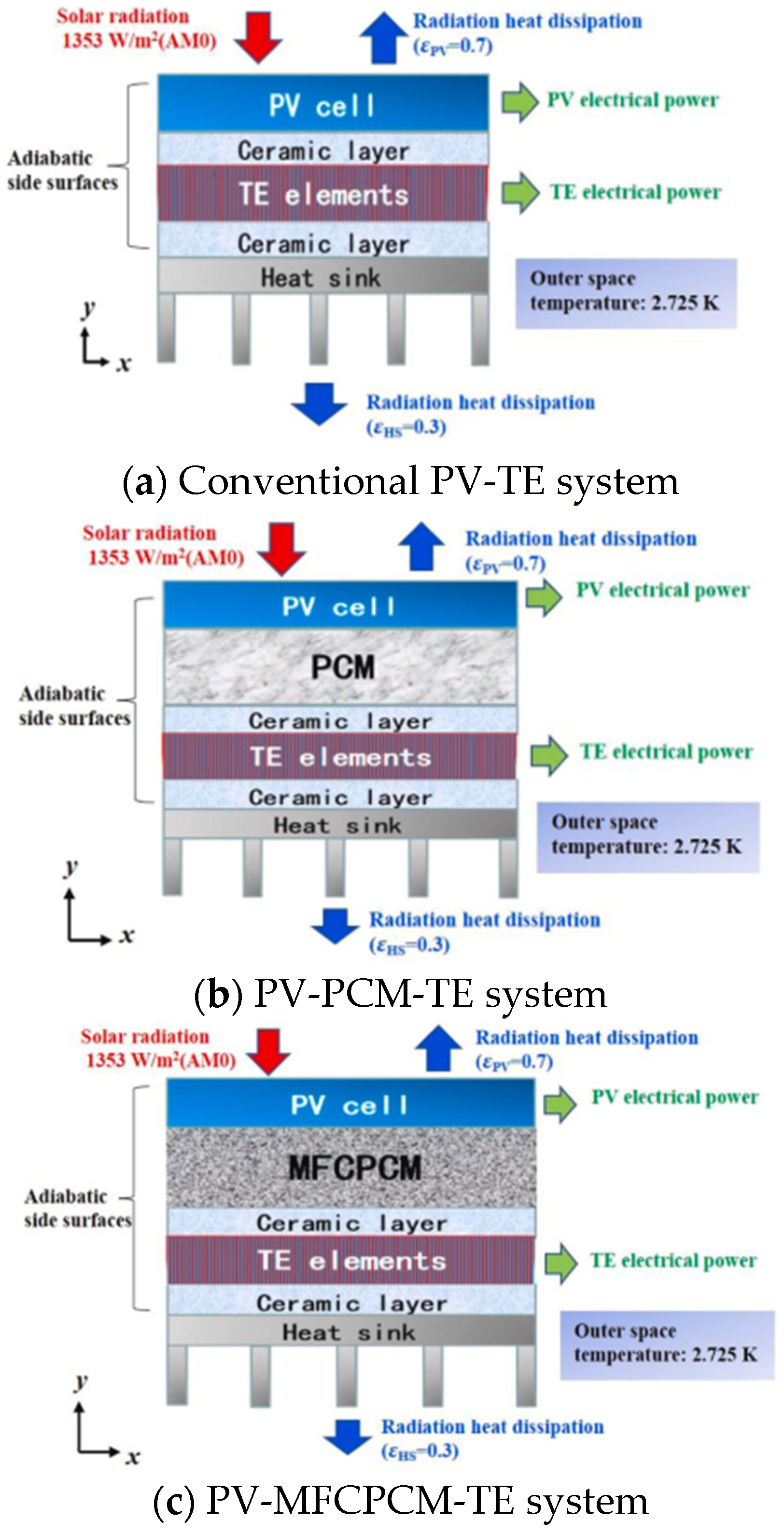
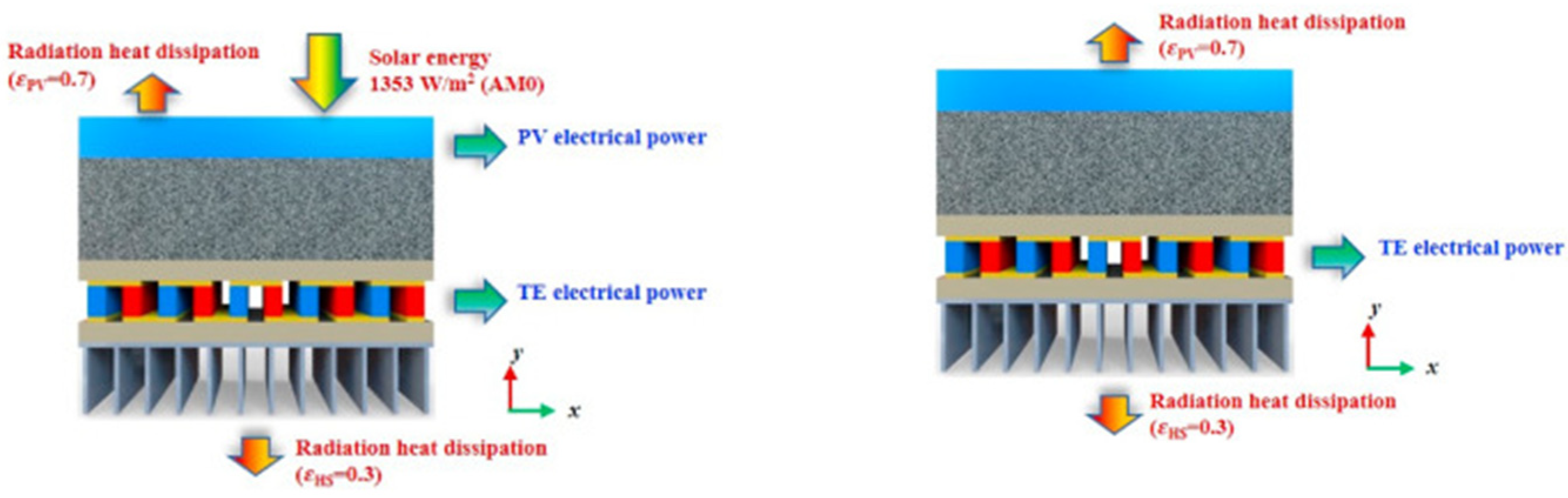
- He et al. [15] developed a two-dimensional model for PV-PCM-TE coupling system to investigate the energy transfer and conversion performance under space conditions. A suitable PCM was selected and it was integrated into the system both in pure form as well as a MFCPCM. A combined performance criterion was defined considering the power generation efficiency and the system mass, which is a critical evaluation index for space systems. The system design is presented in Figure 2b (PCM only) and Figure 2c (MFCPCM). The average total efficiencies for PV-TE, PV-PCM-TE and PV-MFCPCM-TE were 29.50%, 30.50% and 30.61%, and the corresponding average power density were 30.34 W/kg, 22.39 W/kg and 21.20 W/kg, respectively. The authors designed an orthogonal experiment in order to assess the effect of the PV-MFCPCM-TE on the power generation efficiency and power density. After running all cases of the orthogonal experiment, it was found that the mass of the ceramic layer and PCM layer had the highest weight of the PV-PCM-TE system total mass. It was concluded that reducing ceramic layer mass and using high melting enthalpy PCM was an efficient way to further reduce the mass of PV-PCM-TE coupling system and promote its application in space system.
2.3. Temperature Control of Electronics
2.4. Biomedical Applications
2.5. Thermal Storage in Building Applications
2.5.1. Passive Storage Systems
2.5.2. Active Storage Systems
2.6. Increasing the Storage Capacity of Hot/Cold Water Tanks
2.7. Two-Phase LHFTF
- Phase Change Slurries; the PCM is microencapsulation by means of chemical processes by creating a shell that contains the PCM. The microcapsules must not form agglomerations, deposits or fouling so the PCS maintains its homogeneity and can be pumped through tubes and ducts.
- Emulsion of fusible components, with the PCM dispersed in a carrier fluid and kept in suspension by a surfactant agent
- Ice slurries
2.8. Textiles with Thermoregulation Features
2.9. Automotive Applications
2.9.1. Pre-Heating Catalytic Convertors
2.9.2. Engine Cooling/Pre-Heating
2.9.3. Fuel/Air Preheating
2.9.4. Thermal Comfort in the Cabin
2.9.5. Electric Vehicles
2.10. Food Processing/Agriculture
2.11. Waste Heat Recovery
2.11.1. Data Centres
2.11.2. Adiabatic Compression of Gases
2.11.3. Greenhouses
3. PCM Systems Design Considerations
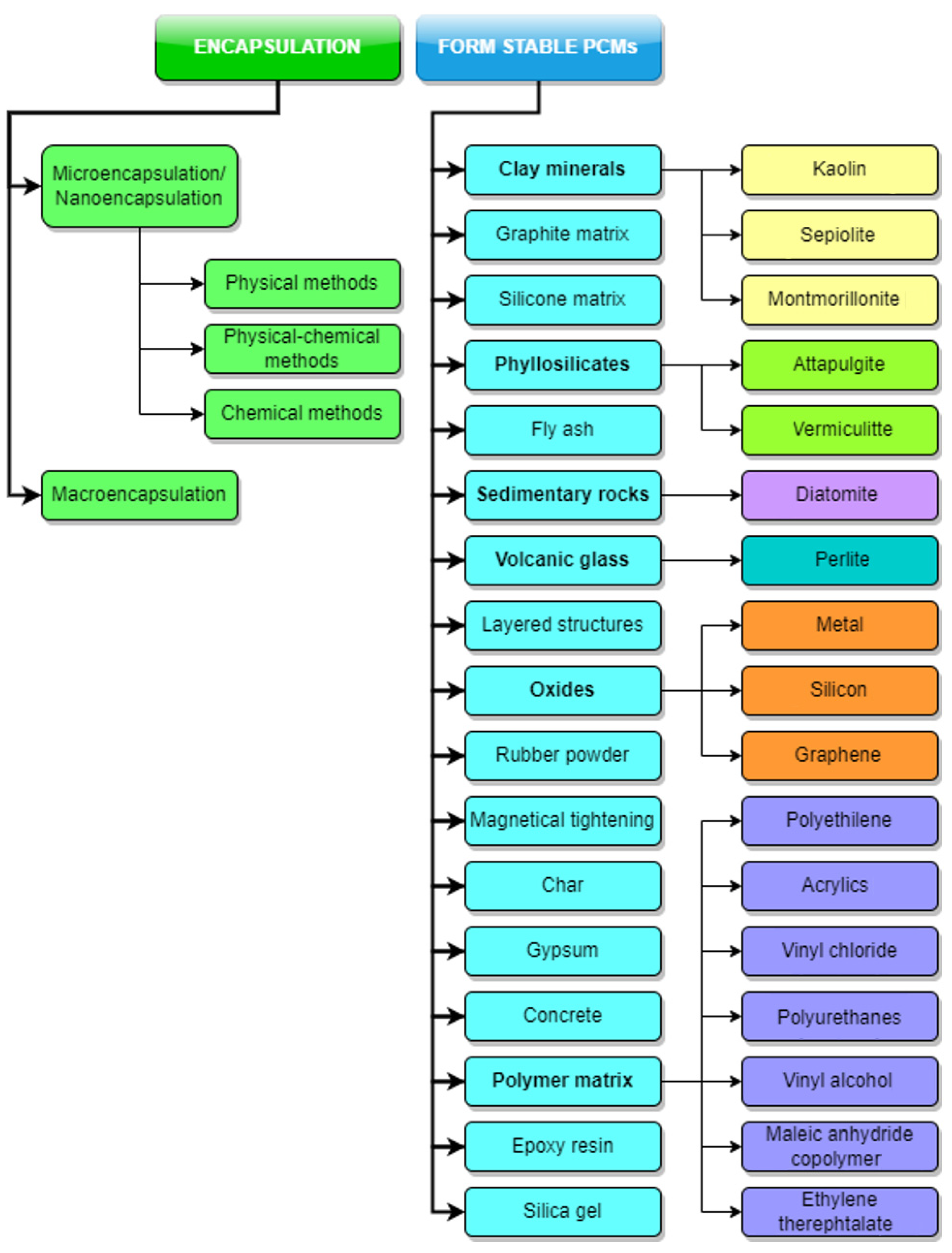
3.1. PCM Encapsulation
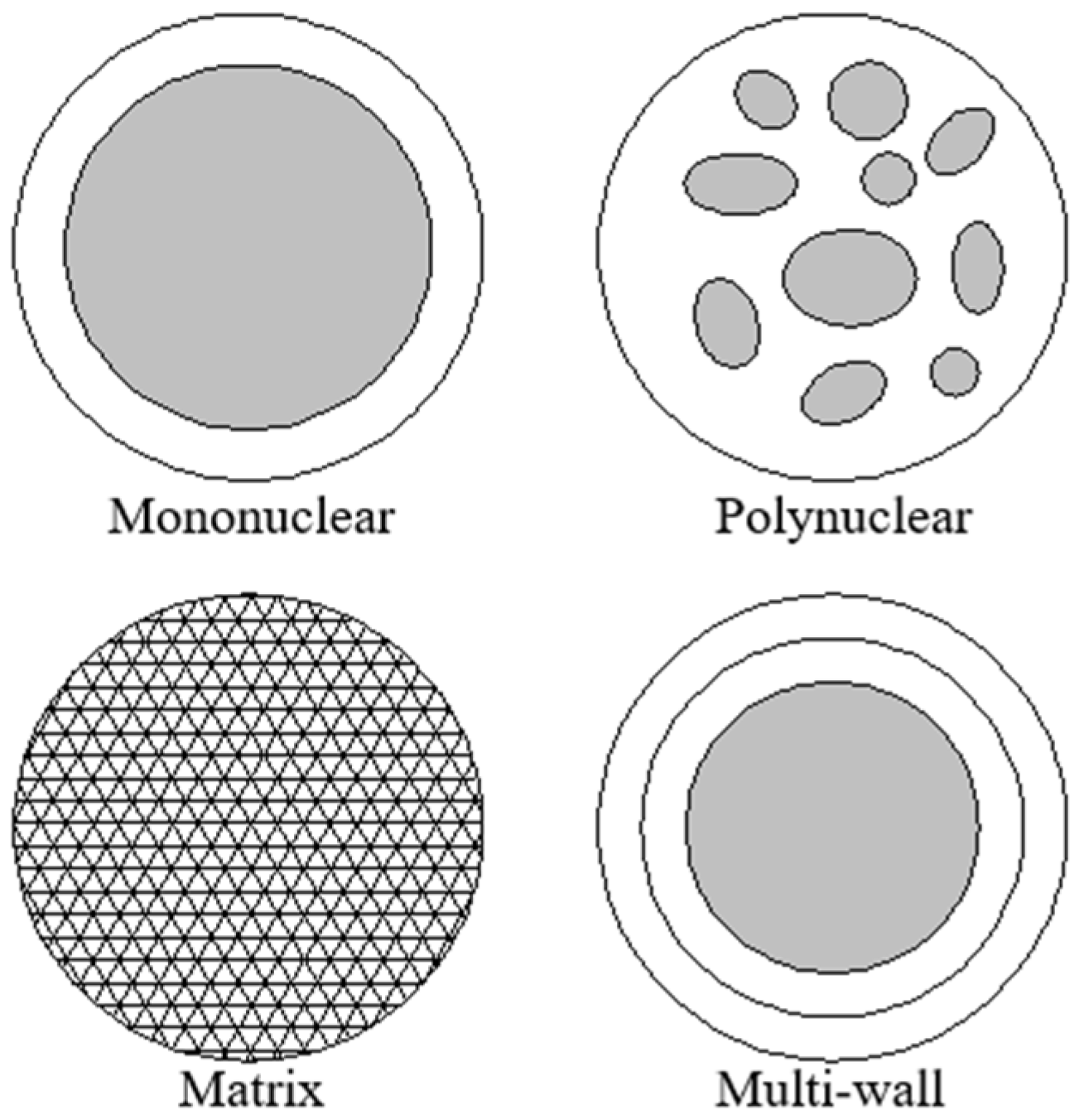
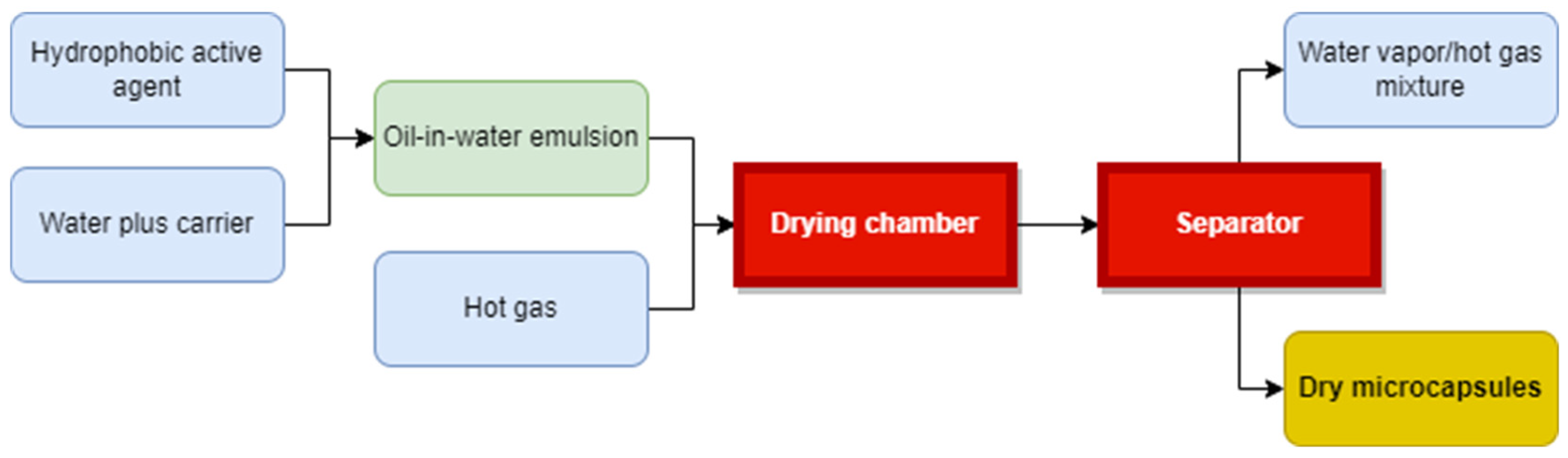
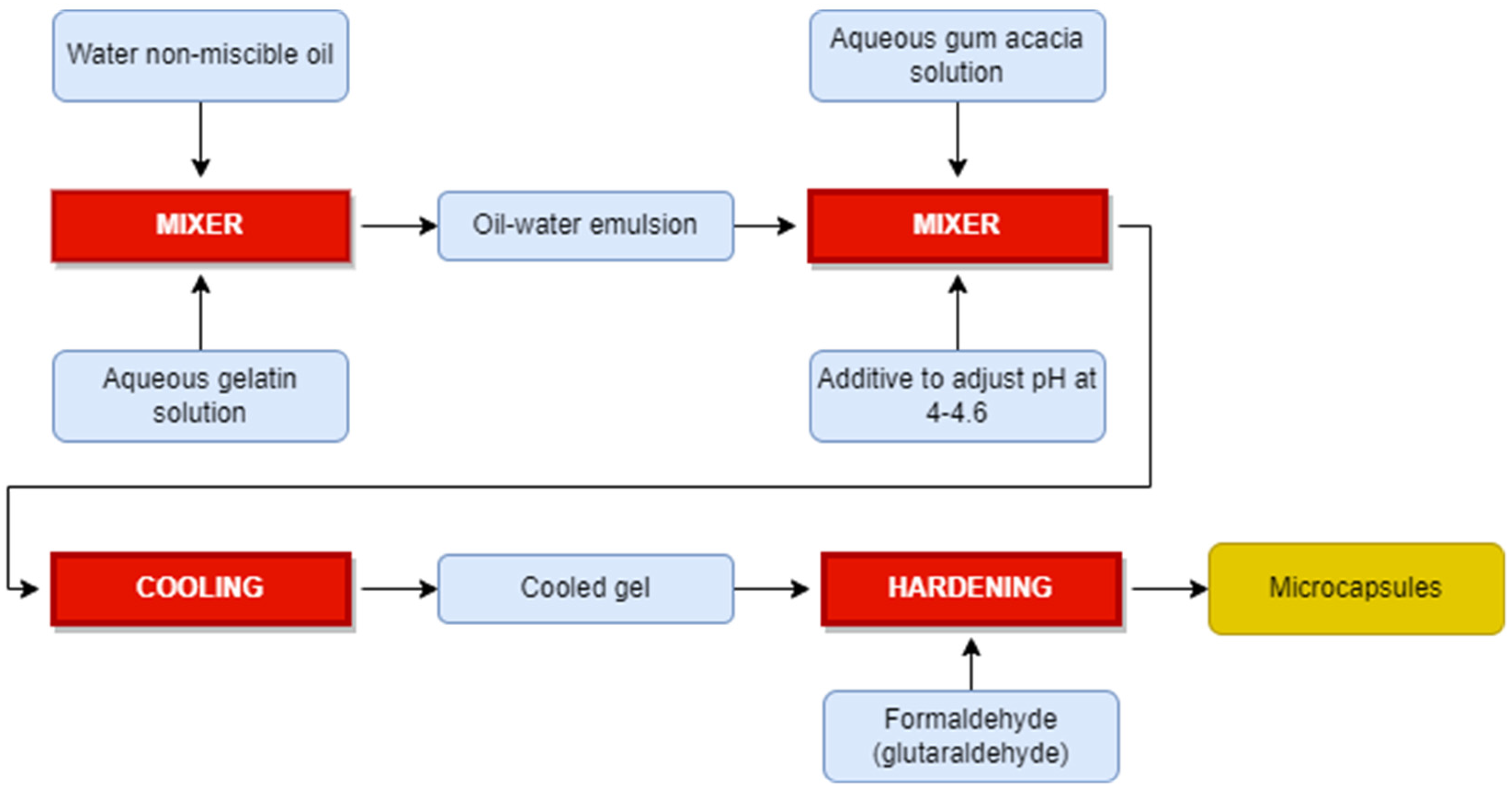
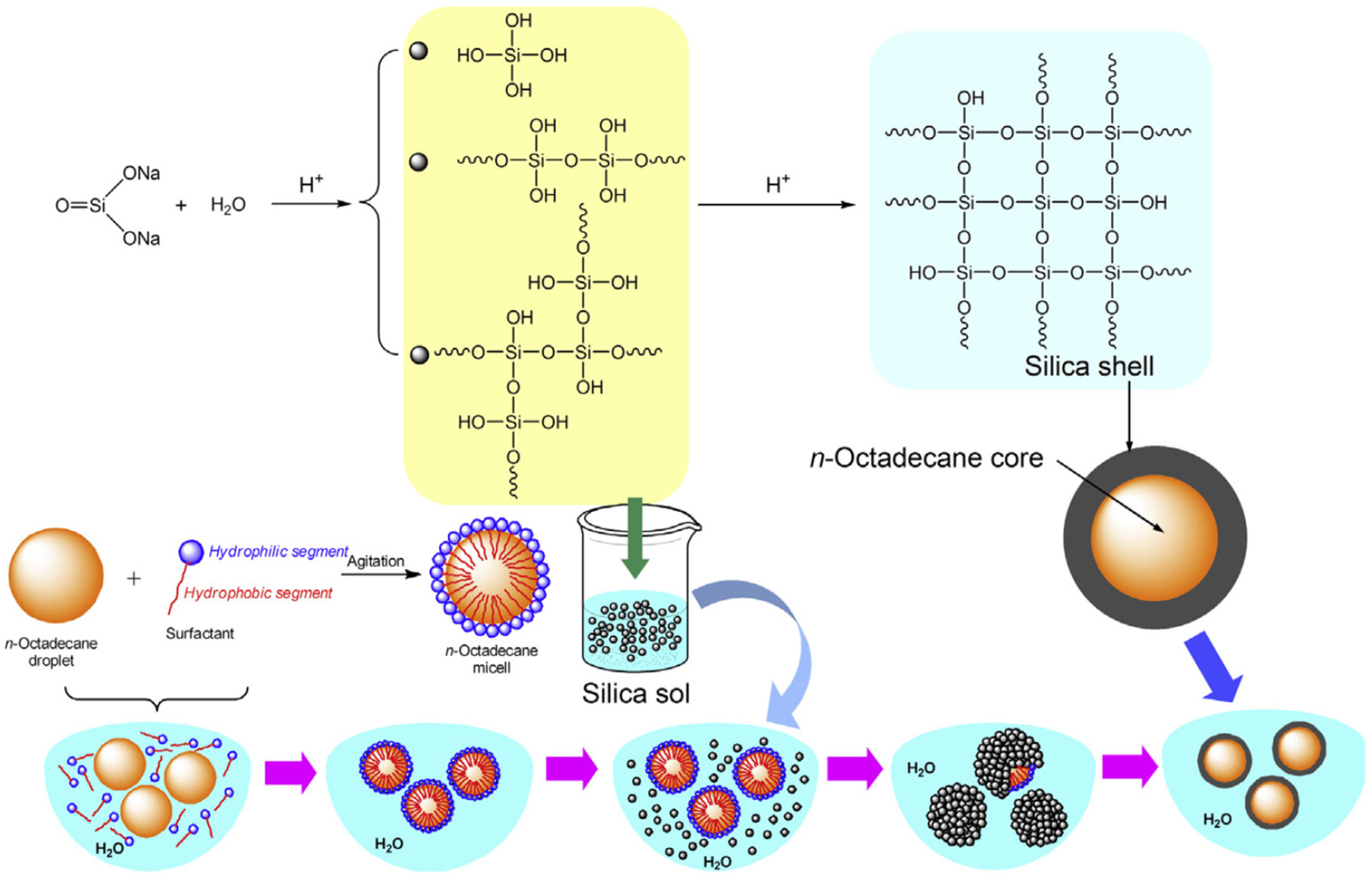
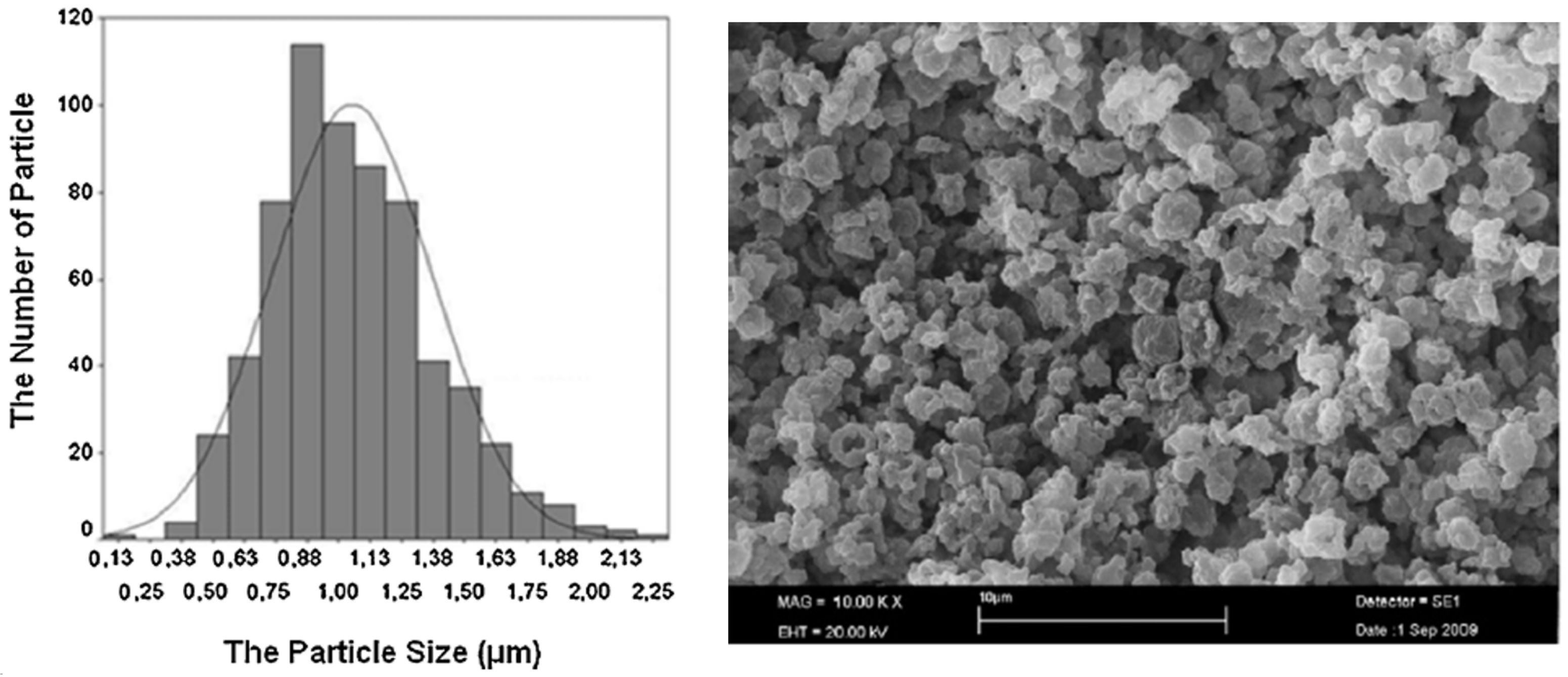
3.1.1. Microencapsulation
- -
- Preparation of a sol solution; this is usually achieved through hydrolysis of one of the precursor compounds like Tetraethoxysilane, Tetraethylorthosilicate, sodium silicate or methyl triethoxysilane. The sol solution is maintained at pH values between 2 and 3 in order to create conditions for hydrolysis reaction to occur.
- -
- The silicate sol solution is added to a PCM O/W emulsion drop by drop while emulsion is kept stirring to prevent formation of any continuous gel and allowing the formation of only discrete silica gel walls around PCM droplets.
- -
- The silica shell will be formed on the surface of the PCM droplets by the condensation polymerization of the silica solid particles. High pH (9–10) condition is maintained in the emulsion to promote condensation reaction.
Emulsion Polymerization
Interfacial Polymerization
In-Situ Polymerization
Microencapsulated Phase Change Slurries
3.1.2. Macro-Encapsulation
- Volumetric expansion/contraction of the PCM occurring during the melting and solidification, respectively
- The pressure build-up occurring in the macro-capsule if no proper expansion mean is provided
- Chemical compatibility between the PCM and the capsule material
3.2. Form-Stable PCMs
- A.
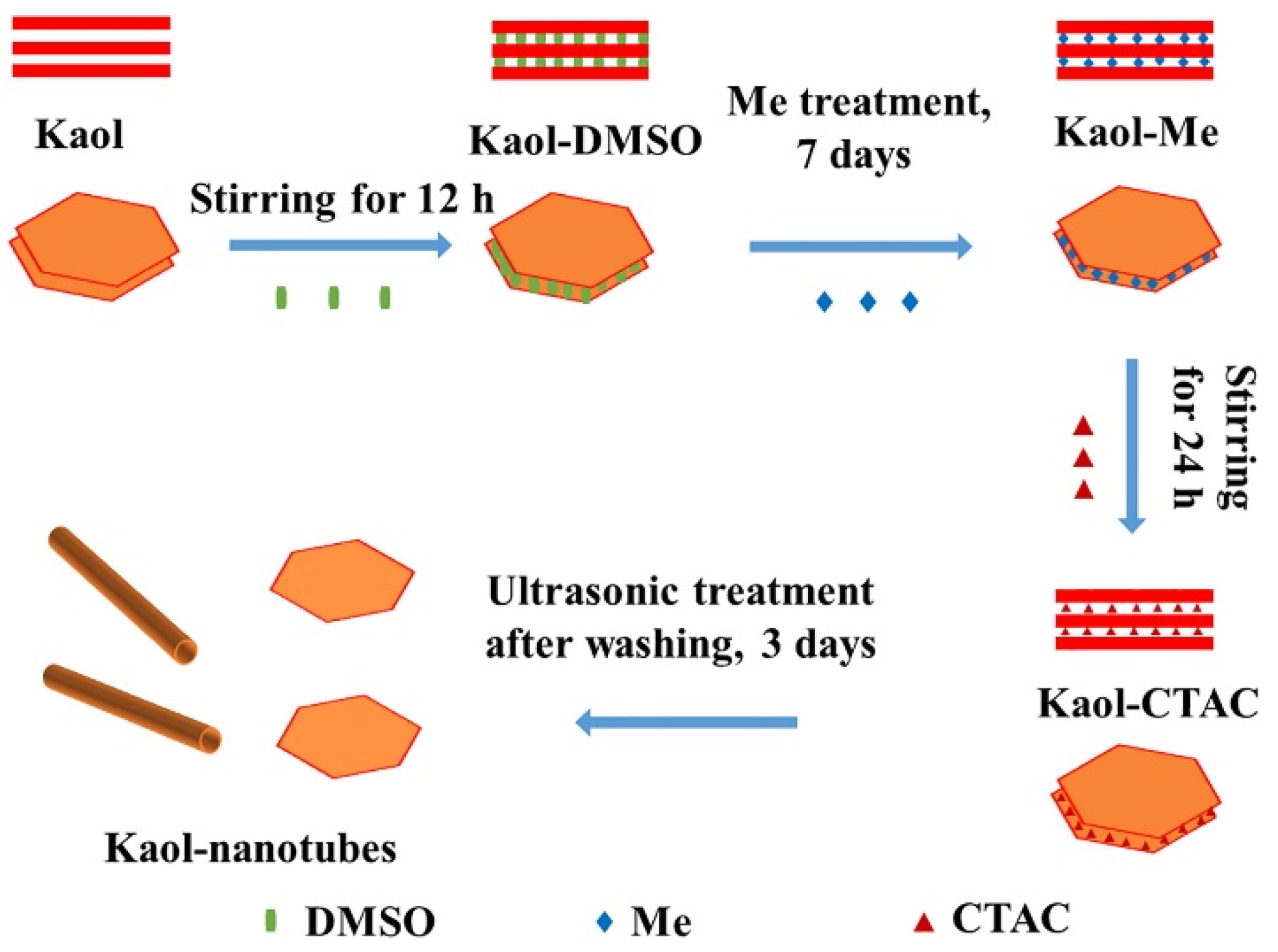
- Layered-packaging/Tubular packaging. Zhang et al. [82] designed a process represented schematically in Figure 18 to prepare (Kaol)–cetyl trimethylammonium chloride intercalation compound (Kaol-nanotubes) through the intercalation method and ultrasonic treatment. This material was used to prepare a SA/Kaol-nanotube composite form-stable phase change material (FSPCM) using a vacuum impregnation method. It was found that after the modification, the morphologies of some of the lamellar structures in the Kaol changed from nanoplates to nanotubes. Hence, the melted stearic acid easily adhered to the surfaces of the Kaol-nanotubes by physical effects, which included surface tension, hydrogen bonding, and the capillary forces during the phase transition.
- B.
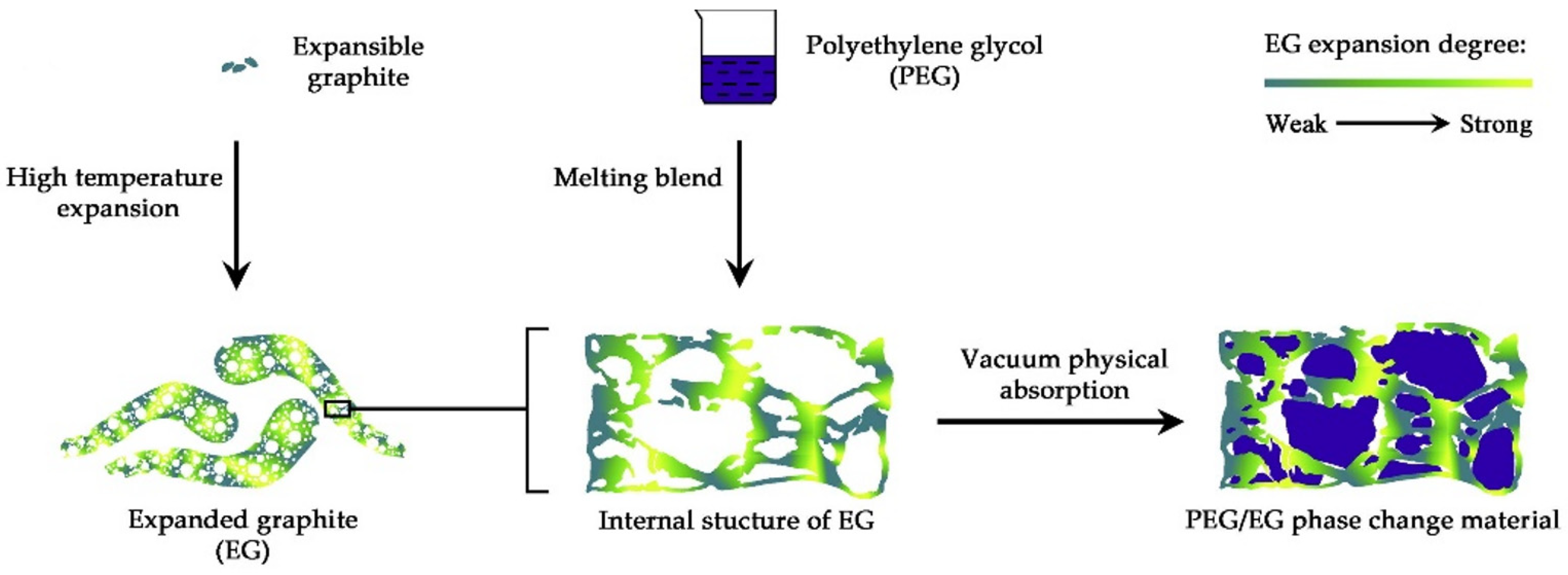
- Porous packaging. Yang et al. [150] prepared a form-stable PCM through high-temperature expansion at 800 °C for 60 s, the over-dried flake graphite was developed into expanded graphite and cooled down naturally in a desiccator. PEG was configured as 20% anhydrous ethanol solution which was blended with EG by spray at mass ratios of 6:1, 7:1, 8:1, 9:1, 10:1 and 11:1. The samples were subject to a thermal treatment in a vacuum oven at 85 °C for 12 h under pressure of 0.1 MPa. After the vacuum adsorption process, the PEG/EG PCMs were prepared. The process stages are shown in Figure 19.
- C.
- Core-shell packaging. Yi et al. [151] used MMT nanosheets (2D-MMT) to encapsulating SA latex particles resulting in composite PCMs with very thin shell and high content of core material prepared through the self-assembly of positively charged MMT nanosheets on the negatively SA latex surfaces under a strong electrostatic attraction, leading to the formation of a core–shell structural composite PCM. The microstructure, thermal performances and cycling stability of this 2D-MMT/SA composites have been investigated through FTIR spectroscopy, SEM, DSC, zeta potential measurement and thermal constant analyser. The experimental results have shown that 2D-MMT/SA composite contained high mass fraction (>80%) of SA due to the very thin MMT nanosheets shell. This feature resulted in a high latent heat storage capacity of approximately 185 J/g). In addition, the thermal conductivity of this PCM composite can reach to 159.46% of SA at most on account of the relatively high thermal conductivity of MMT nanosheets.
- D.
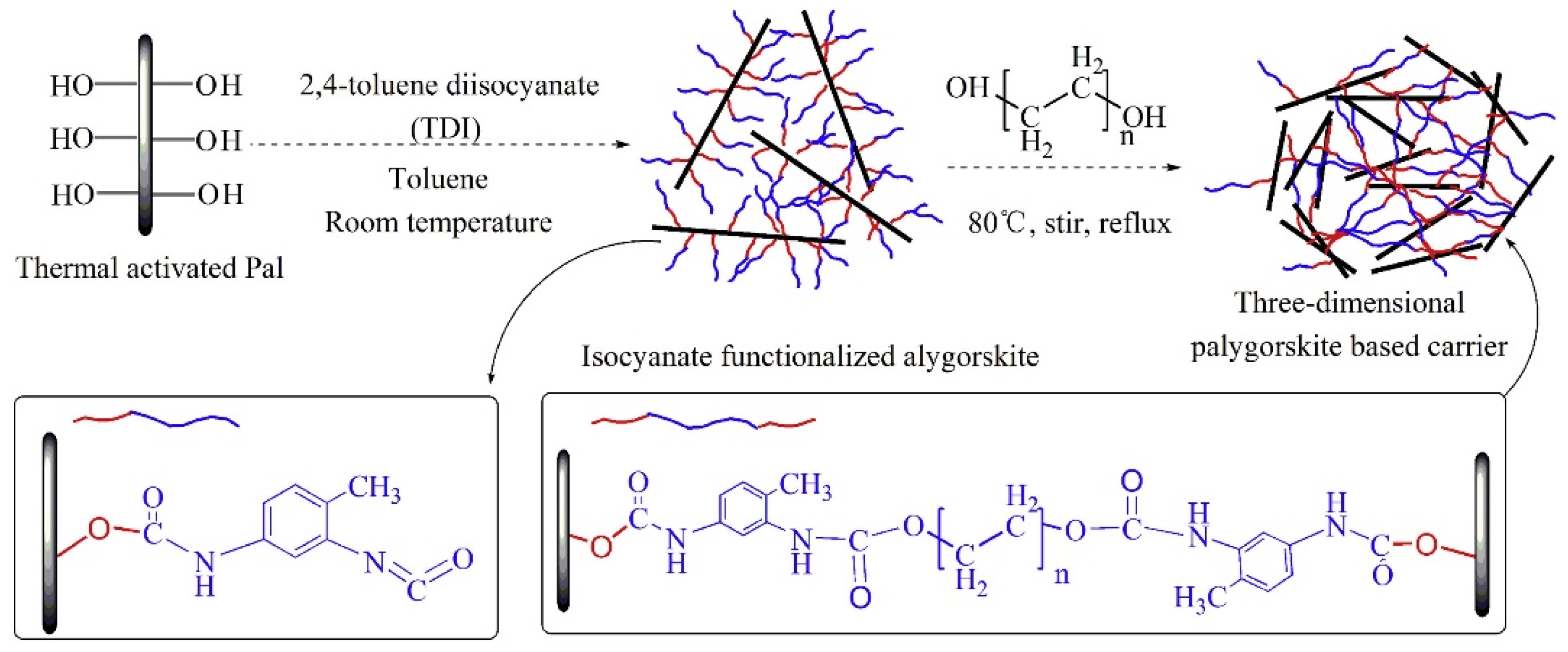
- Network packaging. Wang et al. [152] fabricated three-dimensional palygorskite carriers (TDI-HO-Pal, “HO” stands for different diol molecules) with different pore size by the reaction of isocyanate functionalized palygorskite with dihydric alcohol through a process presented in Figure 20. It was found that by coupling the mineral with isocyanates, it could be grafted by substances containing active hydrogen atoms, or cross-bonded with dihydric alcohols or binary amines. The phase change temperature and phase transition enthalpy of SA/TDI-HO-Pal showed a size dependent property. The melting temperature and fusion latent heat increased with the enlargement of the support pore size. The thermal storage properties of SA/TDI-HO-Pal were significantly higher than that of SA/Pal, increased by 67.4% to 100.7% within the experimental range.
4. Conclusions and Future Research
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Nomenclature
| ASHW | Active Solar Heating Wall |
| CA | Capric Acid |
| CCPCM | Cement-based Composite Phase Change Material |
| CFD | Computational Fluid Dynamics |
| CNF | Chitin Nanofibers |
| CTES | Cold Thermal Energy Storage |
| DC | Data Center |
| DHW | Domestic Hot Water |
| DSC | Differential Scanning Calorimetry |
| FTIR | Fourier Transform Infrared Spectroscopy |
| FR | Flame Retardant |
| GSHP | Ground Source Heat Pump |
| GW | Gypsum Wallboard |
| HVAC | Heat, Ventilation and Air Conditioning |
| LA | Lauric Acid |
| LCPM | Lifecycle Particulate Matter |
| LHS | Latent Heat Storage |
| LHFTF | Latent Heat Functional Thermal Fluids |
| MA | Myristic Acid |
| MDI | Methylene Diisocyanate |
| MEP | Macro-encapsulated PCM |
| MEPCM | Microencapsulated Phase Change Material |
| MFCPCM | Photovoltaic-Metal Foam/Paraffin Composite PCM |
| MMT | Montmorillonite |
| OSS | Organic Silicon Shell |
| O/W | Oil-Water |
| PCM | Phase Change Material |
| PCMSW | Phase Change Material Energy Storage Wallboard |
| PCS | Phase Change Slurry |
| PEG | Polyethylene Glycol |
| PMMA | Poly(methyl-methacrylate) |
| PSD | Particle Size Distribution |
| PV | Photovoltaic |
| SA | Stearic Acid |
| SEM | Scanning Electronic Microscopy |
| SSPCM | Shape-Stabilized Phase Change Materials |
| TE | Thermoelectric |
| TEM | Transmission Electronic Microscope |
| TES | Thermal Energy Storage |
| TESC | Thermal Energy Storage Concrete |
| TGA | Thermogravimetric Analysis |
| VRC | Variable Resistance–Capacitance |
References
- Geisz, J.F.; France, R.M.; Schulte, K.L.; Steiner, M.A.; Norman, A.G.; Guthrey, H.L.; Young, M.R.; Song, T.; Moriarty, T. Six-junction III–V solar cells with 47.1% conversion efficiency under 143 Suns concentration. Nat. Energy 2020, 5, 326–335. [Google Scholar] [CrossRef]
- Mishnaevsky, L., Jr.; Branner, K.; Petersen, H.N.; Beauson, J.; McGugan, M.; Sørensen, B.F. Materials for Wind Turbine Blades: An Overview. Materials 2017, 10, 1285. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kalaiselvam, S.; Parameshwaran, R. Thermal Energy Storage Technologies for Sustainability. In Systems Design, Assessment and Applications; Academic Press: Cambridge, MA, USA, 2014; ISBN 978-0-12-417291-3. [Google Scholar]
- Lawag, R.A.; Ali, H.M. Phase change materials for thermal management and energy storage: A review. J. Energy Storage 2022, 55, 105602. [Google Scholar] [CrossRef]
- Safari, A.; Saidur, R.; Sulaiman, F.; Xu, Y.; Dong, J. A review on supercooling of Phase Change Materials in thermal energy storage systems. Renew. Sustain. Energy Rev. 2017, 70, 905–919. [Google Scholar] [CrossRef]
- Matuszeka, K.; Hattona, C.; Mega Karb, J.M.; Pringleb, D.; MacFarlane, R. Molecular patterns in the thermophysical properties of pyridinium ionic liquids as phase change materials for energy storage in the intermediate temperature range. J. Non-Crystalline Solids X 2022, 15, 100108. [Google Scholar] [CrossRef]
- Dinter, F.; Geyer, M.; Tamme, R. Thermal Energy Storage for Commercial Application (TESCA), a Feasibility Study on Economic Storage Systems; Springer: Berlin, Germany, 1991. [Google Scholar]
- Michels, H.; Pitz-Paal, R. Cascaded latent heat storage for parabolic trough solar power plants. Sol. Energy 2007, 81, 829–837. [Google Scholar] [CrossRef]
- Pielichowska, K.; Pielichowski, K. Phase change materials for thermal energy storage. Prog. Mater. Sci. 2014, 65, 67–123. [Google Scholar] [CrossRef]
- Chidambaram, L.; Ramana, A.; Kamaraj, G.; Velraj, R. Review of solar cooling methods and thermal storage options. Renew. Sustain. Energy Rev. 2011, 15, 3220–3228. [Google Scholar] [CrossRef]
- He, Y.; Tao, Y.; Ye, H. Periodic energy transmission and regulation of photovoltaic-phase change material-thermoelectric coupled system under space conditions. Energy 2022, 263, 125916. [Google Scholar] [CrossRef]
- Yimer, B.; Adami, M. Parametric study of phase change thermal energy storage systems for space application. Energy Convers. Manag. 1997, 38, 253–262. [Google Scholar] [CrossRef]
- Lafdi, K.; Mesalhy, O.; Elgafy, A. Graphite foams infiltrated with phase change materials as alternative materials for space and terrestrial thermal energy storage applications. Carbon 2008, 46, 159–168. [Google Scholar] [CrossRef]
- Cui, H.; Xing, Y.; Guo, Y.; Wang, Z.; Cui, H.; Yuan, X. Numerical simulation and experiment investigation on unit heat exchange tube for solar heat receiver. Sol. Energy 2008, 82, 1229–1234. [Google Scholar] [CrossRef]
- He, Y.; Tao, Y.; Zhao, C.; Yu, X. Structure parameter analysis and optimization of photovoltaic-phase change material-thermoelectric coupling system under space conditions. Renew. Energy 2022, 200, 320–333. [Google Scholar] [CrossRef]
- Krishnan, S.; Garimella, S.; Kang, S. A novel hybrid heat sink using phase change materials for transient thermal management of electronics. IEEE Trans. Compon. Packag. Technol. 2005, 28, 281–289. [Google Scholar] [CrossRef] [Green Version]
- Akhilesh, R.; Narasimhan, A.; Balaji, C. Method to improve geometry for heat transfer enhancement in PCM composite heat sinks. Int. J. Heat Mass Transfer 2005, 48, 2759–2770. [Google Scholar] [CrossRef]
- Qasim, M.A.; Ali, H.M.; Khan, M.N.; Arshad, N.; Khaliq, D.; Ali, Z.; Janjua, M.M. The effect of using hybrid phase change materials on thermal management of photovoltaic panels—An experimental study. Sol. Energy 2020, 209, 415–423. [Google Scholar] [CrossRef]
- Zhang, X. Heat-storage and thermo-regulated textiles and clothing. In Smart Fibers, Fabrics and Clothing; Woodhead Publishing Ltd.: Cambridge, UK; CRC Press LLC: Boca Raton, FL, USA, 2001. [Google Scholar]
- Mondieig, D.; Rajabalee, F.; Laprie, A.; Oonk, H.A.J.; Calvet, T.; Cuevas-Diarte, M.A. Protection of temperature sensitive biomedical products using molecular alloys as phase change material. Transfus. Apher. Sci. 2003, 28, 143–148. [Google Scholar] [CrossRef] [Green Version]
- De Santis, R.; Ambrogi, V.; Carfagna, C.; Ambrosio, L.; Nicolais, L. Effect of microencapsulated phase change materials on the thermo-mechanical properties of poly(methyl-methacrylate) based biomaterials. J. Mater. Sci. Mater. Med. 2006, 17, 1219–1226. [Google Scholar] [CrossRef]
- Pielichowska, K.; Błazewicz, S. Inventors Acrylic Bone Cement/Akrylanowy Cement Kostny. PL Patent 406713 A1, 6 July 2015. [Google Scholar]
- Lv, Y.; Zou, Y.; Yang, L. Feasibility study for thermal protection by microencapsulated phase change micro/nanoparticles during cryosurgery. Chem. Eng. Sci. 2011, 66, 3941–3953. [Google Scholar] [CrossRef]
- Feldman, D.; Banu, D. DSC analysis for the evaluation of an energy storing wallboard. Thermochim. Acta 1996, 272, 243–251. [Google Scholar] [CrossRef]
- Zhang, D.; Li, Z.; Zhou, J.; Wu, K. Development of thermal energy storage concrete. Cem. Concr. Res. 2004, 34, 927–934. [Google Scholar] [CrossRef]
- Weinlader, H.; Beck, A.; Fricke, J. PCM-facade-panel for daylighting and room heating. Sol. Energy 2005, 78, 177–186. [Google Scholar] [CrossRef]
- Darkwa, K.; O’Callaghan, P.W. Simulation of phase change drywalls in a passive solar building. Appl. Therm. Eng. 2006, 26, 853–858. [Google Scholar] [CrossRef]
- Shilei, L.; Neng, Z.; Guohui, F. Eutectic mixtures of capric acid and lauric acid applied in building wallboards for heat energy storage. Energy Build. 2006, 38, 708–711. [Google Scholar] [CrossRef]
- Sari, A.; Karaipekli, A.; Kaygusuz, K. Capric Acid and Myristic Acid for Latent Heat Thermal Energy Storage. Energy Sources Part A 2008, 30, 1498–1507. [Google Scholar] [CrossRef]
- Diaconu, B.M.; Cruceru, M. Novel concept of composite phase change material wall system for year-round thermal energy savings. Energy Build. 2010, 42, 1759–1772. [Google Scholar] [CrossRef]
- Diaconu, B.M. Thermal energy savings in buildings with PCM-enhanced envelope: Influence of occupancy pattern and ventilation. Energy Build. 2011, 43, 101–107. [Google Scholar] [CrossRef]
- Entrop, A.G.; Brouwers, H.J.H.; Reinders, A. Experimental research on the use of micro-encapsulated Phase Change Materials to store solar energy in concrete floors and to save energy in Dutch houses. Sol. Energy 2011, 85, 1007–1020. [Google Scholar] [CrossRef]
- Wijesena, R.N.; Tissera, N.D.; Rathnayaka, V.; Rajapakse, H.; de Silva, R.M.; de Silva, K.N. Shape-stabilization of polyethylene glycol phase change materials with chitin nanofibers for applications in “smart” windows. Carbohydr. Polym. 2020, 237, 116132. [Google Scholar] [CrossRef]
- Du, Y.; Liu, P.; Quan, X.; Ma, C. Characterization and cooling effect of a novel cement-based composite phase change material. Sol. Energy 2020, 208, 573–582. [Google Scholar] [CrossRef]
- Jeon, J.; Park, J.H.; Wi, S.; Yang, S.W.; Ok, Y.S.; Kim, S. Latent heat storage biocomposites of phase change material-biochar as feasible eco-friendly building materials. Environ. Res. 2019, 172, 637–648. [Google Scholar] [CrossRef] [PubMed]
- Leang, E.; Tittelein, P.; Zalewski, L.; Lassue, S. Impact of a Composite Trombe Wall Incorporating Phase Change Materials on the Thermal Behavior of an Individual House with Low Energy Consumption. Energies 2020, 13, 4872. [Google Scholar] [CrossRef]
- Bogatu, D.-I.; Kazanci, O.B.; Olesen, B.W. An experimental study of the active cooling performance of a novel radiant ceiling panel containing phase change material (PCM). Energy Build. 2021, 243, 110981. [Google Scholar] [CrossRef]
- Li, H.; Li, J.; Kong, X.; Long, H.; Yang, H.; Yao, C. A novel solar thermal system combining with active phase-change material heat storage wall (STS-APHSW): Dynamic model, validation and thermal performance. Energy 2020, 201, 117610. [Google Scholar] [CrossRef]
- Ouhsaine, L.; Ramenah, H.; El Ganaoui, M.; Mimet, A. Dynamic state-space model and performance analysis for solar active walls embedded phase change material. Sustain. Energy Grids Netw. 2020, 24, 100401. [Google Scholar] [CrossRef]
- Alam, M.; Zou, P.X.; Sanjayan, J.; Ramakrishnan, S. Energy saving performance assessment and lessons learned from the operation of an active phase change materials system in a multi-storey building in Melbourne. Appl. Energy 2019, 238, 1582–1595. [Google Scholar] [CrossRef]
- Qiao, X.; Kong, X.; Li, H.; Wang, L.; Long, H. Performance and optimization of a novel active solar heating wall coupled with phase change material. J. Clean. Prod. 2020, 250, 119470. [Google Scholar] [CrossRef]
- Lin, K.; Zhang, Y.; Xu, X.; Di, H.; Yang, R.; Qin, P. Experimental study of under-floor electric heating system with shape-stabilized PCM plates. Energy Build. 2005, 37, 215–220. [Google Scholar] [CrossRef]
- Wan, H.; Xu, X.; Xu, T.; Yuen, K.K.R.; Huang, G. Development of a quasi-2D variable resistance–capacitance model for tube-encapsulated phase change material storage tanks. Appl. Therm. Eng. 2022, 214, 118868. [Google Scholar] [CrossRef]
- Jahangir, M.H.; Labbafi, S. Optimization of ground source heat pump system along with energy storage tank armed with phase change materials to improve the microalgae open culture system performance. J. Energy Storage 2022, 51, 104436. [Google Scholar] [CrossRef]
- Liang, H.; Liu, L.; Zhong, Z.; Gan, Y.; Wu, J.-Y.; Niu, J. Towards idealized thermal stratification in a novel phase change emulsion storage tank. Appl. Energy 2022, 310, 118526. [Google Scholar] [CrossRef]
- Wu, F.; Wang, Z.; Zhang, H.; Qin, Y.; You, X.; Lu, J. Experimental and simulation analysis on thermal stratification characteristics in solar storage tanks with phase change materials. J. Energy Storage 2022, 46, 103722. [Google Scholar] [CrossRef]
- Diaconu, B.M.; Varga, S.; Oliveira, A.C. Experimental assessment of heat storage properties and heat transfer characteristics of a phase change material slurry for air conditioning applications. Appl. Energy 2010, 87, 620–628. [Google Scholar] [CrossRef]
- Diaconu, B.M.; Varga, S.; Oliveira, A.C. Experimental study of natural convection heat transfer in a microencapsulated phase change material slurry. Energy 2010, 35, 2688–2693. [Google Scholar] [CrossRef]
- Ikutegbe, C.A.; Al-Shannaq, R.; Farid, M.M. Microencapsulation of low melting phase change materials for cold storage applications. Appl. Energy 2022, 321, 119347. [Google Scholar] [CrossRef]
- Guo, Y.; Gao, Y.; Han, N.; Zhang, X.; Li, W. Facile microencapsulation of phase change material with organic silicon shell used for energy storage. Sol. Energy Mater. Sol. Cells 2022, 240, 111718. [Google Scholar] [CrossRef]
- Chen, H.; Zhao, R.; Wang, C.; Feng, L.; Li, S.; Gong, Y. Preparation and Characterization of Microencapsulated Phase Change Materials for Solar Heat Collection. Energies 2022, 15, 5354. [Google Scholar] [CrossRef]
- Al-Shannaq, R.; Farid, M.M.; Ikutegbe, C.A. Methods for the Synthesis of Phase Change Material Microcapsules with Enhanced Thermophysical Properties—A State-of-the-Art Review. Micro 2022, 2, 426–474. [Google Scholar] [CrossRef]
- Mondal, S. Phase change materials for smart textiles—An overview. Appl. Therm. Eng. 2008, 28, 1536–1550. [Google Scholar] [CrossRef]
- Lu, Y.; Xiao, X.; Fu, J.; Huan, C.; Qi, S.; Zhan, Y.; Zhu, Y.; Xu, G. Novel smart textile with phase change materials encapsulated core-sheath structure fabricated by coaxial electrospinning. Chem. Eng. J. 2019, 355, 532–539. [Google Scholar] [CrossRef]
- Gao, J.; Tian, G.; Sorniotti, A.; Karci, A.E.; Di Palo, R. Review of thermal management of catalytic converters to decrease engine emissions during cold start and warm up. Appl. Therm. Eng. 2019, 147, 177–187. [Google Scholar] [CrossRef]
- Ugurlu, A.; Gokcol, C. A review on thermal energy storage systems with phase change materials in vehicles. Electron. J. Vocat. Coll. 2012, 2, 1–14. [Google Scholar]
- Korin, E.; Reshef, R.; Tshernichovesky, D.; Sher, E. Reducing cold-start emission from internal combustion engines by means of a catalytic converter embedded in a phase-change material. Proc. Inst. Mech. Eng. 1999, 213, 575–583. [Google Scholar] [CrossRef]
- Kim, K.-B.; Choi, K.-W.; Kim, Y.-J.; Lee, K.-H.; Lee, K.-S. Feasibility study on a novel cooling technique using a phase change material in an automotive engine. Energy 2010, 35, 478–484. [Google Scholar] [CrossRef]
- Park, S.; Woo, S.; Shon, J.; Lee, K. Experimental study on heat storage system using phase-change material in a diesel engine. Energy 2017, 119, 1108–1118. [Google Scholar] [CrossRef]
- Gumus, M. Reducing cold-start emission from internal combustion engines by means of thermal energy storage system. Appl. Therm. Eng. 2009, 29, 652–660. [Google Scholar] [CrossRef]
- Soliman, A.S.; Radwan, A.; Xu, L.; Dong, J.; Cheng, P. Energy harvesting in diesel engines to avoid cold start-up using phase change materials. Case Stud. Therm. Eng. 2022, 31, 101807. [Google Scholar] [CrossRef]
- Soliman, A.S.; Zhu, S.; Xu, L.; Dong, J.; Cheng, P. Efficient waste heat recovery system for diesel engines using nano-enhanced phase change materials. Case Stud. Therm. Eng. 2021, 28, 101390. [Google Scholar] [CrossRef]
- Krishnamoorthi, S.; Prabhu, L.; Kuriakose, G.; Lewis, D.J.; Harikrishnan, J. Air-cooling system based on phase change materials for a vehicle cabin. Mater. Today Proc. 2020, 45, 5991–5996. [Google Scholar] [CrossRef]
- Afzal, A.; Saleel, C.A.; Badruddin, I.A.; Khan, T.M.Y.; Kamangar, S.; Mallick, Z.; Samuel, O.D.; Soudagar, M.E.M. Human thermal comfort in passenger vehicles using an organic phase change material—An experimental investigation, neural network modelling, and optimization. Build. Environ. 2020, 180, 107012. [Google Scholar] [CrossRef]
- Youssef, R.; Hosen, S.; He, J.; Al-Saadi, M.; Van Mierlo, J.; Berecibar, M. Novel design optimization for passive cooling PCM assisted battery thermal management system in electric vehicles. Case Stud. Therm. Eng. 2022, 32, 101896. [Google Scholar] [CrossRef]
- Luo, J.; Zou, D.; Wang, Y.; Wang, S.; Huang, L. Battery thermal management systems (BTMs) based on phase change material (PCM): A comprehensive review. Chem. Eng. J. 2021, 430, 132741. [Google Scholar] [CrossRef]
- Devahastin, S.; Pitaksuriyarat, S. Use of latent heat storage to conserve energy during drying and its effect on drying kinetics of a food product. Appl. Therm. Eng. 2006, 26, 1705–1713. [Google Scholar] [CrossRef]
- Bal, L.M.; Satya, S.; Naik, S.N. Solar dryer with thermal energy storage systems for drying agricultural food products: A review. Renew. Sustain. Energy Rev. 2010, 14, 2298–2314. [Google Scholar] [CrossRef]
- Battocchio, C.; Bruni, F.; Di Nicola, G.; Gasperi, T.; Iucci, G.; Tofani, D.; Varesano, A.; Venditti, I. Solar Cookers and Dryers: Environmental Sustainability and Nutraceutical Content in Food Processing. Foods 2021, 10, 2326. [Google Scholar] [CrossRef]
- Simão, C.; Murta-Pina, J.; Oliveira, J.P.; Coelho, L.; Pássaro, J.; Ferreira, D.; Reboredo, F.; Jorge, T.; Figueiredo, P. A Case Study for Decentralized Heat Storage Solutions in the Agroindustry Sector Using Phase Change Materials. AgriEngineering 2022, 4, 255–278. [Google Scholar] [CrossRef]
- Zhang, X.; Shi, Q.; Luo, L.; Fan, Y.; Wang, Q.; Jia, G. Research Progress on the Phase Change Materials for Cold Thermal Energy Storage. Energies 2021, 14, 8233. [Google Scholar] [CrossRef]
- Meng, B.; Zhang, X.; Hua, W.; Liu, L.; Ma, K. Development and application of phase change material in fresh e-commerce cold chain logistics: A review. J. Energy Storage 2022, 55, 105373. [Google Scholar] [CrossRef]
- Calati, M.; Hooman, K.; Mancin, S. Thermal Storage based on Phase Change Materials (PCMs) for refrigerated transport and distribution applications along the cold chain: A review. Int. J. Thermofluids 2022. [Google Scholar] [CrossRef]
- Selvens, H.; Allouche, Y.; Hafner, A.; Schlemminger, C.; Tolstorebrov, I. Cold thermal energy storage for industrial CO2 refrigeration systems using phase change material: An experimental study. Appl. Therm. Eng. 2022, 212, 118543. [Google Scholar] [CrossRef]
- Mu, M.; Zhang, S.; Yang, S.; Wang, Y. Phase change materials applied in agricultural greenhouses. J. Energy Storage 2022, 49, 104100. [Google Scholar] [CrossRef]
- Luo, Y.; Andresen, J.; Clarke, H.; Rajendra, M.; Maroto-Valer, M. A framework for waste heat energy recovery within data centre. Energy Procedia 2019, 158, 3788–3794. [Google Scholar] [CrossRef]
- Liu, L.; Zhang, Q.; Zhai, Z.; Yue, C.; Ma, X. State-of-the-art on thermal energy storage technologies in data center. Energy Build. 2020, 226, 110345. [Google Scholar] [CrossRef]
- Ljungdahl, V.; Jradi, M.; Veje, C. A decision support model for waste heat recovery systems design in Data Center and High-Performance Computing clusters utilizing liquid cooling and Phase Change Materials. Appl. Therm. Eng. 2022, 201, 117671. [Google Scholar] [CrossRef]
- Mousavi, S.B.; Adib, M.; Soltani, M.; Razmi, A.R.; Nathwani, J. Transient thermodynamic modeling and economic analysis of an adiabatic compressed air energy storage (A-CAES) based on cascade packed bed thermal energy storage with encapsulated phase change materials. Energy Convers. Manag. 2021, 243, 114379. [Google Scholar] [CrossRef]
- Yan, S.-R.; Fazilati, M.A.; Samani, N.; Ghasemi, H.R.; Toghraie, D.; Nguyen, Q.; Karimipour, A. Energy efficiency optimization of the waste heat recovery system with embedded phase change materials in greenhouses: A thermo-economic-environmental study. J. Energy Storage 2020, 30, 101445. [Google Scholar] [CrossRef]
- Nishad, S.; Krupa, I. Phase change materials for thermal energy storage applications in greenhouses: A review. Sustain. Energy Technol. Assessments 2022, 52, 102241. [Google Scholar] [CrossRef]
- Zhang, M.; Cheng, H.; Wang, C.; Zhou, Y. Kaolinite nanotube-stearic acid composite as a form-stable phase change material for thermal energy storage. Appl. Clay Sci. 2021, 201, 105930. [Google Scholar] [CrossRef]
- Jafaripour, M.; Sadrameli, S.; Pahlavanzadeh, H.; Mousavi, S.S. Fabrication and optimization of kaolin/stearic acid composite as a form-stable phase change material for application in the thermal energy storage systems. J. Energy Storage 2021, 33, 102155. [Google Scholar] [CrossRef]
- Xie, N.; Gao, X.; Zhong, Y.; Ye, R.; Chen, S.; Ding, L.; Zhong, T. Enhanced thermal performance of Na2HPO4·12H2O composite phase change material supported by sepiolite fiber for floor radiant heating system. J. Build. Eng. 2022, 56, 104747. [Google Scholar] [CrossRef]
- Gao, N.; Tang, T.; Xiang, H.; Zhang, W.; Li, Y.; Yang, C.; Xia, T.; Liu, X. Preparation and structure-properties of crosslinking organic montmorillonite/polyurethane as solid-solid phase change materials for thermal energy storage. Sol. Energy Mater. Sol. Cells 2022, 244, 111831. [Google Scholar] [CrossRef]
- Wen, R.; Zhu, X.; Yang, C.; Sun, Z.; Zhang, L.; Xu, Y.; Qiao, J.; Wu, X.; Min, X.; Huang, Z. A novel composite phase change material from lauric acid, nano-Cu and attapulgite: Preparation, characterization and thermal conductivity enhancement. J. Energy Storage 2022, 46, 103921. [Google Scholar] [CrossRef]
- Shi, J.; Li, M. Surface modification effects in phase change material-infiltrated attapulgite. Mater. Chem. Phys. 2020, 254, 123521. [Google Scholar] [CrossRef]
- Li, M.; Shi, J. Mechanical and thermal performance assessment of paraffin/expanded vermiculite-diatomite composite phase change materials integrated mortar: Experimental and numerical approach. Sol. Energy 2021, 227, 343–353. [Google Scholar] [CrossRef]
- Gencel, O.; Sarı, A.; Ustaoglu, A.; Hekimoglu, G.; Erdogmus, E.; Yaras, A.; Sutcu, M.; Cay, V.V. Eco-friendly building materials containing micronized expanded vermiculite and phase change material for solar based thermo-regulation applications. Constr. Build. Mater. 2021, 308, 125062. [Google Scholar] [CrossRef]
- Xu, T.; Wu, F.; Zou, T.; Li, J.; Yang, J.; Zhou, X.; Liu, D.; Bie, Y. Development of diatomite-based shape-stabilized composite phase change material for use in floor radiant heating. J. Mol. Liq. 2022, 348, 118372. [Google Scholar] [CrossRef]
- Ren, M.; Zhao, H.; Gao, X. Effect of modified diatomite based shape-stabilized phase change materials on multiphysics characteristics of thermal storage mortar. Energy 2022, 241, 122823. [Google Scholar] [CrossRef]
- Fan, Z.; Zhao, Y.; Ding, Y.; Shi, Y.; Liu, X.; Jiang, D. Fabrication and comprehensive analysis of expanded perlite impregnated with myristic acid-based phase change materials as composite materials for building thermal management. J. Energy Storage 2022, 55, 105710. [Google Scholar] [CrossRef]
- Bian, Y.; Wang, K.; Wang, J.; Yu, Y.; Liu, M.; Lv, Y. Preparation and properties of capric acid: Stearic acid/hydrophobic expanded perlite-aerogel composite phase change materials. Renew. Energy 2021, 179, 1027–1035. [Google Scholar] [CrossRef]
- Chen, X.; Tang, Z.; Liu, P.; Gao, H.; Chang, Y.; Wang, G. Smart Utilization of Multifunctional Metal Oxides in Phase Change Materials. Matter 2020, 3, 708–741. [Google Scholar] [CrossRef]
- Kumar, P.M.; Mylsamy, K. Experimental investigation of solar water heater integrated with a nanocomposite phase change material. J. Therm. Anal. 2019, 136, 121–132. [Google Scholar] [CrossRef]
- Ding, J.; Wu, X.; Shen, X.; Cui, S.; Chen, X. Form-stable phase change material embedded in three-dimensional reduced graphene aerogel with large latent heat for thermal energy management. Appl. Surf. Sci. 2020, 534, 147612. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhang, K.; Min, X.; Xiao, J.; Xu, Z.; Huang, Z.; Liu, Y.; Wu, X.; Fang, M. Graphene aerogel stabilized phase change material for thermal energy storage. Case Stud. Therm. Eng. 2022, 40, 102497. [Google Scholar] [CrossRef]
- Rezaie, A.B.; Montazer, M. Shape-stable thermo-responsive nano Fe3O4/fatty acids/PET composite phase-change material for thermal energy management and saving applications. Appl. Energy 2020, 262. [Google Scholar] [CrossRef]
- Li, Y.; Fu, Z.; Xue, M.; Shao, Z.; Li, Y.; Zhu, Q. Experimental study on preparation and thermal storage properties of expanded graphite/paraffin wax as a shape-stabilized phase change material. Energy Rep. 2022, 8, 324–331. [Google Scholar] [CrossRef]
- Wang, T.; Qiu, X.; Chen, X.; Lu, L.; Zhou, B. Sponge-like form-stable phase change materials with embedded graphene oxide for enhancing the thermal storage efficiency and the temperature response in transport packaging applications. Appl. Energy 2022, 325, 119832. [Google Scholar] [CrossRef]
- Amaral, C.; Gama, N.; Mohseni, F.; Amaral, J.; Marques, P.; Barros-Timmons, A.; Vicente, R. Development of structural layers PVC incorporating phase change materials for thermal energy storage. Appl. Therm. Eng. 2020, 179, 115707. [Google Scholar] [CrossRef]
- Jin, X.; Li, J.; Xue, P.; Jia, M. Preparation and characterization of PVC-based form-stable phase change materials. Sol. Energy Mater. Sol. Cells 2014, 130, 435–441. [Google Scholar] [CrossRef]
- Kenisarin, M.M.; Kenisarina, K.M. Form-stable phase change materials for thermal energy storage. Renew. Sustain. Energy Rev. 2012, 16, 1999–2040. [Google Scholar] [CrossRef]
- Laza, J.M.; Veloso-Fernández, A.; Sanchez-Bodon, J.; Martín, A.; Goitandia, A.M.; Monteserín, C.; Mendibil, X.; Vidal, K.; Lambarri, J.; Aranzabe, E.; et al. Analysis of the influence of microencapsulated phase change materials on the behavior of a new generation of thermo-regulating shape memory polyurethane fibers. Polym. Test. 2022, 116, 107807. [Google Scholar] [CrossRef]
- Zhou, L.; Shi, F.; Liu, G.; Ye, J.P.; Han, P.S.; Zhang, G. Fabrication and characterization of in situ cross-linked electrospun Poly(vinyl alcohol)/phase change material nanofibers. Sol. Energy 2021, 213, 339–349. [Google Scholar] [CrossRef]
- Marske, F.; Dasler, J.; Haupt, C.; Bacia, K.; Hahn, T.; Enke, D. Influence of surfactants and organic polymers on monolithic shape-stabilized phase change materials synthesized via sol-gel route. J. Energy Storage 2022, 49, 104127. [Google Scholar] [CrossRef]
- Sari, A.; Alkan, C.; Karaipekli, A.; Önal, A. Preparation, characterization and thermal properties of styrene maleic anhydride copolymer (SMA)/fatty acid composites as form stable phase change materials. Energy Convers. Manag. 2008, 49, 373–380. [Google Scholar]
- Liu, L.; Kong, L.; Wang, H.; Niu, R.; Shi, H. Effect of graphene oxide nanoplatelets on the thermal characteristics and shape-stabilized performance of poly(styrene-co-maleic anhydride)-g-octadecanol comb-like polymeric phase change materials. Sol. Energy Mater. Sol. Cells 2016, 149, 40–48. [Google Scholar] [CrossRef]
- Wang, H.; Shi, H.; Qi, M.; Zhang, L.; Zhang, X.; Qi, L. Structure and thermal performance of poly(styrene-co-maleic anhydride)-g-alkyl alcohol comb-like copolymeric phase change materials. Thermochim. Acta 2013, 564, 34–38. [Google Scholar] [CrossRef]
- Sarı, A.; Bicer, A.; Alkan, C. Thermal energy storage characteristics of poly(styrene-co-maleic anhydride)-graft-PEG as polymeric solid–solid phase change materials. Sol. Energy Mater. Sol. Cells 2017, 161, 219–225. [Google Scholar] [CrossRef]
- Chen, C.; Wang, L.; Huang, Y. Ultrafine electrospun fibers based on stearyl stearate/polyethylene terephthalate composite as form stable phase change materials. Chem. Eng. J. 2009, 150, 269–274. [Google Scholar] [CrossRef]
- Jamekhorshid, A.; Sadrameli, S.M.; Farid, M. A review of microencapsulation methods of phase change materials (PCMs) as a thermal energy storage (TES) medium. Renew. Sustain. Energy Rev. 2014, 31, 531–542. [Google Scholar] [CrossRef]
- Borreguero, A.; Valverde, J.; Rodríguez, J.; Barber, A.; Cubillo, J.; Carmona, M. Synthesis and characterization of microcapsules containing Rubitherm® RT27 obtained by spray drying. Chem. Eng. J. 2011, 166, 384–390. [Google Scholar] [CrossRef]
- Mishra, M.K. Microencapsulation. In Kirk-Othmer Encyclopedia of Chemical Technology; Wiley Online Library: Hoboken, NJ, USA, 2019. [Google Scholar]
- Zhang, P.; Ma, Z.; Wang, R. An overview of phase change material slurries: MPCS and CHS. Renew. Sustain. Energy Rev. 2010, 14, 598–614. [Google Scholar] [CrossRef]
- Alva, G.; Lin, Y.; Liu, L.; Fang, G. Synthesis, characterization and applications of microencapsulated phase change materials in thermal energy storage: A review. Energy Build. 2017, 144, 276–294. [Google Scholar] [CrossRef]
- He, F.; Wang, X.; Wu, D. New approach for sol–gel synthesis of microencapsulated n-octadecane phase change material with silica wall using sodium silicate precursor. Energy 2014, 67, 223–233. [Google Scholar] [CrossRef]
- Alay, S.; Alkan, C.; Gode, F. Synthesis and characterization of poly(methyl methacrylate)/n-hexadecane microcapsules using different cross-linkers and their application to some fabrics. Thermochim. Acta 2011, 518, 1–8. [Google Scholar] [CrossRef]
- Salaün, F.; Bedek, G.; Devaux, E.; Dupont, D.; Gengembre, L. Microencapsulation of a cooling agent by interfacial polymerization: Influence of the parameters of encapsulation on poly(urethane–urea) microparticles characteristics. J. Membr. Sci. 2011, 370, 23–33. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, X. Fabrication and performances of microencapsulated phase change materials based on n-octadecane core and resorcinol-modified melamine–formaldehyde shell. Colloids Surfaces A Physicochem. Eng. Asp. 2009, 332, 129–138. [Google Scholar] [CrossRef]
- Ghasemi, K.; Tasnim, S.; Mahmud, S. PCM, nano/microencapsulation and slurries: A review of fundamentals, categories, fabrication, numerical models and applications. Sustain. Energy Technol. Assess. 2022, 52, 102084. [Google Scholar] [CrossRef]
- Abdeali, G.; Bahramian, A.R. A comprehensive review on rheological behavior of phase change materials fluids (slurry and emulsion): The way toward energy efficiency. J. Energy Storage 2022, 55, 105549. [Google Scholar] [CrossRef]
- Datta, P.; Sengupta, S.; Roy, S.K. Natural Convection Heat Transfer in an Enclosure with Suspensions of Microencapsulated Phase Change Materials; American Society of Mechanical Engineers, Heat Transfer Division: New York, NY, USA, 1992; Volume 204. [Google Scholar]
- Inaba, H.; Dai, C.; Horibe, A. Natural convection heat transfer in enclosures with microemulsion phase change material slurry. Heat Mass Transf. 2003, 40, 179–189. [Google Scholar] [CrossRef]
- Nazir, H.; Batool, M.; Osorio, F.J.B.; Isaza-Ruiz, M.; Xu, X.; Vignarooban, K.; Phelan, P.; Inamuddin; Kannan, A.M. Recent developments in phase change materials for energy storage applications: A review. Int. J. Heat Mass Transf. 2019, 129, 491–523. [Google Scholar] [CrossRef]
- Zhang, G.; Li, J.; Chen, Y.; Xiang, H.; Ma, B.; Xu, Z.; Ma, X. Encapsulation of copper-based phase change materials for high temperature thermal energy storage. Sol. Energy Mater. Sol. Cells 2014, 128, 131–137. [Google Scholar] [CrossRef]
- Zheng, Y.; Zhao, W.; Sabol, J.C.; Tuzla, K.; Neti, S.; Oztekin, A.; Chen, J.C. Encapsulated phase change materials for energy storage—Characterization by calorimetry. Sol. Energy 2012, 87, 117–126. [Google Scholar] [CrossRef]
- Zhang, H.; Baeyens, J.; Degrève, J.; Cáceres, G.; Segal, R.; Pitié, F. Latent heat storage with tubular-encapsulated phase change materials (PCMs). Energy 2014, 76, 66–72. [Google Scholar] [CrossRef]
- Vicente, R.; Silva, T. Brick masonry walls with PCM macrocapsules: An experimental approach. Appl. Therm. Eng. 2014, 67, 24–34. [Google Scholar] [CrossRef]
- Alam, T.E.; Dhau, J.S.; Goswami, D.Y.; Stefanakos, E. Macroencapsulation and characterization of phase change materials for latent heat thermal energy storage systems. Appl. Energy 2015, 154, 92–101. [Google Scholar] [CrossRef] [Green Version]
- Abbas, H.M.; Jalil, J.M.; Ahmed, S.T. Experimental and numerical investigation of PCM capsules as insulation materials inserted into a hollow brick wall. Energy Build. 2021, 246, 111127. [Google Scholar] [CrossRef]
- Mukram, A.; Daniel, J. IOP Conference Series: Earth and Environmental Science; IOP Publishing Ltd.: Bristol, UK, 2020; Volume 573. [Google Scholar]
- Silva, T.; Vicente, R.; Soares, N.; Ferreira, V. Experimental testing and numerical modelling of masonry wall solution with PCM incorporation: A passive construction solution. Energy Build. 2012, 49, 235–245. [Google Scholar] [CrossRef]
- Alawadhi, E.M.; Alqallaf, H.J. Building roof with conical holes containing PCM to reduce the cooling load: Numerical study. Energy Convers. Manag. 2011, 52, 2958–2964. [Google Scholar] [CrossRef]
- Castell, A.; Martorell, I.; Medrano, M.; Pérez, G.; Cabeza, L.F. Experimental study of using PCM in brick constructive solutions for passive cooling. Energy Build. 2010, 42, 534–540. [Google Scholar] [CrossRef]
- Zayed, M.E.; Zhao, J.; Li, W.; Elsheikh, A.; Elbanna, A.M.; Jing, L.; Geweda, A. Recent progress in phase change materials storage containers: Geometries, design considerations and heat transfer improvement methods. J. Energy Storage 2020, 30, 101341. [Google Scholar] [CrossRef]
- Rathore, P.K.S.; Shukla, S.K. Potential of macroencapsulated PCM for thermal energy storage in buildings: A comprehensive review. Constr. Build. Mater. 2019, 225, 723–744. [Google Scholar] [CrossRef]
- Akeiber, H.J.; Hosseini, S.E.; Hussen, H.M.; Wahid, M.A.; Mohammad, A.T. Thermal performance and economic evaluation of a newly developed phase change material for effective building encapsulation. Energy Convers. Manag. 2017, 150, 48–61. [Google Scholar] [CrossRef]
- Izadi, M.; Hajjar, A.; Alshehri, H.M.; Saleem, A.; Galal, A.M. Analysis of applying fin for charging process of phase change material inside H-shaped thermal storage. Int. Commun. Heat Mass Transf. 2022, 139, 106421. [Google Scholar] [CrossRef]
- Yan, P.; Fan, W.; Yang, Y.; Ding, H.; Arshad, A.; Wen, C. Performance enhancement of phase change materials in triplex-tube latent heat energy storage system using novel fin configurations. Appl. Energy 2022, 327, 120064. [Google Scholar] [CrossRef]
- Kumirai, T.; Dirker, J.; Meyer, J. Experimental analysis for thermal storage performance of three types of plate encapsulated phase change materials in air heat exchangers for ventilation applications. J. Build. Eng. 2019, 22, 75–89. [Google Scholar] [CrossRef]
- Yang, X.; Zheng, X.; Yang, Z.; Bai, Y.; Chen, H. Effects of morphology and internal voids of copper ribs on heat transfer performance in copper foam/paraffin composite phase change materials. Int. J. Heat Mass Transf. 2020, 152, 119526. [Google Scholar] [CrossRef]
- Iranmanesh, A. Intensifying the melting process of a triple-tube latent heat energy storage unit via inserting a middle plate into the phase change material container. J. Energy Storage 2022, 56, 105982. [Google Scholar] [CrossRef]
- Younis, O.; Abderrahmane, A.; Hatami, M.; Mourad, A.; Kamel, G. Thermal energy storage using nano phase change materials in corrugated plates heat exchangers with different geometries. J. Energy Storage 2022, 55, 105785. [Google Scholar] [CrossRef]
- Kahwaji, S.; Johnson, M.B.; Kheirabadi, A.C.; Groulx, D.; White, M.A. A comprehensive study of properties of paraffin phase change materials for solar thermal energy storage and thermal management applications. Energy 2018, 162, 1169–1182. [Google Scholar] [CrossRef]
- Ushak, S.; Marín, P.; Galazutdinova, Y.; Cabeza, L.F.; Farid, M.M.; Grágeda, M. Compatibility of materials for macroencapsulation of inorganic phase change materials: Experimental corrosion study. Appl. Therm. Eng. 2016, 107, 410–419. [Google Scholar] [CrossRef] [Green Version]
- Chen, W.; Liang, X.; Han, W.; Wang, S.; Gao, X.; Zhang, Z.; Fang, Y. 3D shape-stable temperature-regulated macro-encapsulated phase change material: KAl(SO4)2·12H2O-C2H2O4·2H2O-CO(NH2)2 eutectic/polyurethane foam as core and carbon modified silicone resin as shell. J. Mater. Sci. Technol. 2022, 100, 27–35. [Google Scholar] [CrossRef]
- Mehling, H.; Brütting, M.; Haussmann, T. PCM products and their fields of application—An overview of the state in 2020/2021. J. Energy Storage 2022, 51, 104354. [Google Scholar] [CrossRef]
- Gao, D.-C.; Sun, Y.; Fong, A.M.; Gu, X. Mineral-based form-stable phase change materials for thermal energy storage: A state-of-the art review. Energy Storage Mater. 2022, 46, 100–128. [Google Scholar] [CrossRef]
- Yang, Y.; Pang, Y.; Liu, Y.; Guo, H. Preparation and thermal properties of polyethylene glycol/expanded graphite as novel form-stable phase change material for indoor energy saving. Mater. Lett. 2018, 216, 220–223. [Google Scholar] [CrossRef]
- Yi, H.; Zhan, W.; Zhao, Y.; Qu, S.; Wang, W.; Chen, P.; Song, S. A novel core-shell structural montmorillonite nanosheets/stearic acid composite PCM for great promotion of thermal energy storage properties. Sol. Energy Mater. Sol. Cells 2019, 192, 57–64. [Google Scholar] [CrossRef]
- Wang, Y.; Song, Y.; Li, S.; Zhang, T.; Zhang, D.; Guo, P. Thermophysical properties of three-dimensional palygorskite based composite phase change materials. Appl. Clay Sci. 2020, 184, 105367. [Google Scholar] [CrossRef]
- Zhang, Y.; Jia, Z.; Hai, A.M.; Zhang, S.; Tang, B. Shape-stabilization micromechanisms of form-stable phase change materials-A review. Compos. Part A Appl. Sci. Manuf. 2022, 160, 107047. [Google Scholar] [CrossRef]
- Yin, G.-Z.; Hobson, J.; Duan, Y.; Wang, D.-Y. Polyrotaxane: New generation of sustainable, ultra-flexible, form-stable and smart phase change materials. Energy Storage Mater. 2021, 40, 347–357. [Google Scholar] [CrossRef]
- Maleki, B.; Khadang, A.; Maddah, H.; Alizadeh, M.; Ali Kazemian, A.; Muhamma Ali, H. Development and thermal performance of nano-encapsulated PCM/ plaster wallboard for thermal energy storage in buildings. J. Build. Eng. 2020, 32, 101727. [Google Scholar] [CrossRef]
- Soo, X.Y.D.; Png, Z.M.; Chua, M.H.; Yeo, J.C.C.; Ong, P.J.; Wang, S.; Wang, X.; Suwardi, A.; Cao, J.; Chen, Y.; et al. A highly flexible form-stable silicone-octadecane PCM composite for heat harvesting. Mater. Today Adv. 2022, 14, 100227. [Google Scholar] [CrossRef]
- Dobri, A.; Tsiantis, A.; Papathanasiou, T.; Wang, Y. Investigation of transient heat transfer in multi-scale PCM composites using a semi-analytical model. Int. J. Heat Mass Transf. 2021, 175, 121389. [Google Scholar] [CrossRef]
- Zhao, Y.; Min, X.; Huang, Z.; Liu, Y.; Wu, X.; Fang, M. Honeycomb-like structured biological porous carbon encapsulating PEG: A shape-stable phase change material with enhanced thermal conductivity for thermal energy storage. Energy Build. 2018, 158, 1049–1062. [Google Scholar] [CrossRef]
- Ran, X.; Wang, H.; Zhong, Y.; Zhang, F.; Lin, J.; Zou, H.; Dai, Z.; An, B. Thermal properties of eutectic salts/ceramics/expanded graphite composite phase change materials for high-temperature thermal energy storage. Sol. Energy Mater. Sol. Cells 2021, 225, 111047. [Google Scholar] [CrossRef]
- Xie, N.; Li, Z.; Gao, X.; Fang, Y.; Zhang, Z. Preparation and performance of modified expanded graphite/eutectic salt composite phase change cold storage material. Int. J. Refrig. 2020, 110, 178–186. [Google Scholar] [CrossRef]
- Lu, W.; Liu, G.; Xiong, Z.; Wu, Z.; Zhang, G. An experimental investigation of composite phase change materials of ternary nitrate and expanded graphite for medium-temperature thermal energy storage. Sol. Energy 2020, 195, 573–580. [Google Scholar] [CrossRef]
- Zhong, L.; Zhang, X.; Luan, Y.; Wang, G.; Feng, Y.; Feng, D. Preparation and thermal properties of porous heterogeneous composite phase change materials based on molten salts/expanded graphite. Sol. Energy 2014, 107, 63–73. [Google Scholar] [CrossRef]
- Aridi, R.; Yehya, A. Review on the sustainability of phase-change materials used in buildings. Energy Convers. Manag. X 2022, 15, 100237. [Google Scholar] [CrossRef]
- Jahangir, M.H.; Ziyaei, M.; Kargarzadeh, A. Evaluation of thermal behavior and life cycle cost analysis of greenhouses with bio-phase change materials in multiple locations. J. Energy Storage 2022, 54, 105176. [Google Scholar] [CrossRef]
- Souayfane, F.; Biwole, P.H.; Fardoun, F.; Achard, P. Energy performance and economic analysis of a TIM-PCM wall under different climates. Energy 2019, 169, 1274–1291. [Google Scholar] [CrossRef]
- Singh, D.; Marrocco, A.; Wohlleben, W.; Park, H.-R.; Diwadkar, A.R.; Himes, B.E.; Lu, Q.; Christiani, D.C.; Demokritou, P. Release of particulate matter from nano-enabled building materials (NEBMs) across their lifecycle: Potential occupational health and safety implications. J. Hazard. Mater. 2022, 422, 126771. [Google Scholar] [CrossRef]
- Chandel, S.; Agarwal, T. Review of current state of research on energy storage, toxicity, health hazards and commercialization of phase changing materials. Renew. Sustain. Energy Rev. 2017, 67, 581–596. [Google Scholar] [CrossRef]
- McLaggan, M.S.; Hadden, R.M.; Gillie, M. Fire Performance of Phase Change Material Enhanced Plasterboard. Fire Technol. 2018, 54, 117–134. [Google Scholar] [CrossRef]
- Png, Z.M.; Soo, X.Y.D.; Chua, M.H.; Ong, P.J.; Suwardi, A.; Tan, C.K.I.; Xu, J.; Zhu, Q. Strategies to reduce the flammability of organic phase change Materials: A review. Sol. Energy 2022, 231, 115–128. [Google Scholar] [CrossRef]




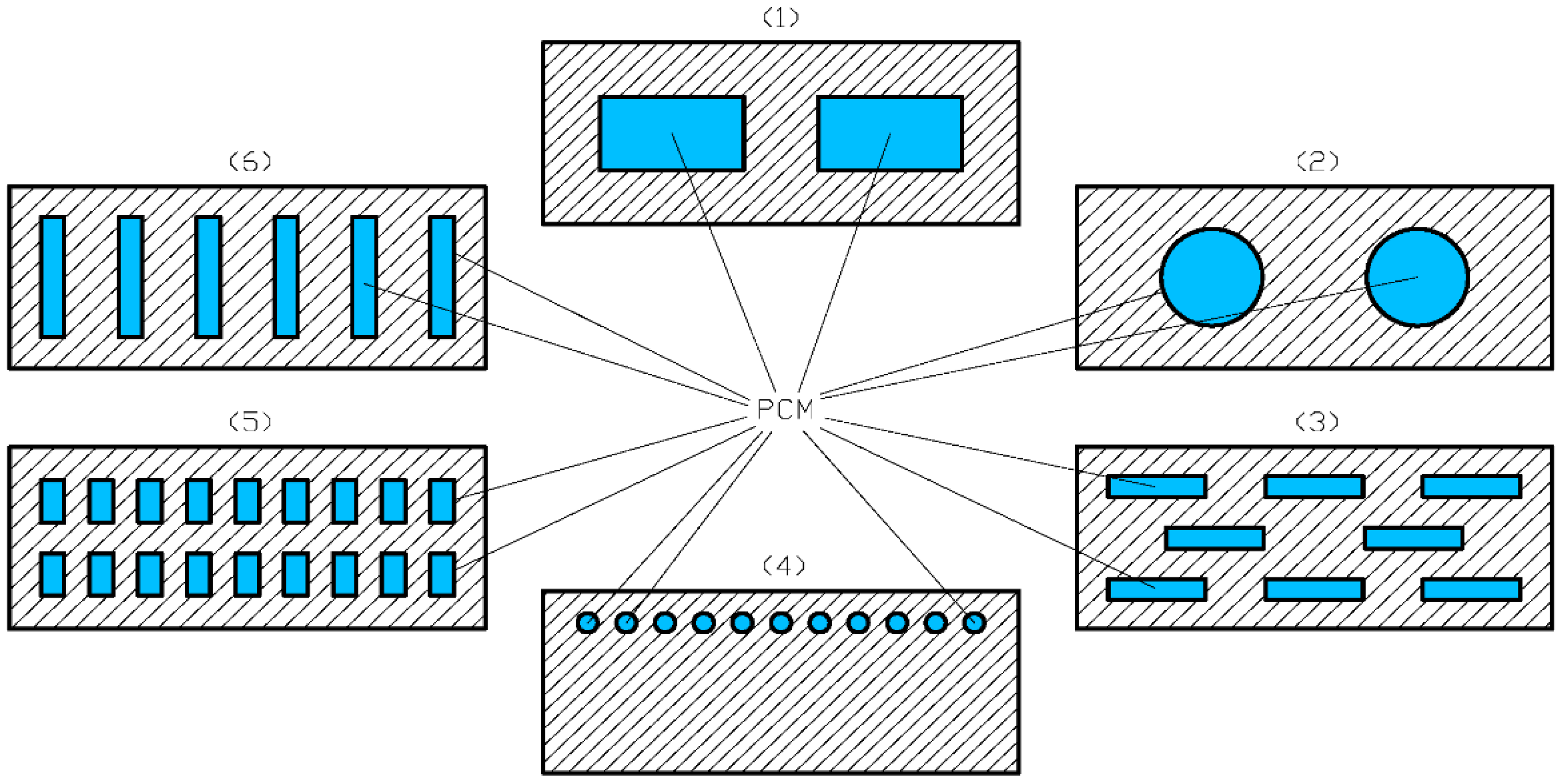




| Ref. | System Design and Conditions | Findings, Conclusions |
|---|---|---|
| [24] | Gypsum wallboard impregnated with a mixture of methyl palmitate/methyl stearate with a melting range 23–26.5 C and latent heat of the phase transition 180 kJ/kg | Peak load shifting |
| [25] | A procedure to produce TES concrete (TESC) by infiltrating porous aggregates with molten PCM under vacuum conditions | The storage capacity of the TESC was comparable with that of a commercially available PCM. |
| [26] | Facade panels with double glazing combined with PCM | A more equalized energy balance in the during the day, when moderate heat gains with very low heat losses were experienced. This suggests lightweight construction appropriateness |
| [27] | Laminated wallboard with a narrow phase change temperature range. | Moderating night time temperature in a passively designed room. |
| [28] | Mixtures of CA and LA as the PCM to impregnate wallboards for building energy storage | After 360 thermal cycles the melting temperature and latent heat of the PCM wallboards showed no obvious changes |
| [29] | Phase change GW incorporating the eutectic mixture of fatty acids | A maximum of 25% of the total mass of CA/MA eutectic could be incorporated into the GW; after 1000 cycles PCM leakage occurred |
| [30] | Three-layer sandwich type wall system consisting of two PCM wallboards, impregnated with PCMs with different values of the melting point as outer layers and a middle layer of conventional thermal insulation | Annual energy savings; reduces the peak value of the cooling/heating loads. The melting point values for the two PCMs resulting in the highest value of the energy savings were identified. |
| [31] | PCM-enhanced wall system and a simplified model for the heat exchange between the indoor environment and ambient was developed | The occupancy pattern influenced the value of the PCM melting point for which maximum energy savings value was reached and; the ventilation and its pattern reduced the relative value of the energy savings. |
| [32] | PCM incorporated in concrete floors, in which solar thermal energy is stored in a concrete/PCM composite | Reduction of the maximum floor temperatures by 16% and an increase in the minimum temperatures of up to 7%. |
| [33] | PEG based shape-stabilized PCMs were successfully prepared using CNFs isolated from crab shells | The device remained opaque (∼3.5%) below the melting point of the PEG-CNF composite and became gradually transparent as the temperature of the device increased ultimately stabilizing at a transmittance value of ∼88%. CNF phase had an effect on the thermal properties of the PEG-CNF material |
| [34] | A fabrication approach of cement-based composite phase change materials (CCPCMs) by means of the water solubility of polyethylene glycol | Compared to control cement paste, the surface temperature of CCPCMs sample reduced by a maximum of 6.2 °C. |
| [35] | Latent heat storage bio-composite | The maximum energy consumption of reference building models dropped by ~530 kWh per year |
| [36] | (1) the model house without the solar Trombe wall, defined as the reference configuration; (2) the model house integrating the concrete solar Trombe wall; and (3) the model house integrating the PCM solar Trombe wall. three different climate conditions were considered for simulations: Paris-Orly, Lyon, and Nice | Similar minimizations of the one-year energy heating demand inside the bedroom, equal to roughly 20% (i.e., 20.45% of concrete storage wall and 19.90% of PCM storage wall) compared to the reference configuration. The heating energy demand in the bedroom, through application of the two storage walls (concrete and PCM) could be minimized by 20.34% in Paris, 20.20% in Lyon, and 68.10% in Nice (for the concrete storage wall) vs. the reference configuration; and by 18.79% in Paris, 19.56% in Lyon, and 55.15% in Nice (for the PCM storage wall) compared to the reference configuration |
| Ref. | System Design and Conditions | Findings, Conclusions |
|---|---|---|
| [37] | Macro-encapsulated PCM panel with embedded pipes as an active ceiling cooling component as opposed to commercially available radiant cooling technologies | PCM was fully discharged, the installed heat storage capacity was enough to shift the cooling demand to off-peak hours. The MEP’s specific cooling power during melting was between 5.3 and 27.7 W/m2, with 11.3 W/m2 on average over the active ceiling surface for a water supply temperature and flow rate of 20 °C and 140 kg/h, respectively |
| [38] | Solar thermal system coupling with active PCM heat storage wall. The effect of the solar collector area, hot water flow rate, initial melting temperature and thickness of PCMSW on the indoor temperature maintained by STS-APHSW was assessed. Economic comfort ratio was proposed as assessment criteria | Optimal configuration was achieved when: the solar collector area per square unit building area was 0.024 m2, hot water flow rate was 2.8 kg/s, initial melting temperature was 27 °C and the thickness of PCMSW was 20 mm. STS-APHSW can balance the time mismatch between the solar supply and demand, thus increasing the solar fraction and solar energy application potential |
| [39] | A state-space model for solar active wall-based (PCM) was proposed. The proposed numerical model was applied for a multi-layer wall with PCM Wallboards (PCMW) embedded between indoor and outdoor environments | The state-space model was able to estimate the thermal behaviour of the system, as well as the thermal characteristics of embedding PCM in the internal face of the wall. The PCM presence significantly contributed to stabilizing the indoor temperature. |
| [40] | Active PCM system installed in an eleven-storey building in Melbourne. Macro-encapsulated PCM with the phase transition temperature of 15 °C was installed in a large PCM tank. Water was used as the heat transfer fluid to extract and store cooling energy from the PCM tank. The performance of the active PCM system was monitored for 25 consecutive months, and the results were analysed on a seasonal basis | The active PCM system reduced cooling load on the chiller by 12–37% only during colder months, but remained inactive during the summer. In the case of maximum effectiveness, the PCM tank only utilized 15% of its available heat storage capacity to reduce the cooling load. The factors that contributed to the underperformance of active PCM system were identified: mismatch between designed and actual operation of the PCM system, inefficient system control, material quality, lack of maintenance staff training |
| [41] | An experiment was conducted to study the thermal performance and energy efficiency of an ASHW-PCM system. Comparative trials were then carried out to complete its systematic optimization using CFD, considering the dominating factors that may have an effect on the systematic thermal performance: the number and location of the water capillary pipes, the PCM wallboard thickness, and the heating water temperature | The test room with the ASHW-PCM system reached an energy conservation of 1.93 kWh and an indoor temperature 21.9 °C, which are higher than that of the reference room. Numerical results showed that the number of water capillary pipes mainly affects the homogenization of the ASHW-PCM wallboard surface heating temperature, and the pipe location mostly influences the heating temperature. |
| [42] | A new structure of under-floor electric heating system with SSPCM plates. The SS-PCM consisted of 75 wt.% paraffin as a dispersed PCM and of 25 wt.% polyethylene as the supporting material. | The system increased the indoor temperature of an experimental house without increasing the temperature difference; the temperature of the PCM plates was maintained at the phase transition temperature for a long period after the heaters stopped working. More than 50% of the total electric heat energy was shifted from the peak period to the off-peak hours |
| Ref | PCM | Shell Material | Temperature Range (°C) | Shell Shape | Capsule Size | PCM Mass (g) | Operational Cycles | Findings |
|---|---|---|---|---|---|---|---|---|
| [126] | Copper 99.9% | Cr-Ni | 1050–1150 | Cylinder | 2 mm | 1000 | No leakage | |
| [127] | NaNO3, NaCl–MgCl2 | Stainless steel 304. Carbon steel 1018 | 350–490 | Cylinder | 50.8, 25.4 mm(Ø) 127, 50.8 mm (height) | 269.9 28.8 23.9 284.6 282.5 | 50 | Storage performance maintained |
| [128] | NaNO3 KNO3 | AISI 321 | 160–270 | Cylinder | 27.3, 75, 39 mm(Ø) 27.7, 77, 37 mm (height) | 5000 | No shell cracking | |
| [129] | Paraffin | Steel | 0–36 | Rectangular prism | 30 × 17 × 2.8 cm | 48 h | No leakage | |
| [130] | NaNO3, KNO3, NaNO3–KNO3, NaNO3–KNO3–LiNO3 | Polytetrafluoroethylene-Ni | 120–350 | Spherical | 27.43 mm | 17.4 | 2200 | No leakage |
| [131] | Paraffin | Aluminum | 30–49.2 | Cylinder | 29 mm(Ø) 70 mm (height) | 35 | No leakage at 90 °C |
| Reference | Method | Adsorption Rate | Support | PCM |
|---|---|---|---|---|
| [158] | Vacuum | 85% | Porous carbon | PEG |
| [159] | Sintering | NaCl, KCl, MgCl2 | Eutectic salt chloride | |
| [160] | Adsorption | 81% | Expanded graphite | K2HPO4⋅3H2O– NaH2PO4⋅2H2O–Na2S2O3⋅5H2O–H2O |
| [161] | Adsorption | - | Expanded graphite | KNO3-LiNO3-Ca(NO3)2 |
| [162] | Solution impregnation | 82% | Expanded graphite | LiNO3–KCl, LiNO3–NaNO3 LiNO3–NaCl |
| [95] | Vacuum-assisted melting infiltration | 97% | Graphene oxide | PEG-6000 |
| Hydrothermal reductio | 86% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Diaconu, B.M.; Cruceru, M.; Anghelescu, L. Phase Change Materials—Applications and Systems Designs: A Literature Review. Designs 2022, 6, 117. https://doi.org/10.3390/designs6060117
Diaconu BM, Cruceru M, Anghelescu L. Phase Change Materials—Applications and Systems Designs: A Literature Review. Designs. 2022; 6(6):117. https://doi.org/10.3390/designs6060117
Chicago/Turabian StyleDiaconu, Bogdan Marian, Mihai Cruceru, and Lucica Anghelescu. 2022. "Phase Change Materials—Applications and Systems Designs: A Literature Review" Designs 6, no. 6: 117. https://doi.org/10.3390/designs6060117







