The Historical Case for and the Future Study of Antibiotic-Resistant Scrub Typhus
Abstract
:1. Introduction
2. Scrub Typhus and Antibiotic Resistance
2.1. Reports of Antibiotic-Resistant Scrub Typhus
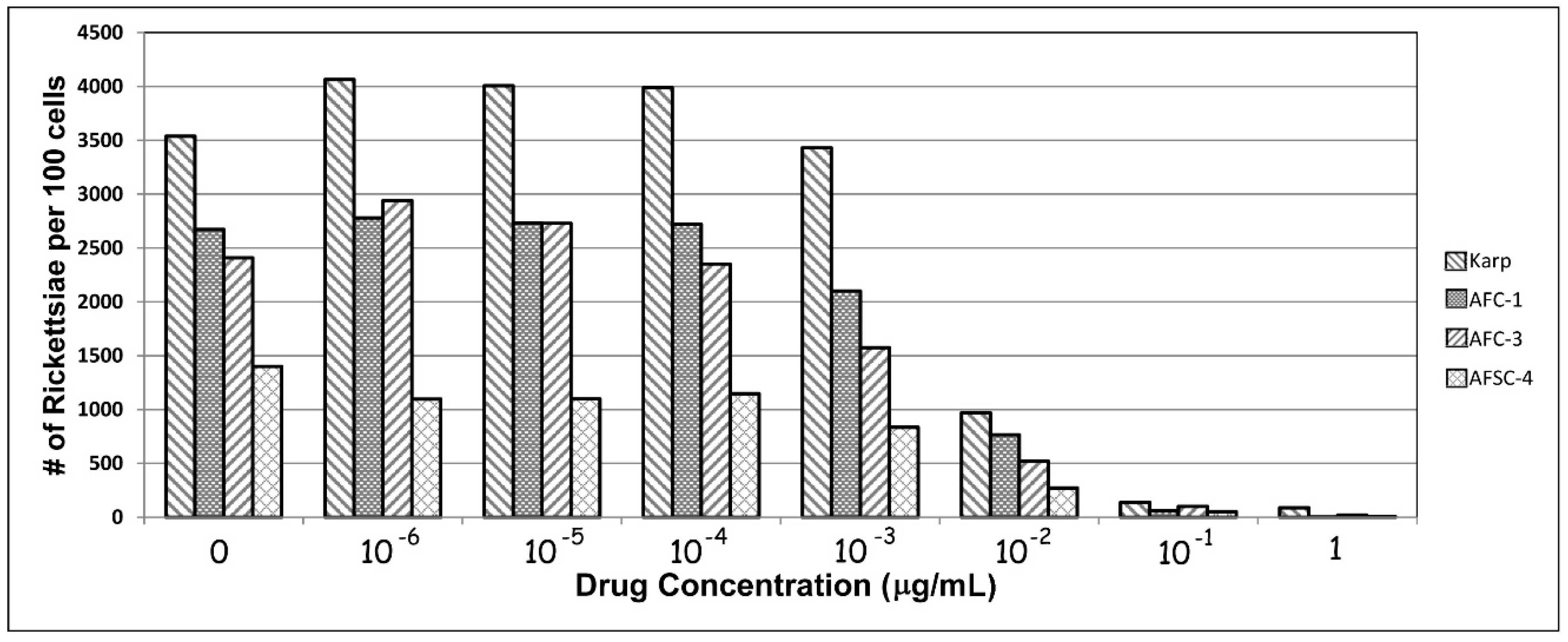
2.2. Measuring Antibiotic-Resistant Scrub Typhus
2.3. Reports of Human Antibiotic Resistance
2.4. Theoretical Aspects of Antibiotic-Resistant Scrub Typhus
2.5. Genomics of Antibiotic Resistance
2.6. Search for Specific Genes That Can Contribute to Resistance
2.7. Horizontal Transfer of Resistance
3. Discussion/Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References and Note
- Kelly, D.J. Orientia tsutsugamushi. In Antimicrobial Therapy and Vaccines; Williams & Wilkins Inc.: Baltimore, MD, USA, 1999; pp. 551–554. ISBN 0-683-30061-X. [Google Scholar]
- Kelly, D.J.; Fuerst, P.A.; Ching, W.-M.; Richards, A.L. Scrub typhus: The geographic distribution of phenotypic and genotypic variants of Orientia tsutsugamushi. Clin. Infect. Dis. 2009, 48, S203–S230. [Google Scholar] [CrossRef] [PubMed]
- Strickman, D.; Sheer, T.; Salata, K.; Hershey, J.; Dasch, G.; Kelly, D.; Kuschner, R. In vitro effectiveness of azithromycin against doxycycline-resistant and susceptible strains of Rickettsia tsutsugamushi, etiologic agent of scrub typhus. Antimicrob. Agent Chemother. 1995, 39, 2406–2410. [Google Scholar]
- Watt, G.; Kantipong, P.; Jongsakul, K.; Watcharapichat, P.; Phulsuksoombati, D. Azithromycin activities against Orientia tsutsugamushi strains isolated in cases of scrub typhus in northern Thailand. Antimicrob. Agents Chemother. 1999, 43, 2817–2818. [Google Scholar] [PubMed]
- Kim, Y.-S.; Yun, H.-J.; Shim, S.K.; Koo, S.H.; Kim, S.Y.; Kim, S. A comparative trial of a single dose of azithromycin versus doxycycline for the treatment of mild scrub typhus. Clin. Infect. Dis. 2004, 39, 1329–1335. [Google Scholar] [CrossRef] [PubMed]
- Jones, W.S. Chinese liaison detail. In Crisis Fleeting; Stone, J.H., Ed.; Office of the Surgeon General: Washington, DC, USA, 1969; pp. 123–124. [Google Scholar]
- Balcells, M.E.; Rabagliati, R.; Garcia, P.; Poggi, H.; Oddo, D.; Concha, M.; Abarca, K.; Jiang, J.; Kelly, D.J.; Richards, A.L.; et al. Endemic scrub typhus-like illness, Chile. Emer. Infect. Dis. 2011, 17, 1659–1663. [Google Scholar] [CrossRef]
- Kelly, D.J.; Foley, D.H.; Richards, A.L. A spatiotemporal database to track human scrub typhus using the VectorMap application. PLoS Negl. Trop. Dis. 2015, 9, e0004161. [Google Scholar] [CrossRef]
- Watt, G.; Chouriyagune, C.; Ruangweerayud, R.; Watcharapichat, P.; Phulsuksombati, D.; Jongsakul, K.; Teja-Isavadharm, P.; Bhodhidatta, D.; Corcoran, K.D.; Dasch, G.A.; et al. Scrub typhus infections poorly responsive to antibiotics in northern Thailand. Lancet 1996, 348, 86–89. [Google Scholar] [CrossRef] [PubMed]
- Watt, G.; Parola, P. Scrub typhus and tropical rickettsioses. Curr. Opin. Infect. Dis. 2003, 16, 429–436. [Google Scholar] [CrossRef] [PubMed]
- Mathai, E.; Rolain, J.M.; Verghese, G.M.; Abraham, O.C.; Mathai, D.; Mathai, M.; Raoult, D. Outbreak of scrub typhus in southern India during the cooler months. Ann. N. Y. Acad. Sci. 2003, 990, 359–364. [Google Scholar] [CrossRef] [PubMed]
- Tantibhedhyangkul, W.; Angelakis, E.; Tongyoo, N.; Newton, P.N.; Moore, C.E.; Phetsouvanh, R.; Raoult, D.; Rolain, J.-M. Intrinsic fluoroquinolone resistance in Orientia tsutsugamushi. Int. J. Antimicrob. Agents 2010, 35, 338–341. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, M.S.; Baek, J.H.; Lee, J.-S.; Chung, M.-H.; Lee, S.M.; Kang, J.-S. High in vitro infectivity of a doxycycline-insensitive strain of Orientia tsutsugamushi. Infect. Chemother. 2013, 45, 431–434. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.-H.; Chung, E.J.; Kim, E.-G.; Sea, J.H. A case of doxycycline-resistant tsutsugamushi meningoencephalitis. Neurol. Asia 2014, 19, 205–206. [Google Scholar]
- Thipmontree, W.; Tantibhedhyangkul, W.; Silpasakorn, S.; Wongsawat, E.; Waywa, D.; Suputtamongkol, Y. Scrub typhus in northeastern Thailand: eschar distribution, abnormal electrocardiographic findings and predictors of fatal outcome. Am. J. Trop. Med. Hyg. 2016, 95, 769–773. [Google Scholar] [CrossRef] [PubMed]
- Izzard, L.; Fuller, A.; Blacksell, S.D.; Paris, D.H.; Richards, A.L.; Aukkanit, N.; Nguyen, C.; Jiang, J.; Fenwick, S.; Day, N.P. Isolation of a novel Orientia species (O. chuto sp. nov.) from a patient infected in Dubai. J. Clin. Microbiol. 2010, 48, 4404–4409. [Google Scholar] [CrossRef] [PubMed]
- Weitzel, T.; Dittrich, S.; López, J.; Phuklia, W.; Martinez-Valdebenito, C.; Velásquez, K.; Blacksell, S.D.; Paris, D.H.; Abarca, K. Endemic scrub typhus in South America. N. Engl. J. Med. 2016, 375, 954–961. [Google Scholar] [CrossRef] [PubMed]
- Kocher, C.; Jiang, J.; Morrison, A.C.; Castillo, R.; Leguia, M.; Loyola, S.; Ampuero, J.S.; Cespedes, M.; Halsey, E.S.; Bausch, D.G.; et al. Serologic evidence of scrub typhus in the Peruvian Amazon. Emerg. Infect. Dis. 2017, 23, 1389–1391. [Google Scholar] [CrossRef] [PubMed]
- Giroud, P.; Jadin, J. The presence of antibodies against Rickettsia orientalis in indigenous and Asian (peoples) living in Ruanda-Urundi (Belgian Congo). Bull. Soc. Pathol. Exot. 1951, 44, 50–51. [Google Scholar]
- Osuga, K.; Kimura, M.; Goto, H.; Shimada, K.; Suto, T. A case of tsutsugamushi disease probably contracted in Africa. Eur. J. Clin. Microbiol. Infect. Dis. 1991, 10, 95–96. [Google Scholar] [CrossRef] [PubMed]
- Ghorbani, R.P.; Ghorbani, A.J.; Jain, M.K.; Walker, D.H. A case of scrub typhus probably acquired in Africa. Clin. Infect. Dis. 1997, 25, 1473–1474. [Google Scholar] [CrossRef] [PubMed]
- Groen, J.; Dolmans, W.; Ligthelm, R.J. Scrub and murine typhus among Dutch travellers. Infection 1999, 27, 291–292. [Google Scholar] [PubMed]
- Cosson, J.F.; Galan, M.; Bard, E.; Razzauti, M.; Bernard, M.; Morand, S.; Brouat, C.; Dalecky, A.; Ba, K.; Charbonnel, N.; et al. Detection of Orientia sp. DNA in rodents from Asia, West Africa, and Europe. Parasites Vectors 2015, 8, 14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thiga, J.W.; Mutai, B.; Eyako, W.; Ng’ang’a, Z.; Jiang, J.; Richards, A.L.; Waitumbi, J.N. High sero-prevalence and IgG titers for spotted fever and scrub typhus in patients with febrile illness in Kenya. Emerg. Infect. Dis. 2015, 21, 688–691. [Google Scholar] [CrossRef] [PubMed]
- Horton, K.C.; Jiang, J.; Maina, A.; Dueger, E.; Zayed, A.; Ahmed, A.A.; Guillermo Pimentel, G.; Richards, A.L. Evidence of Rickettsia and Orientia infections among abattoir workers in Djibouti. Am. J. Trop. Med. Hyg. 2016, 95, 462–465. [Google Scholar] [CrossRef] [PubMed]
- Maina, A.N.; Farris, C.N.; Odhiambo, A.; Jiang, J.; Laktabai, J.; Armstrong, J.; Holland, T.; Richards, A.L.; O’Meara, W.P. Q fever, scrub typhus, and rickettsial diseases in children, Kenya, 2011–2012. Emerg. Infect. Dis. 2016, 22, 883–886. [Google Scholar] [CrossRef] [PubMed]
- Luce-Fedrow, A.; Mullins, K.; Jiang, J.; Richards, A. Strategies for detecting rickettsiae and diagnosing rickettsial diseases. Future Microbiol. 2015, 10, 537–564. [Google Scholar] [CrossRef] [PubMed]
- Brown, G.W.; Robinson, D.M.; Huxsoll, D.L. Scrub typhus: A common cause of illness in indigenous populations. Trans. R. Soc. Trop. Med. Hyg. 1976, 70, 444–448. [Google Scholar] [CrossRef]
- Watt, G.; Strickman, D. Life threatening scrub typhus in a traveler returning from Thailand. Clin. Infect. Dis. 1994, 18, 624–626. [Google Scholar] [CrossRef] [PubMed]
- Silpapojakul, K. Scrub typhus in the western Pacific region. Ann. Acad. Med. Singap. 1997, 26, 794–800. [Google Scholar] [PubMed]
- Corwin, A.; Sonderquist, R.; Suwanabun, N.; Sattabongkot, J.; Martin, L.; Kelly, D.; Beecham, J. Scrub typhus and military operations in Indochina. Clin. Infect. Dis. 1999, 29, 940–941. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Marienau, K.J.; May, L.A.; Beecham, H.J., III; Wilkinson, R.; Ching, W.M.; Richards, A.L. Laboratory diagnosis of two scrub typhus outbreaks at Camp Fuji, Japan in 2000 and 2001 by enzyme-linked immunosorbent assay, rapid flow assay, and Western blot assay using outer membrane 56-kD recombinant proteins. Am. J. Trop. Med. Hyg. 2003, 69, 60–66. [Google Scholar] [PubMed]
- Rodkvamtook, W.; Ruang-areerate, T.; Gaywee, J.; Richards, A.L.; Jeamwattanalert, P.; Bodhidatta, D.; Sangjun, N.; Prasartvit, A.; Jatisatienr, A.; Jatisatienr, C. Isolation and characterization of Orientia tsutsugamushi from rodents captured following a scrub typhus outbreak at a military training base, Bothong District, Chonburi Province, central Thailand. Am. J. Trop. Med. Hyg. 2011, 84, 599–607. [Google Scholar] [CrossRef] [PubMed]
- Sheehy, T.W.; Hazlett, D.; Turk, R.E. Scrub typhus: A comparison of chloramphenicol and tetracycline in its treatment. Arch. Intern. Med. 1973, 132, 77–80. [Google Scholar] [CrossRef] [PubMed]
- Koh, G.C.K.W.; Maude, R.J.; Paris, D.H.; Newton, P.N.; Blacksell, S.D. Review: Diagnosis of scrub typhus. Am. J. Trop. Med. Hyg. 2010, 82, 368–370. [Google Scholar] [CrossRef] [PubMed]
- Taylor, A.J.; Paris, D.H.; Newton, P.N. A systematic review of mortality from untreated scrub typhus (Orientia tsutsugamushi). PLoS Negl. Trop. Dis. 2015, 9, e0003971. [Google Scholar] [CrossRef] [PubMed]
- Philip, C.B. Tsutsugamushi disease (scrub typhus) in World War II. J. Parasitol. 1948, 34, 161–191. [Google Scholar] [CrossRef]
- Sayen, J.J.; Pond, H.S.; Forrester, J.S.; Wood, F.C. Scrub typhus in Assam and Burma: A clinical study of 616 cases. Medicine 1946, 25, 155–214. [Google Scholar] [CrossRef] [PubMed]
- Smadel, J.E.; Woodward, T.E.; Ley, H.L., Jr.; Philip, C.B. Chloromycetin in the treatment of scrub typhus. Science 1948, 108, 160–161. [Google Scholar] [CrossRef] [PubMed]
- Oaks, S.C., Jr.; Ridgway, R.L.; Shirai, A.; Twartz, J.C. Scrub Typhus; Bull No. 21; Institute for Medical Research: Kuala Lumpur, Malaysia, 1983. [Google Scholar]
- Smadel, J.E.; Jackson, E.B.; Cruise, A.B. Chloromycetin in experimental rickettsial infections. J. Immunol. 1949, 62, 49–65. [Google Scholar] [PubMed]
- Bailey, C.A.; Ley, H.L., Jr. The treatment and prophylaxis of scrub typhus with antibiotics. Ann. N. Y. Acad. Sci. 1952, 55, 983–991. [Google Scholar] [CrossRef] [PubMed]
- Smadel, J.E.; Ley, H.L.; Diercks, F.H.; Cameron, J.A.P. Persistence of Rickettsia tsutsugamushi in tissues of patients recovered from scrub typhus. Am. J. Hyg. 1952, 56, 294–302. [Google Scholar] [PubMed]
- Berman, S.J.; Kundin, W.D. Scrub typhus in South Vietnam: A study of 87 cases. Ann. Intern. Med. 1973, 7, 26–30. [Google Scholar] [CrossRef]
- Brown, G.W.; Saunders, J.P.; Singh, S.; Huxsoll, D.L.; Shirai, A. Single dose doxycycline therapy for scrub typhus. Trans. R. Soc. Trop. Med. Hyg. 1978, 72, 412–416. [Google Scholar] [CrossRef]
- Olson, J.G.; Bourgeois, A.L.; Fang, R.C.Y.; Dennis, D. Risk of relapse associated with doxycycline therapy for scrub typhus. In Rickettsiae and Rickettsial Diseases; Academic Press Inc.: Cambridge, MA, USA, 1981; pp. 201–210. [Google Scholar]
- Watt, G.; Kantipong, P.; Jongsakul, K.; Watcharapichat, P.; Phulsuksombati, D.; Strickman, D. Doxycycline and rifampicin for mild scrub-typhus infections in northern Thailand: A randomised trial. Lancet 2000, 356, 1057–1061. [Google Scholar] [CrossRef]
- Brown, G.W.; Shirai, A.; Jegathesan, M.; Burke, D.S.; Twartz, J.C.; Saunders, J.P.; Huxsoll, D.L. Febrile illness in Malaysia—An analysis of 1629 hospitalized patients. Am. J. Trop. Med. Hyg. 1984, 33, 311–315. [Google Scholar] [CrossRef] [PubMed]
- Alanis, A.J. Resistance to antibiotics: Are we in the post-antibiotic era? Arch. Med. Res. 2005, 36, 697–705. [Google Scholar] [CrossRef] [PubMed]
- Kelly, D.J.; Richards, A.L.; Temenak, J.; Strickman, D.; Dasch, G.A. The past and present threat of rickettsial diseases to military medicine and international public health. Clin. Infect. Dis. 2002, 34, S145–S169. [Google Scholar] [CrossRef] [PubMed]
- McClain, J.B.; Joshi, B.; Rice, R. Chloramphenicol, gentamycin, and ciprofloxacin against murine scrub typhus. Antimicrob. Agents Chemother. 1988, 32, 285–286. [Google Scholar] [CrossRef] [PubMed]
- Eaton, M.; Cohen, M.T.; Shlim, D.R.; Innis, B. Ciprofloxacin treatment of typhus. JAMA 1989, 262, 772–773. [Google Scholar] [CrossRef] [PubMed]
- Raoult, D. Antimicrobial activity against obligate intracellular bacteria. Trends Microbiol. 2001, 9, 14. [Google Scholar] [CrossRef]
- Diene, S.M.; Abat, C.; Rolain, J.-M.; Raoult, D. How artificial is the antibiotic resistance definition? Lancet 2017, 17, 690. [Google Scholar] [CrossRef]
- Chosewood, L.C.; Wilson, D.E. (Eds.) Section VIII-D: Rickettsial Agents. In Biosafety in Microbiological and Biomedical Laboratories (BMBL), 5th ed.; HHS Publication: Rockville, MD, USA, 2009; pp. 195–199. [Google Scholar]
- Barker, L.F.; Patt, J.K.; Hopps, H.E. Titrations and neutralization of Rickettsia tsutsugamushi in tissue culture. J. Immunol. 1968, 100, 825–830. [Google Scholar] [PubMed]
- Wisseman, C.L., Jr.; Waddell, A.D.; Walsh, W.T. In vitro studies of the action of antibodies on Rickettsia prowazeki by two basic methods of cell culture. J. Infect. Dis. 1974, 130, 564–574. [Google Scholar] [CrossRef] [PubMed]
- Kelly, D.J.; Strickman, D.; Salata, K.; Hershey, J. Rickettsia tsutsugamushi infection in cell culture: Antibiotic susceptibility determined by flow cytometry. Am. J. Trop. Med. Hyg. 1995, 53, 602–606. [Google Scholar] [CrossRef] [PubMed]
- Raoult, D.; Drancourt, M. Antimicrobial therapy of rickettsial diseases. Antimicrob. Agents Chemother. 1991, 35, 2457–2462. [Google Scholar] [CrossRef] [PubMed]
- McOrist, S. Obligate intracellular bacteria and antibiotic resistance. Trends Microbiol. 2000, 8, 483–485. [Google Scholar] [CrossRef]
- Strickman, D. Drug resistant scrub typhus. In Proceedings of the 4th International Symposium on Public Health, Pusan, Korea, 11 September 1996; Institute of Public Health, Kosin University: Busan, Korea, 1996; pp. 83–92. [Google Scholar]
- Singh, P. Scrub typhus, a case report: Military and regional significance. Med. J. Armed Forces India 2004, 60, 89–90. [Google Scholar] [CrossRef]
- Takahashi, M.; Murata, M.; Misumi, H.; Hori, E.; Kawamura, A., Jr.; Tanaka, H. Failed vertical transmission of Rickettsia tsutsugamushi (Rickettsiales: Rickettsiaceae) acquired from rickettsemic mice by Leptotrombidium pallidum (Acari: Trombiculidae). J. Med. Entomol. 1994, 31, 212–216. [Google Scholar] [CrossRef] [PubMed]
- Rosenberg, R. Drug-resistant scrub typhus: Paradigm and paradox. Parasitol. Today 1997, 13, 131–132. [Google Scholar] [CrossRef]
- Traub, R.; Wisseman, C.L., Jr.; Jones, M.R.; O’Keefe, J.J. The acquisition of Rickettsia tsutsugamushi by chiggers (trombiculid mites) during the feeding process. Ann. N. Y. Acad. Sci. 1975, 266, 91–113. [Google Scholar] [CrossRef] [PubMed]
- D’Costa, V.M.; King, C.E.; Kalan, L.; Morar, M.; Sung, W.W.L.; Schwarz, C.; Froese, D.; Zazula, G.; Calmels, F.; Debruyne, R.; et al. Antibiotic resistance is ancient. Nature 2011, 477, 457–460. [Google Scholar] [CrossRef] [PubMed]
- Olaitan, A.O.; Rolain, J.M. Ancient resistome. Microbiol. Spectr. 2016, 4. [Google Scholar] [CrossRef]
- Perry, J.; Waglechner, N.; Wright, G. The prehistory of antibiotic resistance. Cold Spring Harb. Perspect. Med. 2016, 6, a025197. [Google Scholar] [CrossRef] [PubMed]
- Sonthayanon, P.; Peacock, S.J.; Chierakul, W.; Wuthiekanun, V.; Blacksell, S.D.; Holden, M.T.G.; Bentley, S.D.; Feil, E.J.; Day, N.P.J. High rates of homologous recombination in the mite endosymbiont and opportunistic human pathogen Orientia tsutsugamushi. PLoS Negl. Trop. Dis. 2010, 4, e752. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smadel, J.E.; Jackson, E.B.; Bennett, B.L.; Rights, F.L. A toxic substance associated with the Gilliam strain of R. orientalis. Proc. Soc. Exp. Biol. Med. 1946, 62, 138–140. [Google Scholar] [CrossRef] [PubMed]
- Oaks, S.C., Jr.; Ng, F.K.P.; Elwell, M.R.; Groves, M.G.; Lewis, G.E., Jr. Pathology of toxic death in mice following intravenous injection of Rickettsia tsutsugamushi strain Gilliam: Examination by light and scanning electron microscopy. Jpn. J. Med. Sci. Biol. 1985, 38, 67–72. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.X.; Feng, D.; Suo, J.J.; Xing, Y.B.; Liu, G.; Liu, L.H.; Xiao, H.J.; Jia, N.; Gao, Y.; Yang, H.; et al. Clinical characteristics of the autumn-winter type scrub typhus cases in south of Shandong province, northern China. BMC Infect. Dis. 2009, 9, 82. [Google Scholar] [CrossRef] [PubMed]
- Jeung, Y.S.; Kim, C.M.; Yun, N.R.; Kim, S.W.; Han, M.A.; Kim, D.M. Effect of latitude and seasonal variation on scrub typhus, South Korea, 2001–2013. Am. J. Trop. Med. Hyg. 2016, 94, 22–25. [Google Scholar] [CrossRef] [PubMed]
- Yoshikura, H. Seasonality and geographical distribution of tsutsugamushi diseases in Japan: Analysis of the trends since 1955 till 2014. Jpn. J. Infect. Dis. 2017. [Google Scholar] [CrossRef] [PubMed]
- Cho, N.H.; Kim, H.R.; Lee, J.H.; Kim, S.Y.; Kim, J.; Cha, S.; Kim, S.Y.; Darby, A.C.; Fuxelius, H.H.; Yin, J.; et al. The Orientia tsutsugamushi genome reveals massive proliferation of conjugative type IV secretion system and host-cell interaction genes. Proc. Natl. Acad. Sci. USA 2007, 104, 7981–7986. [Google Scholar] [CrossRef] [PubMed]
- Nakayama, K.; Yamashita, A.; Kurokawa, K.; Morimoto, T.; Ogawa, M.; Fukuhara, M.; Urakami, H.; Ohnish, M.; Uchiyama, I.; Ogura, Y.; et al. The whole-genome sequencing of the obligate intracellular bacterium Orientia tsutsugamushi revealed massive gene amplification during reductive genome evolution. DNA Res. 2008, 15, 185–199. [Google Scholar] [CrossRef] [PubMed]
- Liao, H.M.; Chao, C.C.; Lei, H.; Li, B.; Tsai, S.; Hung, G.C. Genomic sequencing of Orientia tsutsugamushi strain Karp, an assembly comparable to the genome size of the strain Ikeda. Genome Announc. 2016, 4. [Google Scholar] [CrossRef] [PubMed]
- Liao, H.M.; Chao, C.C.; Lei, H.Y.; Li, B.J.; Tsai, S.E.; Hung, G.C.; Ching, W.M.; Lo, S.C. Intraspecies comparative genomics of three strains of Orientia tsutsugamushi with different antibiotic sensitivity. Genom. Data 2017, 12, 84–88. [Google Scholar] [CrossRef] [PubMed]
- Daugherty, S.C.; Su, Q.; Abolude, K.; Beier-Sexton, M.; Carlyon, J.A.; Carter, R.; Day, N.P.; Dumler, S.J.; Dyachenko, V.; Godinez, A.; et al. Genome sequencing of Rickettsiales. Unpublished work 2015. Bioprojects accession numbers in NCBI Genbank: UT144:PRJNA232539; Karp: PRJNA212440; Kato: PRJNA212441; Gilliam: PRJNA212442; Ca. O. chuto: PRJNA212443; TA763: PRJNA212454; Sido: PRJNA212456; UT716: PRJNA212457; Sido: PRJNA212490.
- Fleshman, A.C.; Mullins, K.E.; Sahl, J.W.; Hepp, C.M.; Nieto, N.C.; Wiggins, K.; Hornstra O’Neil, H.; Paris, D.; Dittrich, S.; Kelly, D.; et al. Comparative pan-genomic analyses of Orientia tsutsugamushi demonstrates the unprecedented extent to which gene duplication and divergence drive genomic diversity. BMC Biol. 2017. in revision. [Google Scholar]
- Rolain, J.M.; Raoult, D. Prediction of resistance to erythromycin in the genus Rickettsia by mutations in L22 ribosomal protein. J. Antimicrob. Chemother. 2005, 56, 396–398. [Google Scholar] [CrossRef] [PubMed]
- Rolain, J.M.; Raoult, D. Genome comparison analysis of molecular mechanisms of resistance to antibiotics in the Rickettsia genus. Ann. N. Y. Acad. Sci. 2005, 1063, 222–230. [Google Scholar] [CrossRef] [PubMed]
| Isolate Name | Whole Genome Sequence Project | # of Contigs | Size (bp) |
|---|---|---|---|
| Boryong 1 | NC_009488 | 1 | 2,127,051 |
| Ikeda 1 | NC_010793 | 1 | 2,008,987 |
| AFSC4 | LYMT01 | 452 | 1,295,323 |
| AFSC7 | LYMB01 | 485 | 1,437,566 |
| Karp | LYMA02 | 108 | 2,026,724 |
| Karp | LANM01 | 145 | 1,454,354 |
| UT144 | LAOR01 | 229 | 1,689,193 |
| Sido | LAOM01 | 83 | 712,858 |
| TA716 | LAOA01 | 234 | 2,221,260 |
| UT76 | LANZ01 | 332 | 3,033,399 |
| TA763 | LANY01 | 194 | 2,460,104 |
| Gilliam | LANO01 | 76 | 1,997,698 |
| Kato PP | LANN01 | 137 | 1,478,442 |
| Ca. O. chuto | LANP01 | 47 | 1,092,196 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kelly, D.J.; Fuerst, P.A.; Richards, A.L. The Historical Case for and the Future Study of Antibiotic-Resistant Scrub Typhus. Trop. Med. Infect. Dis. 2017, 2, 63. https://doi.org/10.3390/tropicalmed2040063
Kelly DJ, Fuerst PA, Richards AL. The Historical Case for and the Future Study of Antibiotic-Resistant Scrub Typhus. Tropical Medicine and Infectious Disease. 2017; 2(4):63. https://doi.org/10.3390/tropicalmed2040063
Chicago/Turabian StyleKelly, Daryl J., Paul A. Fuerst, and Allen L. Richards. 2017. "The Historical Case for and the Future Study of Antibiotic-Resistant Scrub Typhus" Tropical Medicine and Infectious Disease 2, no. 4: 63. https://doi.org/10.3390/tropicalmed2040063





