Abstract
Radiation-induced graft polymerization provides industrially superior functionalization schemes by selection of existing polymer substrates and design of graft chains. In this review, by a pre-irradiation method of the radiation-induced graft polymerization and subsequent chemical modifications, charged polymer chains grafted onto various components and shapes of the polymer substrates are described. The charged graft chains immobilized onto a porous hollow-fiber membrane captured proteins in multilayers via multipoint binding. A membrane onto which positively charged graft chains are immobilized, i.e., an anion-exchange porous hollow-fiber membrane, was commercialized in 2011 for the removal of undesirable proteins in the purification of pharmaceuticals. On the other hand, a membrane onto which negatively charged graft chains are immobilized, i.e., a cation-exchange porous hollow-fiber membrane, exhibited a low permeation flux for pure water; however, the prepermeation of an aqueous solution of magnesium chloride through the membrane restored the permeation flux because of ionic crosslinking of graft chains with magnesium ions. The charged graft chains provide a precipitation field for inorganic compounds such as insoluble cobalt ferrocyanide. The graft chains entangle or penetrate a precipitate owing to electrostatic interactions with the surface charge on the precipitate. Braids and wound filters composed of insoluble-cobalt-ferrocyanide-impregnated fibers are used for the removal of radiocesium from contaminated water at Tokyo Electric Power Co. (TEPCO) Fukushima Daiichi Nuclear Power Plant.
1. Introduction of Radiation-Induced Graft Polymerization
Radiation-induced graft polymerization was first reported in the 1950s [1,2,3] and was considered a promising technique to modify polymeric materials. Radiation-induced radical polymerization is widely applicable to various components and shapes of polymeric materials compared with other grafting techniques [4,5,6,7,8,9]. This allows us to select polymer substrates in terms of specific applications. Novel functional materials that demonstrate high performance have been manufactured via radiation-induced graft polymerization. Examples include diaphragms of button batteries, chemical filters for ultraclean rooms, and ion-exchange materials for ultrapure water system. For the preparation scheme of graft-type functional materials, there is an analogy between graftings performed by gardeners and by chemists (Figure 1). In this review, we focused on a pre-irradiation method, which is one of radiation-induced graft polymerization techniques, and its schematic is shown in Figure 1b. Grafting is known as one of the effective agricultural techniques. By cutting a branch with scissors, the gardeners form a graft site on a tree with little or no fruit on its branches. Subsequently, they graft a branch which yields an abundant harvest. Similarly, by cleaving covalent bonds with radiation irradiation, chemists form graft sites (radicals) in polymeric materials suitable for specific applications. Radicals are unpaired electrons and generally show high chemical reactivity. They then graft a polymer branch with suitable capabilities. The successive contact of vinyl monomers, double-bond-containing monomers, with the radicals in the polymeric substrates inducing the growth of the polymer chain before termination occurs. By combining the polymeric materials (substrates) and polymer branches (graft chains), radiation-induced graft polymerization provides us with facile design of desirable and useful functional materials.
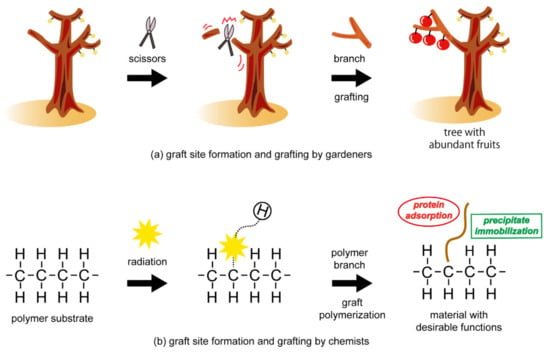
Figure 1.
Analogy between graftings performed by gardeners and chemists. (a) Graft site formation and grafting by gardeners for production of a tree with abundant fruits, (b) Graft site formation and grafting by chemists for preparation of a material with desirable functions.
2. Production and Storage of Radicals
To produce radicals in the polymer substrates, radiations such as electron beam and gamma rays can be applied. An electron beam is emitted from an electron gun at a prescribed electrical voltage and current. Emitted electrons penetrate the polymer substrates placed beneath the gun and produce radicals. For a small-scale irradiation, the polymer substrates are enclosed into a gas barrier bag with deoxygenating agent and put on a conveyer. Cobalt-60 (Co-60) has been widely utilized for gamma ray irradiation. The irradiation dose is adjustable by selecting the appropriate distance and irradiation time. Nowadays, radiation irradiation is primarily applied to the sterilization of various materials at a precise dose with acceptable cost. In addition, there are fifteen thousand electron accelerators for a commercial use worldwide [10]. The grafting process is available on an industrial scale and can be applied without owning the irradiation facilities, which is preferable for radiation-induced graft polymerization.
Radiation-induced graft polymerization are divided into two methods: pre-irradiation and simultaneous irradiation graft polymerization methods [1,2,11,12,13]. The former method needs to irradiate polymer substrates before a vinyl monomer is grafted, whereas the latter method needs to irradiate the polymer substrates and vinyl monomers simultaneously for radical generation and grafting. Basically, the former provides a brush structure because radicals are generated only on the substrate and the polymer grows from the radicals, as shown in Figure 1b. The latter provides a polymer gel to the surface of the substrate. Because radicals are generated not only in the substrate but also in the solvent molecules in the solution, the polymer grows from all the radicals; subsequently, the grafted polymer from the surface and excessive ungrafted polymer in the solution are generated. The grafted and ungrafted polymers generated by the simultaneous irradiation form a three-dimensional crosslinked polymer network or polymer gel. In this review, we focused on the pre-irradiation graft polymerization method because it is advantageous in that the formation of ungrafted polymer can be limited and a graft-polymerization is independently processed from an irradiation step, leading to promoting the industrial production of polymer chain-grafted materials. However, the pre-irradiation grafting requires storage of radicals.
Specifications of the radicals formed on the trunk polymers, such as species, location, and lifetime, are reported to design the adsorbents using radiation-induced graft polymerization technique [14]. For example, electron beam irradiation to polyethylene generates three types of radicals, i.e., alkyl, allyl, and peroxy radicals. Depending on the accessibility of oxygen to the radicals, different decay behavior was observed. Oxygen-accessible alkyl radicals rapidly decayed in air compared to the other two radicals. As the vinyl monomer also diffuses into the oxygen-accessible sites, the alkyl radicals will contribute to the graft polymerization. In addition, long-term radical storage was demonstrated by maintaining the irradiated polymer substrates below its glass transition temperature; for example, almost no decay of alkyl radical was observed by maintaining the irradiated polyethylene substrate at a dry-ice temperature of 195 K, where the matrix polymers have no mobility below a glass transition temperature of 203 K for polyethylene [14]. The capability of radical storage has an industrial significance: The graft polymerization step can be separated from the irradiation step with electron beams or gamma rays.
3. Classification and Evaluation of Graft Chain
For polymeric materials, such as polyethylene, matrices have crystalline and amorphous domains. The crystalline domain is an integrated structure of lamella formed by the tightly folded polymer chains, whereas the amorphous domain is formed by loose polymer chains. As mentioned above, radiation generates radicals throughout the matrix, that is, radicals are produced both in the crystalline and amorphous domains. Graft polymerization starts from the radicals. Therefore, graft chains are classified into two types on the basis of the formation site within the polymer substrate: Graft chains embedded in the polymer matrix of the substrate and graft chains extending from the substrate surface. The former and latter graft chains are referred to as the polymer root and polymer brush, respectively. The polymer root is surrounded by polymers of the substrates, which is characteristic of radiation-induced graft polymerization, whereas the polymer brush is formed by other methods of graft polymerization, such as chemical- [15,16], photo- [17,18], and plasma- [19,20] induced graft polymerization.
Isolating the polymer brush from the polymer root is technically challenging. Thus, the mole percentages of the polymer brush and root in the graft chain have not been fully understood. Ishihara et al. [21,22] proposed a method for determining the mole percentage of the polymer brush and root. The swelling behavior accompanied by the introduction of charged groups into the graft chain with poor solvent can provide the estimation of such percentages (Figure 2). The ratio of polymer brush to polymer root may be affected by the experimental conditions, such as the component and shape of the polymer substrates, irradiation conditions, type of monomer for grafting, and solvents for grafting. To date, the effect of dose [21] and solvent viscosity [22] on the mole percentages of polymer brush and root grafted onto porous polyethylene sheet have been investigated. The polymer brush and root in porous polymeric adsorbents share respective roles. A smaller target (i.e., metal ions) binds to the polymer root as well as the brush, whereas a larger target (a protein) binds to only the polymer brush. The polymer root induces swelling of the polymer matrix, thereby minimizing the pore size reduction associated with the polymer brush. For the preparation of ion-exchange materials represented by polyethylene films [23] and nylon fibers [24,25], the polymer root must penetrate the amorphous domain across the film thickness before ion-exchange groups are introduced into the polymer root. This suggests that the polymer root plays a major role in governing the performance of ion-exchange materials. Control of the molar ratio of polymer brush to polymer root will enable the precise design of polymer chain-grafted functional materials.
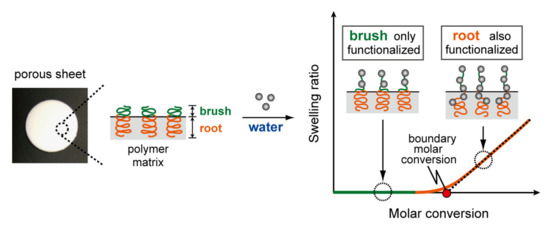
Figure 2.
Schematics of determination method for mole percentage of the polymer brush and root.
4. Preparation of Functional Materials Utilizing Radiation-Induced Graft Polymerization
Radiation generates radicals throughout the matrix of the polymeric substrates and polymerization starts from the radicals, resulting in producing polymer chain-grafted materials. Unlike other grafting techniques [4,5,6,7,8,9], radiation-induced radical polymerization does not require initiators. This enables utilization of various components and shapes of polymer substrates without losing their properties and shapes (Figure 3a). Therefore, we can select the component and shape of the polymer substrate suitable for specific applications for the preparation of adsorbents utilizing radiation-induced graft polymerization. Various polymeric materials such as polyethylene (PE) [26,27], nylon [28], cellulose [29,30], PE and polypropylene (PP) [31], poly(ethylene terephthalate) and PP [32] have been utilized as substrates. Polyimide and polyethylene terephthalate show a weak activity in radiation-induced graft polymerization, as they form stable resonance structures after irradiation. Various shapes of materials such as membranes [33,34,35], nonwoven fabrics [31,36], porous hollow-fiber membranes [37,38], fibers [25], plant fibers [39], and particles [40,41] have been utilized as substrates.
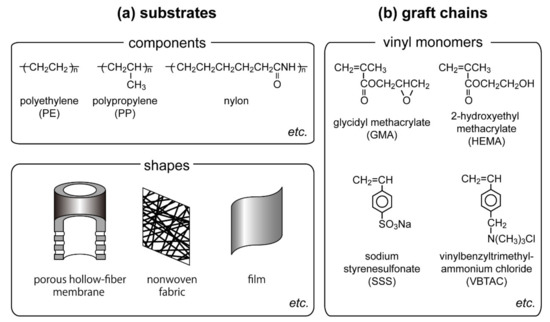
Figure 3.
Constituents for the preparation of functional materials by utilizing radiation-induced graft polymerization. (a) Utilizable components and shapes as substrates, (b) vinyl monomers for graft chains.
As for the graft chain, various vinyl monomers are applicable to graft polymerization (Figure 3b). According to the purpose of the applications, hydrophobic [42], hydrophilic [43], ion-exchangeable [44], and amphiphilic [45] polymer chains have been appended by radiation-induced graft polymerization. The direct introduction of functional groups with functional vinyl monomers is favorable in terms of industrial applications. For example, cation- and anion-exchange materials are readily prepared by grafting sodium styrene sulfonate (SSS, CH2=CHC6H4SO3Na) [44] and vinyl benzyl trimethyl ammonium chloride (VBTAC, CH2=CHC6H4CH2N(CH3)3Cl) [46] onto irradiated polymer substrates, respectively.
5. Charged Graft Chain
For the complex design of the polymer substrate surface, copolymerization or subsequent chemical modifications could be a countermeasure. For example, for obtaining hydrophilic graft chains from hydrophobic polymer substrates, copolymerization of neutral (uncharged) vinyl monomers with hydrophilic vinyl monomers provides ideal performances [47]. Hydrophilic vinyl monomers themselves show poor accessibility to the irradiated hydrophobic polymer substrate, leading to a low grafting rate. Copolymerization of neutral (uncharged) vinyl monomers and the hydrophilic vinyl monomers is an effective way to improve the accessibility [47]. In addition, reactive vinyl monomer grafting and subsequent chemical modification are effective for obtaining hydrophilic graft chains from hydrophobic polymer substrates. Glycidyl methacrylate (GMA, CH2=CCH3COOCH2CHOCH2) is a representative reactive vinyl monomer. Because GMA is a hydrophobic monomer, grafting from the hydrophobic polyethylene substrate can be easily achieved. GMA has an epoxy group which allows to readily react with a wide range of chemical groups such as amino and thiol groups. Therefore, hydrophilic ligands with the groups can be introduced [48,49], resulting in obtaining hydrophilic graft chains from a hydrophobic substrate. Thus, copolymerization or subsequent chemical modifications of the graft chain provides further diversity for the substrates.
Since 1984, we have been preparing polymeric adsorbents via radiation-induced graft polymerization [50]. For example, polymeric adsorbents in porous hollow-fiber forms collect valuable substances such as palladium and remove hazardous substances such as radioactive cesium. In addition, proteins that are partially ionizable as a function of the pH of an aqueous solution can be captured by polymeric adsorbents in porous hollow-fiber forms. Some epoxy groups of GMA graft chains are converted into the sulfonic acid group (SA group, –SO3H) by reaction with sodium sulfite and into the diethylamino group (DEA group, –N(C2H5)2) by reaction with diethylamine, as shown in Figure 4. Both SA and DEA groups are ionizable in an aqueous solution. Graft chains immobilizing the ionizable group are defined as charged or ion-exchange graft chains. Charged graft chains can capture ions in an aqueous solution. Furthermore, extension of the charged graft chains caused via electrostatic repulsion and hydration produces space for adsorption of proteins and for precipitation of inorganic compounds (Figure 5). In enormous combinations of substrates and graft chains, we focus on a charged graft chain in this review and introduce its recent progresses on functions, features, and usages in the following sections.
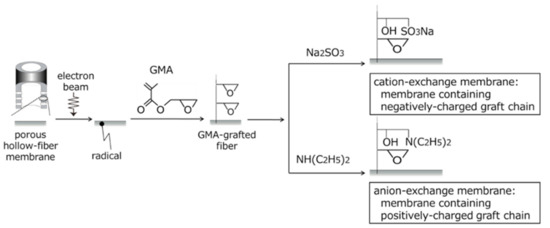
Figure 4.
Immobilization of negatively and positively charged graft chains onto porous hollow-fiber membrane.
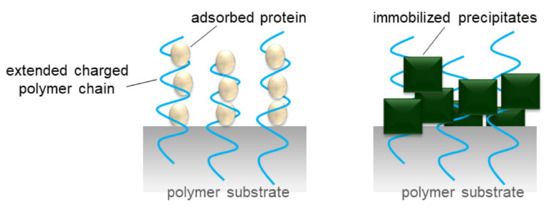
Figure 5.
Protein multilayer adsorption and inorganic-precipitate immobilization to the extended charged polymer chains.
6. Multilayer Adsorption of Protein to Charged Graft Chain
In the purification of proteins such as pharmaceuticals, foods, and biocatalysts, bead-packed columns have been widely used [51,52]. However, the protein adsorption rate decreased with increasing flow rate of the protein solution through the column. In our studies, to prepare a novel adsorbent capable of improving the protein adsorption rate, a commercially available polyethylene porous hollow-fiber membrane was selected as a polymer substrate. A hollow fiber has inner and outer diameters of 2 and 3 mm, respectively, a porosity of 70%, and an average pore size of 0.5 μm. The irradiation of an electron beam onto this hollow fiber produces radicals uniformly throughout the entire volume. Subsequently, an epoxy-group-containing vinyl monomer, GMA, was graft-polymerized uniformly throughout the entire volume of the hollow fiber. A porous hollow-fiber membrane with the DEA-group-containing graft chain (hereafter, DEA fiber) was prepared. One end of the DEA fiber was connected to a syringe containing a protein solution and the other end was sealed, as illustrated in Figure 6. The protein bovine serum albumin (BSA, pI 5) was dissolved in a buffer solution (pH 8.0) to establish a negative surface charge. The BSA solution was fed into the inner surface of the DEA fiber using a syringe pump at a constant flow rate in the range of 20 to 100 mL/h. During the permeation of the BSA solution through the pores, the BSA was captured by the positively charged polymer brush extending from the pore surface. The effluent penetrating the outer surface of the DEA fiber was continuously collected with fraction vials.
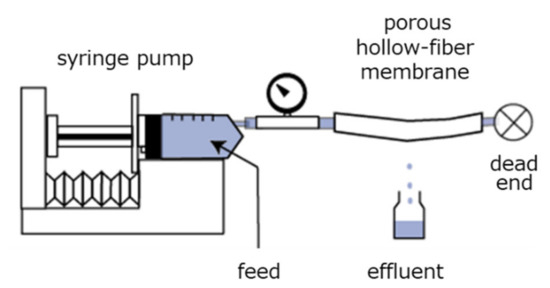
Figure 6.
Experimental apparatus for the determination of breakthrough curves of modified porous hollow-fiber membrane for protein adsorption.
The breakthrough curve, i.e., the BSA concentration of the effluent as a function of the accumulated effluent volume, is shown in Figure 7 [53]. The ordinate is the BSA concentration ratio of the effluent to the feed, whereas the abscissa is the accumulated effluent volume divided by the hollow-fiber volume excluding the lumen volume. The breakthrough curves overlapped irrespective of the flow rate, i.e., the residence time of the protein solution in the pores. This indicates that the higher the flow rate of the protein solution, the higher the overall adsorption rate of the protein. This behavior was ascribed to the negligible diffusional mass-transfer resistance of the protein through the pores.
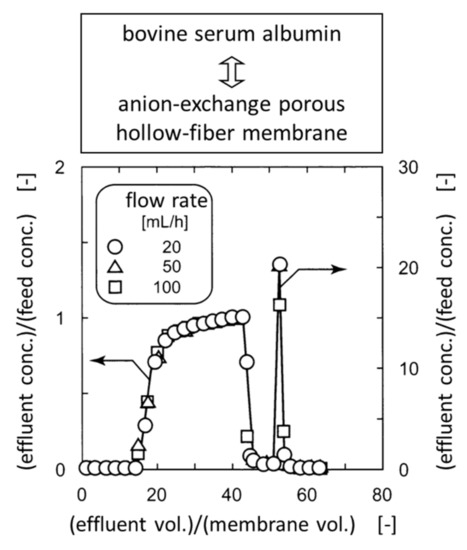
Figure 7.
Dimensionless breakthrough and elution curves of anion-exchange porous hollow-fiber membrane for bovine serum albumin adsorption.
The equilibrium binding capacity of BSA with respect to the BSA concentration of the feed was calculated by integrating the breakthrough curves and was found to be 0.22 g of BSA per gram of the DEA fiber. This value was five times the theoretical monolayer binding capacity, which was calculated on the assumption that BSA molecules are adsorbed onto the pore surface in the monolayer in an end-on orientation. This favorable result indicates that the charged polymer brushp captures BSA in multilayers and probably at multipoints. Other proteins also bind to the charged polymer brush with varying degrees of multilayer binding [54].
After some of the epoxy groups were converted into DEA groups, the remaining epoxy groups were reacted with 2-hydroxyamino ethanol to minimize the nonselective adsorption of proteins. The BSA that bound to the DEA fiber was quantitatively eluted by permeation with a 0.5 mol/L NaCl solution. In addition, the DEA fiber was conditioned with a buffer solution. The DEA fiber retained the same amount of BSA adsorbed during permeation.
High-rate and high-capacity adsorption of proteins was achieved through repeated adsorption and elution using the porous hollow-fiber membrane onto which the charged graft chain was immobilized. In 2011, Asahi Kasei Medical Co. commercialized an anion-exchange porous hollow-fiber membrane with the brand name “QyuSpeed D”. Membrane modules comprising the porous hollow-fiber membranes were used for the purification of antibody-based pharmaceuticals.
7. Extension and Shrinkage of Charged Graft Chain
A lysozyme solution was used to permeate from the inner surface to the outer surface of the SA fiber, i.e., a porous hollow-fiber membrane with immobilized SA-group-containing graft chains. Lysozyme (pI 11) was dissolved in a buffer solution (pH 9.0) to establish a positive charge. However, unlike the permeation of BSA solution through the DEA fiber, even at the upper limit of the operational pressure of the syringe pump that feeds the lysozyme solution to the inner surface of the SA fiber, the solution did not permeate through the pores. This is because the charged polymer brush markedly extends owing to electrostatic repulsions between strongly ionizable SA groups.
To restore the permeability of the SA fiber, a 5.0 mmol/L MgCl2 solution was used to permeate [55]. The volume of the effluent penetrating the outer surface of the SA fiber increased. Subsequently, the feed was switched to the lysozyme solution. As the amount of lysozyme adsorbed increased, the operational pressure required to maintain a constant flow rate of the lysozyme solution decreased. This phenomenon can be explained by considering the following. First, the SA-group-containing polymer brush crosslinked with divalent magnesium ions. Second, magnesium ions were replaced by lysozyme, a macromolecular cation. These two steps resulted in the shrinkage of the charged polymer brush (Figure 8).
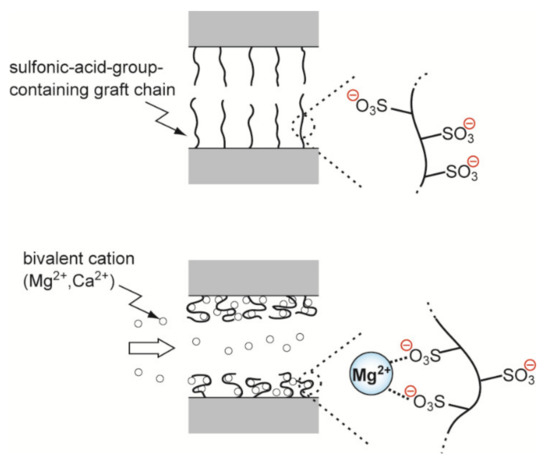
Figure 8.
Ionic crosslinking of sulfonic acid-type charged graft chains with magnesium ions.
The epoxy groups of the GMA graft chains on the porous hollow-fiber membrane were ring-opened with taurine (TA, NH2CH2CH2SO3H) instead of sodium sulfite (NaHSO3) [56]. Taurine is an amphoteric compound that has amino and sulfonic acid groups. The resultant taurine-immobilized porous hollow-fiber membrane (hereafter, TA fiber) exhibited a practical permeation flux without prepermeation of the MgCl2 aqueous solution. The sulfonic acid group is partially shielded by the amino groups. The decrease in the equilibrium binding capacity for lysozyme of the TA fiber by 70% compared with the SA fiber reflects the decrease in the degree of extension of the charged graft chain.
8. Immobilization of Precipitate Using Charged Graft Chain
Potassium metal hexacyanoferrates [57,58] and zeolites [59,60,61] are known as selective adsorbents for cesium ions. However, for the purpose of cesium-ion removal from the river or sea water, these adsorbents are not suitable because they are particle form and their collection from the contaminated water is not easy. A precipitate of insoluble cobalt ferrocyanide (CoFC) was formed in the polymer chain grafted onto a commercially available nylon 6 fiber [62]. Potassium metal ferrocyanide was reported as a selective inorganic adsorbent for cesium [57,63]. As shown in Figure 9, the impregnation scheme for the CoFC-immobilized fiber consists of six steps: (1) irradiation of gamma rays on nylon fiber, (2) graft polymerization of GMA onto the irradiated nylon fiber, (3) addition of triethylene diamine (TEDA) to the epoxy groups of the GMA graft chain, (4) conditioning with HCl aqueous solution, (5) adsorption of ferrocyanide ions (Fe(CN)64−) to the TEDA moiety, and (6) precipitation of insoluble cobalt ferrocyanide with cobalt chloride aqueous solution. The CoFC content of the resultant fiber, defined as a weight percentage of CoFC to the total mass of the product, was 12%.
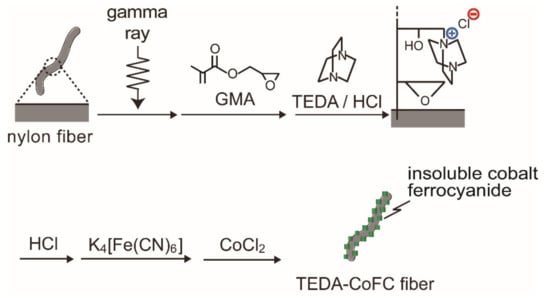
Figure 9.
Impregnation scheme for insoluble cobalt ferrocyanide onto nylon fiber.
The CoFC precipitate formed in the graft chain did not leak into the external liquid when the pH and ionic strength of the liquid varied in the ranges of 0 to 13 and 0 to 1.0 mol/L, respectively, and when rubbing stress was applied by hand. This stable immobilized structure is ascribed to the entanglement and penetration of the CoFC precipitate by the graft chain owing to the electrostatic attraction between the negative surface charge of the CoFC precipitate and the positive charge of the TEDA-moiety-containing graft chain [64].
A bobbin of the CoFC-immobilized fiber has been mass-produced from a bobbin of commercially available nylon 6 fiber by KJK Co. (Takasaki, Japan) by radiation-induced graft polymerization. From a bobbin of the CoFC-immobilized fiber, braids and wound filters are manufactured. Braids were immersed in water contaminated with radioactive cesium ions at the harbor of TEPCO Fukushima Daiichi Nuclear Power Plant, and wound filters were used for the filtration of the contaminated groundwater pumped from the subdrain installed at the Nuclear Power Plant. Since April 2015, the braids have been placed in the drainage system, side ditches, and wells to remove radioactive cesium ions and their colloids. The cesium-bound fiber is incinerated to reduce the volume of radioactive waste because the polymer substrate and graft chain are organic polymeric materials. The incinerability of the adsorbents is an additional advantage of the graft-type polymeric adsorbent over various inorganic adsorbents such as zeolites [65].
9. Conclusions
Radiation-induced graft polymerization is a promising technique to append polymer chains to the various components and shapes of polymer substrates. Especially, charged graft chains show unique functions, features, and usages. The degree of extension and shrinkage of the charged polymer brush or ionizable polymer brush is governed by internal factors such as the length and density of graft chains, and the density of charged groups, and by external factors such as the pH and ionic strength of the liquid surrounding the charged graft chains. In the case of a porous material used as the polymer substrate, the extension of the polymer root imbedded in the matrix of the polymer substrate resulted in the swelling of the entire volume of the polymer substrate, whereas that of the polymer brush stretching from the pore surface provided space for the adsorption of proteins and the precipitation of inorganic compounds. The space led to high-capacity binding and high-content immobilization. Radiation-induced graft polymerization enables us to select various shapes and components of commercially available polymers for the polymer substrate and to design the mass-production of the innovative polymeric adsorbents. The charged graft chains prepared by radiation-induced graft polymerization will be studied further to explore their additional practical applications.
Author Contributions
Conceived and designed the experiments: K.S. Performed the experiments and analyzed the data: R.I., S.A. Wrote the paper: R.I., S.A., K.S. All authors have confirmed the final version of the manuscript.
Funding
This work was partly supported by JSPS KAKENHI Grant Number 18K18404.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Ballantine, D.S.; Glines, A.; Metz, D.J.; Beher, J.; Mesrobian, R.B.; Restaino, A.J. G values of gamma-ray initiation of vinyl polymerization and their relation to graft copolymer formation. J. Polym. Sci. 1956, 19, 219–224. [Google Scholar] [CrossRef]
- Ballantine, D.; Glines, A.; Adler, G.; Metz, D.J. Graft copolymerization by pre-irradiation technique. J. Polym. Sci. 1959, 34, 419–438. [Google Scholar] [CrossRef]
- Chapiro, A. Préparation des copolymères greffés du polytetrafluoroéthylène (Teflon) par voie radiochimique. J. Polym. Sci. 1959, 34, 481–501. [Google Scholar] [CrossRef]
- Ohno, K.; Morinaga, T.; Koh, K.; Tsujii, Y.; Fukuda, T. Synthesis of Monodisperse Silica Particles Coated with Well-Defined, High-Density Polymer Brushes by Surface-Initiated Atom Transfer Radical Polymerization. Macromolecules 2005, 38, 2137–2142. [Google Scholar] [CrossRef]
- Kim, J.-B.; Bruening, M.L.; Baker, G.L. Surface-Initiated Atom Transfer Radical Polymerization on Gold at Ambient Temperature. J. Am. Chem. Soc. 2000, 122, 7616–7617. [Google Scholar] [CrossRef]
- Ishihara, R.; Katagiri, A.; Nakajima, T.; Matsui, R.; Komatsu, S.; Hosokawa, K.; Maeda, M.; Tomooka, Y.; Kikuchi, A. Design of a surface-functionalized power-free microchip for extracellular vesicle detection utilizing UV grafting. React. Funct. Polym. 2019, 142, 183–188. [Google Scholar] [CrossRef]
- Ishihara, R.; Yamaguchi, Y.; Tanabe, K.; Makino, Y.; Nishio, K. Preparation of Pt/WO3-coated polydimethylsiloxane membrane for transparent/flexible hydrogen gas sensors. Mater. Chem. Phys. 2019, 226, 226–229. [Google Scholar] [CrossRef]
- Baskaran, D.; Mays, J.W.; Bratcher, M.S. Polymer-Grafted Multiwalled Carbon Nanotubes through Surface-Initiated Polymerization. Angew. Chem. Int. Edit. 2004, 43, 2138–2142. [Google Scholar] [CrossRef]
- Matsuno, R.; Yamamoto, K.; Otsuka, H.; Takahara, A. Polystyrene- and Poly(3-vinylpyridine)-Grafted Magnetite Nanoparticles Prepared through Surface-Initiated Nitroxide-Mediated Radical Polymerization. Macromolecules 2004, 37, 2203–2209. [Google Scholar] [CrossRef]
- Han, B.; Kim, J.; Kim, Y.; Jeong, K.-Y. Industrial application of e-beam accelerators in Korea. J. Korean Phys. Soc. 2012, 61, 180–184. [Google Scholar] [CrossRef]
- Zhou, T.; Shao, R.; Chen, S.; He, X.; Qiao, J.; Zhang, J. A review of radiation-grafted polymer electrolyte membranes for alkaline polymer electrolyte membrane fuel cells. J. Power Sources 2015, 293, 946–975. [Google Scholar] [CrossRef]
- Chen, X.; Wang, Y.; Dai, G.; Peng, J.; Li, J.; Shi, M.; Zhai, M. Radiation grafting of glycidyl methacrylate and divinylbenzene onto polyethylene terephthalate fabrics for improving anti-dripping performance. Radiat. Phys. Chem. 2016, 127, 256–263. [Google Scholar] [CrossRef]
- Xing, L.; Liu, L.; Xie, F.; Huang, Y. Mutual irradiation grafting on indigenous aramid fiber-3 in diethanolamine and epichlorohydrin and its effect on interfacially reinforced epoxy composite. Appl. Surf. Sci. 2016, 375, 65–73. [Google Scholar] [CrossRef]
- Uezu, K.; Saito, K.; Furusaki, S.; Sugo, T.; Ishigaki, I. Radicals contributing to preirradiation graft polymerization onto porous polyethylene. Int. J. Radiat. Appl. Instrum. C Radiat. Phys. Chem. 1992, 40, 31–36. [Google Scholar] [CrossRef]
- Amamoto, Y.; Kikuchi, M.; Otsuka, H.; Takahara, A. Solvent-Controlled Formation of Star-like Nanogels via Dynamic Covalent Exchange of PSt-b-PMMA Diblock Copolymers with Alkoxyamine Units in the Side Chain. Macromolecules 2010, 43, 5470–5473. [Google Scholar] [CrossRef]
- Jonas, A.M.; Hu, Z.; Glinel, K.; Huck, W.T.S. Effect of Nanoconfinement on the Collapse Transition of Responsive Polymer Brushes. Nano Lett. 2008, 8, 3819–3824. [Google Scholar] [CrossRef] [PubMed]
- Silva, R.; Muniz, E.C.; Rubira, A.F. Maleimide Immobilized on a PE Surface: Preparation, Characterization and Application as a Free-Radical Photoinitiator. Langmuir 2009, 25, 873–880. [Google Scholar] [CrossRef]
- Sergeyeva, T.A.; Matuschewski, H.; Piletsky, S.A.; Bendig, J.; Schedler, U.; Ulbricht, M. Molecularly imprinted polymer membranes for substance-selective solid-phase extraction from water by surface photo-grafting polymerization. J. Chromatogr. A 2001, 907, 89–99. [Google Scholar] [CrossRef]
- Tarducci, C.; Kinmond, E.J.; Badyal, J.P.S.; Brewer, S.A.; Willis, C. Epoxide-Functionalized Solid Surfaces. Chem. Mater. 2000, 12, 1884–1889. [Google Scholar] [CrossRef]
- Gupta, B.; Plummer, C.; Bisson, I.; Frey, P.; Hilborn, J. Plasma-induced graft polymerization of acrylic acid onto poly(ethylene terephthalate) films: Characterization and human smooth muscle cell growth on grafted films. Biomaterials 2002, 23, 863–871. [Google Scholar] [CrossRef]
- Ishihara, R.; Uchiyama, S.; Ikezawa, H.; Yamada, S.; Hirota, H.; Umeno, D.; Saito, K. Effect of Dose on Mole Percentages of Polymer Brush and Root Grafted onto Porous Polyethylene Sheet by Radiation-Induced Graft Polymerization. Ind. Eng. Chem. Res. 2013, 52, 12582–12586. [Google Scholar] [CrossRef]
- Ishihara, R. Design of Capture Materials Utilizing Radiation-Induced Graft Polymerization. Kobunshi Ronbunshu 2018, 75, 456–467. [Google Scholar] [CrossRef]
- Asari, Y.; Shoji, N.; Miyoshi, K.; Umeno, D.; Saito, K. Electrodialysis of Sulfuric Acid with Cation-Exchange Membranes Prepared by Electron-Beam-Induced Graft Polymerization. J. Ion Exch. 2011, 22, 53–57. [Google Scholar] [CrossRef]
- Ikeda, K.; Umeno, D.; Saito, K.; Koide, F.; Miyata, E.; Sugo, T. Removal of Boron Using Nylon-Based Chelating Fibers. Ind. Eng. Chem. Res. 2011, 50, 5727–5732. [Google Scholar] [CrossRef]
- Ting, T.M.; Nasef, M.M.; Hashim, K. Modification of nylon-6 fibres by radiation-induced graft polymerisation of vinylbenzyl chloride. Radiat. Phys. Chem. 2015, 109, 54–62. [Google Scholar] [CrossRef]
- Choi, S.-H.; Nho, Y.C. Radiation-induced graft copolymerization of binary monomer mixture containing acrylonitrile onto polyethylene films. Radiat. Phys. Chem. 2000, 58, 157–168. [Google Scholar] [CrossRef]
- Terada, A.; Yuasa, A.; Tsuneda, S.; Hirata, A.; Katakai, A.; Tamada, M. Elucidation of dominant effect on initial bacterial adhesion onto polymer surfaces prepared by radiation-induced graft polymerization. Colloids Surf. B 2005, 43, 99–107. [Google Scholar] [CrossRef]
- Odian, G.; Sobel, M.; Rossi, A.; Klein, R.; Acker, T. Radiation-induced graft polymerization of styrene to nylon. J. Polym. Sci. Part A Gen. Pap. 1963, 1, 639–654. [Google Scholar] [CrossRef]
- Imrišováa, D.; Maryška, S. Study of radiation-induced graft polymerization of vinyl monomers to cellulose by infrared spectroscopy. II. Cellulose–polystyrene copolymers. J. Appl. Polym. Sci. 1968, 12, 2007–2011. [Google Scholar] [CrossRef]
- Guthrie, J.T. Developments in radiation-induced graft polymerization to cellulosics. Polymer 1975, 16, 134–150. [Google Scholar] [CrossRef]
- Kavaklı, P.A.; Seko, N.; Tamada, M.; Güven, O. Radiation-induced graft polymerization of glycidyl methacrylate onto PE/PP nonwoven fabric and its modification toward enhanced amidoximation. J. Appl. Polym. Sci. 2007, 105, 1551–1558. [Google Scholar] [CrossRef]
- Shtanko, N.I.; Kabanov, V.Y.; Apel, P.Y.; Yoshida, M. The use of radiation-induced graft polymerization for modification of polymer track membranes. Nucl. Instrum. Methods Phys. Res. B Beam Interact. Mater. Atoms. 1999, 151, 416–422. [Google Scholar] [CrossRef]
- Shin, I.H.; Hong, S.; Lim, S.J.; Son, Y.-S.; Kim, T.-H. Surface modification of PVDF membrane by radiation-induced graft polymerization for novel membrane bioreactor. J. Ind. Eng. Chem. 2017, 46, 103–110. [Google Scholar] [CrossRef]
- Omichi, H.; Okamoto, J. Synthesis of ion-exchange membranes by radiation-induced multiple grafting of methyl α,β,β-trifluoroacrylate. J. Polym. Sci. Polym. Chem. Ed. 1982, 20, 521–528. [Google Scholar] [CrossRef]
- Yamagishi, H.; Saito, K.; Furusaki, S.; Sugo, T.; Okamoto, J. Permeability of methyl methacrylate grafted cellulose triacetate membrane. Chem. Mater. 1990, 2, 705–708. [Google Scholar] [CrossRef]
- Kim, M.; Saito, K. Preparation of silver-ion-loaded nonwoven fabric by radiation-induced graft polymerization. React. Funct. Polym. 1999, 40, 275–279. [Google Scholar] [CrossRef]
- Kim, M.; Saito, K. Radiation-induced graft polymerization and sulfonation of glycidyl methacrylate on to porous hollow-fiber membranes with different pore sizes. Radiat. Phys. Chem. 2000, 57, 167–172. [Google Scholar] [CrossRef]
- Kubota, N.; Konno, Y.; Saito, K.; Sugita, K.; Watanabe, K.; Sugo, T. Module performance of anion-exchange porous hollow-fiber membranes for high-speed protein recovery. J. Chromatogr. A 1997, 782, 159–165. [Google Scholar] [CrossRef]
- Madrid, J.F.; Nuesca, G.M.; Abad, L.V. Gamma radiation-induced grafting of glycidyl methacrylate (GMA) onto water hyacinth fibers. Radiat. Phys. Chem. 2013, 85, 182–188. [Google Scholar] [CrossRef]
- Pasanphan, W.; Rattanawongwiboon, T.; Rimdusit, P.; Piroonpan, T. Radiation-induced graft copolymerization of poly(ethylene glycol) monomethacrylate onto deoxycholate-chitosan nanoparticles as a drug carrier. Radiat. Phys. Chem. 2014, 94, 199–204. [Google Scholar] [CrossRef]
- Uchiyama, S.; Sasaki, T.; Ishihara, R.; Fujiwara, K.; Sugo, T.; Umeno, D.; Saito, K. High-resolution separation of neodymium and dysprosium ions utilizing extractant-impregnated graft-type particles. J. Chromatogr. A 2018, 1533, 10–16. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, W.-C.; Wada, Y.; Mitobe, T.; Mitomo, H.; Seko, N.; Tamada, M. Effect of hydrophilic and hydrophobic monomers grafting on microbial poly(3-hydroxybutyrate). J. Taiwan Inst. Chem. Eng. 2009, 40, 413–417. [Google Scholar] [CrossRef]
- Kim, M.; Kojima, J.; Saito, K.; Furusaki, S.; Sugo, T. Reduction of Nonselective Adsorption of Proteins by Hydrophilization of Microfiltration Membranes by Radiation-Induced Grafting. Biotechnol. Progr. 1994, 10, 114–120. [Google Scholar] [CrossRef] [PubMed]
- Sugiyama, S.; Tsuneda, S.; Saito, K.; Furusaki, S.; Sugo, T.; Makuuchi, K. Attachment of sulfonic acid groups to various shapes of polyethylene, polypropylene and polytetrafluoroethylene by radiation-induced graft polymerization. React. Polym. 1993, 21, 187–191. [Google Scholar] [CrossRef]
- Tanaka, H.; Iwasaki, I.; Kunai, Y.; Sato, N.; Matsuyama, T. Radiation-induced graft polymerization of amphiphilic monomers with different polymerization characteristics onto hydrophobic polysilane. Radiat. Phys. Chem. 2011, 80, 884–889. [Google Scholar] [CrossRef][Green Version]
- Kolhe, S.M.; Kumar, A. Radiation-induced grafting of vinyl benzyl trimethyl ammonium chloride onto nylon-6 fabric. Radiat. Phys. Chem. 2007, 76, 901–906. [Google Scholar] [CrossRef]
- Tsuneda, S.; Saito, K.; Furusaki, S.; Sugo, T.; Makuuichi, K. Simple Introduction of Sulfonic Acid Group onto Polyethylene by Radiation-Induced Cografting of Sodium Styrenesulfonate with Hydrophilic Monomers. Ind. Eng. Chem. Res. 1993, 32, 1464–1470. [Google Scholar] [CrossRef]
- Kim, M.; Kiyohara, S.; Konishi, S.; Tsuneda, S.; Saito, K.; Sugo, T. Ring-opening reaction of poly-GMA chain grafted onto a porous membrane. J. Membrane Sci. 1996, 117, 33–38. [Google Scholar] [CrossRef]
- Asai, S.; Hanzawa, Y.; Konda, M.; Suzuki, D.; Magara, M.; Kimura, T.; Ishihara, R.; Saito, K.; Yamada, S.; Hirota, H. Preparation of Microvolume Anion-Exchange Cartridge for Inductively Coupled Plasma Mass Spectrometry-Based Determination of 237Np Content in Spent Nuclear Fuel. Anal. Chem. 2016, 88, 3149–3155. [Google Scholar] [CrossRef]
- Saito, K.; Fujiwara, K.; Sugo, T. Innovative Polymeric Adsorbents; Springer: Singapore, 2018; pp. 1–200. [Google Scholar]
- Luo, J.; Zhou, W.; Su, Z.; Ma, G.; Gu, T. Comparison of fully-porous beads and cored beads in size exclusion chromatography for protein purification. Chem. Eng. Sci. 2013, 102, 99–105. [Google Scholar] [CrossRef]
- Jain, P.; Vyas, M.K.; Geiger, J.H.; Baker, G.L.; Bruening, M.L. Protein Purification with Polymeric Affinity Membranes Containing Functionalized Poly(acid) Brushes. Biomacromolecules 2010, 11, 1019–1026. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Tsuneda, S.; Saito, K.; Furusaki, S.; Sugo, T. High-throughput processing of proteins using a porous and tentacle anion-exchange membrane. J. Chromatogr. A 1995, 689, 211–218. [Google Scholar] [CrossRef]
- Matoba, S.; Tsuneda, S.; Saito, K.; Sugo, T. Highly Efficient Enzyme Recovery Using a Porous Membrane with Immobilized Tentacle Polymer Chains. Nat. Biotechnol. 1995, 13, 795–797. [Google Scholar] [CrossRef]
- Sasagawa, N.; Saito, K.; Sugita, K.; Kunori, S.I.; Sugo, T. Ionic crosslinking of SO3H-group-containing graft chains helps to capture lysozyme in a permeation mode. J. Chromatogr. A 1999, 848, 161–168. [Google Scholar] [CrossRef]
- Miyoshi, K.; Saito, K.; Shiraishi, T.; Sugo, T. Introduction of taurine into polymer brush grafted onto porous hollow-fiber membrane. J. Membrane Sci. 2005, 264, 97–103. [Google Scholar] [CrossRef]
- Barton, G.B.; Hepworth, J.L.; McClanahan, E.D.; Moore, R.L.; Tuyl, H.H.V. Chemical Processing Wastes. Recovering Fission Products. Ind. Eng. Chem. 1958, 50, 212–216. [Google Scholar] [CrossRef]
- Lehto, J.; Paajanen, R.; Harjula, R. Selectivity of potassium cobalt hexacyanoferrate (II) for alkali and alkaline earth metal ions. J. Radioanal. Nucl. Chem. 1992, 164, 39–46. [Google Scholar] [CrossRef]
- Mimura, H.; Kanno, T. Distribution and Fixation of Cesium and Strontium in Zeolite A and Chabazite. J. Nucl. Sci. Technol. 1985, 22, 284–291. [Google Scholar] [CrossRef]
- Borai, E.H.; Harjula, R.; malinen, L.; Paajanen, A. Efficient removal of cesium from low-level radioactive liquid waste using natural and impregnated zeolite minerals. J. Hazard. Mater. 2009, 172, 416–422. [Google Scholar] [CrossRef]
- El-Kamash, A.M.; El-Naggar, M.R.; El-Dessouky, M.I. Immobilization of cesium and strontium radionuclides in zeolite-cement blends. J. Hazard. Mater. 2006, 136, 310–316. [Google Scholar] [CrossRef]
- Ishihara, R.; Fujiwara, K.; Harayama, T.; Okamura, Y.; Uchiyama, S.; Sugiyama, M.; Someya, T.-a.; Amakai, W.; Umino, S.; Ono, T.; et al. Removal of Cesium Using Cobalt-Ferrocyanide-Impregnated Polymer-Chain-Grafted Fibers. J. Nucl. Sci. Technol. 2011, 48, 1281–1284. [Google Scholar] [CrossRef][Green Version]
- Watari, K.; Izawa, M. Separation of Radiocesium by Copper Ferrocyanide-Anion Exchange Resin. J. Nucl. Sci. Technol. 1965, 2, 321–322. [Google Scholar] [CrossRef]
- Goto, S.; Umino, S.; Amakai, W.; Fujiwara, K.; Sugo, T.; Kojima, T.; Kawai-Noma, S.; Umeno, D.; Saito, K. Impregnation structure of cobalt ferrocyanide microparticles by the polymer chain grafted onto nylon fiber. J. Nucl. Sci. Technol. 2016, 53, 1251–1255. [Google Scholar] [CrossRef]
- Mimura, H.; Kimura, M.; Akiba, K.; Onodera, Y. Separation of Cesium and Strontium by Potassium Nickel. J. Nucl. Sci. Technol. 1999, 36, 307–310. [Google Scholar] [CrossRef][Green Version]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).