Clinical Features and Laboratory Findings of Travelers Returning to South Australia with Dengue Virus Infection
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection
2.2. Serological Testing for DENV
2.3. RNA Extraction and pan-DENV RT-PCR
2.4. Serotype-Specific DENV RT-PCR and ZIKV RT-PCR
2.5. Data Analysis
3. Results
3.1. Dengue Positive Samples Represent a Minority of DENV Test Requests and Are Mostly NS1/IgM Positive
3.2. Demographics of the Patient Cohort with Clinical Dengue Serology Requests
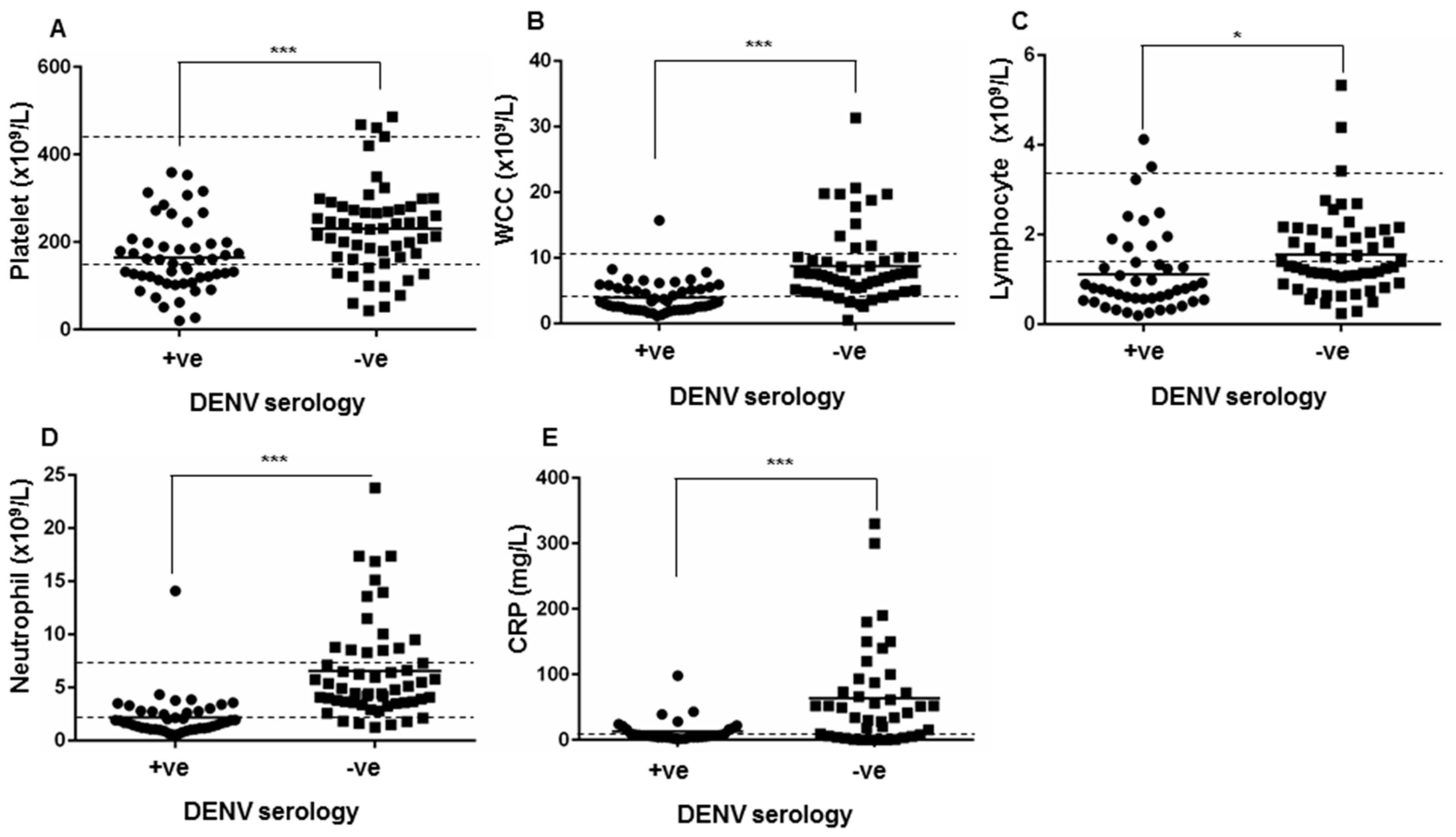
3.3. Clinical Presentations and Laboratory Measurements in the Patient Cohort with Clinical DENV Serology Requests
3.4. Reliable Detection of DENV by RT-PCR Requires Multiple PCR Approaches and Detects DENV-1, -2 and -3 Serotypes
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- World Health Organization (WHO). World Health Organization Global Strategey for Dengue Prevention and Control, 2012–2020; WHO: Geneva, Switzerland, 2012. [Google Scholar]
- Bhatt, S.; Gething, P.W.; Brady, O.J.; Messina, J.P.; Farlow, A.W.; Moyes, C.L.; Drake, J.M.; Brownstein, J.S.; Hoen, A.G.; Sankoh, O.; et al. The global distribution and burden of dengue. Nature 2013, 496, 504–507. [Google Scholar] [CrossRef] [PubMed]
- Guzman, M.G.; Halstead, S.B.; Artsob, H.; Buchy, P.; Farrar, J.; Gubler, D.J.; Hunsperger, E.; Kroeger, A.; Margolis, H.S.; Martinez, E.; et al. Dengue: A continuing global threat. Nat. Rev. Microbiol. 2010, 8, S7–S16. [Google Scholar] [CrossRef] [PubMed]
- Guzman, M.G.; Harris, E. Dengue. Lancet 2015, 385, 453–465. [Google Scholar] [CrossRef]
- Knope, K.; National, A.; Malaria Advisory, C.; Giele, C. Increasing notifications of dengue in Australia related to overseas travel, 1991 to 2012. Commun. Dis. Intell. Q. Rep. 2013, 37, E55–E59. [Google Scholar] [PubMed]
- Munoz-Jordan, J.L. Diagnosis of Zika virus infections: Challenges and opportunities. J. Infect. Dis. 2017, 216, S951–S956. [Google Scholar] [CrossRef] [PubMed]
- Van Meer, M.P.A.; Mogling, R.; Klaasse, J.; Chandler, F.D.; Pas, S.D.; van der Eijk, A.A.; Koopmans, M.P.G.; Reusken, C.; GeurtsvanKessel, C.H. Re-evaluation of routine dengue virus serology in travelers in the era of Zika virus emergence. J. Clin. Virol. 2017, 92, 25–31. [Google Scholar] [CrossRef] [PubMed]
- Russell, R.C.; Currie, B.J.; Lindsay, M.D.; Mackenzie, J.S.; Ritchie, S.A.; Whelan, P.I. Dengue and climate change in Australia: Predictions for the future should incorporate knowledge from the past. Med. J. Aust. 2009, 190, 265–268. [Google Scholar] [PubMed]
- Halstead, S.B.; Cohen, S.N. Dengue hemorrhagic fever at 60 years: Early evolution of concepts of causation and treatment. Microbiol. Mol. Biol. Rev. 2015, 79, 281–291. [Google Scholar] [CrossRef] [PubMed]
- Simmons, C.P.; Farrar, J.J.; van Nguyen, V.C.; Wills, B. Dengue. N. Engl. J. Med. 2012, 366, 1423–1432. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization (WHO). Dengue Guidelines for Diagnosis, Treatment, Prevention and Control; World Health Organization: Geneva, Switzerland, 2009. [Google Scholar]
- Muller, D.A.; Depelsenaire, A.C.; Young, P.R. Clinical and laboratory diagnosis of dengue virus infection. J. Infect. Dis. 2017, 215, S89–S95. [Google Scholar] [CrossRef] [PubMed]
- Yacoub, S.; Wills, B. Predicting outcome from dengue. BMC Med. 2014, 12, 147. [Google Scholar] [CrossRef] [PubMed]
- Halstead, S.B. Pathogenesis of dengue: Dawn of a new era. F1000Res. 2015, 4. [Google Scholar] [CrossRef] [PubMed]
- Warrilow, D.; Northill, J.A.; Pyke, A.; Smith, G.A. Single rapid TaqMan fluorogenic probe-based PCR assay that detects all four dengue serotypes. J. Med. Virol. 2002, 66, 524–528. [Google Scholar] [CrossRef] [PubMed]
- Gualano, R.C.; Pryor, M.J.; Cauchi, M.R.; Wright, P.J.; Davidson, A.D. Identification of a major determinant of mouse neurovirulence of dengue virus type 2 using stably cloned genomic-length cDNA. J. Gen. Virol. 1998, 79, 437–446. [Google Scholar] [CrossRef] [PubMed]
- Callahan, J.D.; Wu, S.J.; Dion-Schultz, A.; Mangold, B.E.; Peruski, L.F.; Watts, D.M.; Porter, K.R.; Murphy, G.R.; Suharyono, W.; King, C.C.; et al. Development and evaluation of serotype- and group-specific fluorogenic reverse transcriptase PCR (TaqMan) assays for dengue virus. J. Clin. Microbiol. 2001, 39, 4119–4124. [Google Scholar] [CrossRef] [PubMed]
- Musso, D.; Gubler, D.J. Zika Virus. Clin. Microbiol. Rev. 2016, 29, 487–524. [Google Scholar] [CrossRef] [PubMed]
- Hoffmeister, B.; Suttorp, N.; Zoller, T. The revised dengue fever classification in German travelers: Clinical manifestations and indicators for severe disease. Infection 2015, 43, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Tai, A.Y.; McGuinness, S.L.; Robosa, R.; Turner, D.; Huang, G.K.; Leder, K.; Korman, T.M.; Thevarajan, I.; Stewardson, A.J.; Padiglione, A.A.; et al. Management of dengue in Australian travellers: A retrospective multicentre analysis. Med. J. Aust. 2017, 206, 295–300. [Google Scholar] [CrossRef] [PubMed]
- Tavakolipoor, P.; Schmidt-Chanasit, J.; Burchard, G.D.; Jordan, S. Clinical features and laboratory findings of dengue fever in German travellers: A single-centre, retrospective analysis. Travel Med. Infect. Dis. 2016, 14, 39–44. [Google Scholar] [CrossRef] [PubMed]
- Trojanek, M.; Maixner, J.; Sojkova, N.; Kyncl, J.; Rohacova, H.; Maresova, V.; Stejskal, F. Dengue fever in Czech travellers: A 10-year retrospective study in a tertiary care centre. Travel Med. Infect. Dis. 2016, 14, 32–38. [Google Scholar] [CrossRef] [PubMed]
- Jazuli, F.; Lynd, T.; Mah, J.; Klowak, M.; Jechel, D.; Klowak, S.; Ovens, H.; Sabbah, S.; Boggild, A.K. Evaluation of a programme for ‘Rapid Assessment of Febrile Travelers’ (RAFT): A clinic-based quality improvement initiative. BMJ Open 2016, 6, e010302. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Vegas, C.; Hamer, D.H.; Chen, L.H.; Wilson, M.E.; Benoit, C.; Hunsperger, E.; Macleod, W.B.; Jentes, E.S.; Ooi, W.W.; Karchmer, A.W.; et al. Prevalence of dengue virus infection in US travelers who have lived in or traveled to dengue-endemic countries. J. Travel Med. 2013, 20, 352–360. [Google Scholar] [CrossRef] [PubMed]
- Odolini, S.; Parola, P.; Gkrania-Klotsas, E.; Caumes, E.; Schlagenhauf, P.; Lopez-Velez, R.; Burchard, G.D.; Santos-O’Connor, F.; Weld, L.; von Sonnenburg, F.; et al. Travel-related imported infections in Europe, EuroTravNet 2009. Clin. Microbiol. Infect. 2012, 18, 468–474. [Google Scholar] [CrossRef] [PubMed]
- Alcon, S.; Talarmin, A.; Debruyne, M.; Falconar, A.; Deubel, V.; Flamand, M. Enzyme-linked immunosorbent assay specific to dengue virus type 1 nonstructural protein NS1 reveals circulation of the antigen in the blood during the acute phase of disease in patients experiencing primary or secondary infections. J. Clin. Microbiol. 2002, 40, 376–381. [Google Scholar] [CrossRef] [PubMed]
- Sa-Ngasang, A.; Anantapreecha, S.; A-Nuegoonpipat, A.; Chanama, S.; Wibulwattanakij, S.; Pattanakul, K.; Sawanpanyalert, P.; Kurane, I. Specific IgM and IgG responses in primary and secondary dengue virus infections determined by enzyme-linked immunosorbent assay. Epidemiol. Infect. 2006, 134, 820–825. [Google Scholar] [CrossRef] [PubMed]
- Warrilow, D.; Northill, J.A.; Pyke, A.T. Sources of dengue viruses imported into Queensland, Australia, 2002–2010. Emerg. Infect. Dis. 2012, 18, 1850–1857. [Google Scholar] [CrossRef] [PubMed]
- Jentes, E.S.; Lash, R.R.; Johansson, M.A.; Sharp, T.M.; Henry, R.; Brady, O.J.; Sotir, M.J.; Hay, S.I.; Margolis, H.S.; Brunette, G.W. Evidence-based risk assessment and communication: A new global dengue-risk map for travellers and clinicians. J. Travel Med. 2016, 23. [Google Scholar] [CrossRef] [PubMed]
- Verschueren, J.; Cnops, L.; van Esbroeck, M. Twelve years of dengue surveillance in Belgian travellers and significant increases in the number of cases in 2010 and 2013. Clin. Microbiol. Infect. 2015, 21, 867–872. [Google Scholar] [CrossRef] [PubMed]
- Ratnam, I.; Black, J.; Leder, K.; Biggs, B.A.; Matchett, E.; Padiglione, A.; Woolley, I.; Panagiotidis, T.; Gherardin, T.; Pollissard, L.; et al. Incidence and seroprevalence of dengue virus infections in Australian travellers to Asia. Eur. J. Clin. Microbiol. Infect. Dis. 2012, 31, 1203–1210. [Google Scholar] [CrossRef] [PubMed]
- Rocklov, J.; Lohr, W.; Hjertqvist, M.; Wilder-Smith, A. Attack rates of dengue fever in Swedish travellers. Scand. J. Infect. Dis. 2014, 46, 412–417. [Google Scholar] [CrossRef] [PubMed]
- Nascimento, E.J.; Silva, A.M.; Cordeiro, M.T.; Brito, C.A.; Gil, L.H.; Braga-Neto, U.; Marques, E.T. Alternative complement pathway deregulation is correlated with dengue severity. PLoS ONE 2009, 4, e6782. [Google Scholar] [CrossRef] [PubMed]
- Soo, K.M.; Khalid, B.; Ching, S.M.; Tham, C.L.; Basir, R.; Chee, H.Y. Meta-analysis of biomarkers for severe dengue infections. PeerJ 2017, 5, e3589. [Google Scholar] [CrossRef] [PubMed]
- Kotaki, T.; Yamanaka, A.; Mulyatno, K.C.; Churrotin, S.; Sucipto, T.H.; Labiqah, A.; Ahwanah, N.L.; Soegijanto, S.; Kameoka, M.; Konishi, E. Divergence of the dengue virus type 2 cosmopolitan genotype associated with two predominant serotype shifts between 1 and 2 in Surabaya, Indonesia, 2008–2014. Infect. Genet. Evol. 2016, 37, 88–93. [Google Scholar] [CrossRef] [PubMed]
- Tian, H.; Sun, Z.; Faria, N.R.; Yang, J.; Cazelles, B.; Huang, S.; Xu, B.; Yang, Q.; Pybus, O.G.; Xu, B. Increasing airline travel may facilitate co-circulation of multiple dengue virus serotypes in Asia. PLoS Negl. Trop. Dis. 2017, 11, e0005694. [Google Scholar] [CrossRef] [PubMed]
- Kusmintarsih, E.S.; Hayati, R.F.; Turnip, O.N.; Yohan, B.; Suryaningsih, S.; Pratiknyo, H.; Denis, D.; Sasmono, R.T. Molecular characterization of dengue viruses isolated from patients in Central Java, Indonesia. J. Infect. Public Health 2017. [Google Scholar] [CrossRef] [PubMed]
- Megawati, D.; Masyeni, S.; Yohan, B.; Lestarini, A.; Hayati, R.F.; Meutiawati, F.; Suryana, K.; Widarsa, T.; Budiyasa, D.G.; Budiyasa, N.; et al. Dengue in Bali: Clinical characteristics and genetic diversity of circulating dengue viruses. PLoS Negl. Trop. Dis. 2017, 11, e0005483. [Google Scholar] [CrossRef] [PubMed]
- Gubler, D.J.; Kuno, G.; Sather, G.E.; Waterman, S.H. A case of natural concurrent human infection with two dengue viruses. Am. J. Trop. Med. Hyg. 1985, 34, 170–173. [Google Scholar] [CrossRef] [PubMed]
- Laille, M.; Deubel, V.; Sainte-Marie, F.F. Demonstration of concurrent dengue 1 and dengue 3 infection in six patients by the polymerase chain reaction. J. Med. Virol. 1991, 34, 51–54. [Google Scholar] [CrossRef] [PubMed]
- Dewi, B.E.; Naiggolan, L.; Putri, D.H.; Rachmayanti, N.; Albar, S.; Indriastuti, N.T.; Sjamsuridzal, W.; Sudiro, T.M. Characterization of dengue virus serotype 4 infection in Jakarta, Indonesia. Southeast Asian J. Trop. Med. Public Health 2014, 45, 53–61. [Google Scholar] [PubMed]
- Panchaud, A.; Stojanov, M.; Ammerdorffer, A.; Vouga, M.; Baud, D. Emerging role of Zika virus in adverse fetal and neonatal outcomes. Clin. Microbiol. Rev. 2016, 29, 659–694. [Google Scholar] [CrossRef] [PubMed]
- Bosch, I.; de Puig, H.; Hiley, M.; Carre-Camps, M.; Perdomo-Celis, F.; Narvaez, C.F.; Salgado, D.M.; Senthoor, D.; O’Grady, M.; Phillips, E.; et al. Rapid antigen tests for dengue virus serotypes and Zika virus in patient serum. Sci. Transl. Med. 2017, 9. [Google Scholar] [CrossRef] [PubMed]
- Kwong, J.C.; Druce, J.D.; Leder, K. Zika virus infection acquired during brief travel to Indonesia. Am. J. Trop. Med. Hyg. 2013, 89, 516–517. [Google Scholar] [CrossRef] [PubMed]
- Perkasa, A.; Yudhaputri, F.; Haryanto, S.; Hayati, R.F.; Ma’roef, C.N.; Antonjaya, U.; Yohan, B.; Myint, K.S.; Ledermann, J.P.; Rosenberg, R.; et al. Isolation of Zika virus from febrile patient, Indonesia. Emerg. Infect. Dis. 2016, 22, 924–925. [Google Scholar] [CrossRef] [PubMed]
| RT-PCR | Sequence | Ref. | |
|---|---|---|---|
| Pan-DENV | Forward | AAGGACTAGAGGTTAKAGGAGACCC | |
| Reverse | CGYTCTGTGCCTGGAWTGATG | [15] | |
| Probe | FAM-TCTGGTCTTTCCAGCGTCAATATGCTGTT-BHQ | ||
| DENV-1 | Forward | GACACCACACCCTTTGGACAA | [17] |
| Reverse | CACCTGGCTGCTACCTCCAT | ||
| DENV-2 | Forward | GACCACACGYAACGGAGAA | [17] |
| Reverse | TCTGTTTTRAACAGRAGACTTTT | ||
| DENV-3 | Forward | GGGAARACCGTCTATCAATA | [17] |
| Reverse | CGCCATAACCAAYTTCATTGG | ||
| DENV-4 | Forward | TGAAGAGATTCTCAACCGGAC | [17] |
| Reverse | AATCCCTGCTGTTGGTGGG | ||
| ZIKV | Forward | CCTTGGATTCTTGAACGAGGA | [18] |
| Reverse | AGAGCTTCATTCTCCAGATCAA |
| Diagnostic Parameter | # Number (%) Seropositive | RT-PCR | |||
|---|---|---|---|---|---|
| # of samples | Pan-DENV PCR positive | Positive by any PCR | |||
| NS1 positive | 18 (37%) | 12 | 5 (42%) | 10 (83.3%) | |
| IgM positive | 9 (18%) | 3 | 0 | 3 (100%) | |
| IgM & NS1 positive | 16 (33%) | 12 | 7 (58%) | 10 (83.3%) | |
| IgM & IgG positive | 3 (6%) | 2 | 0 | 2 (100%) | |
| IgG & NS1 positive | 1 (2%) | 1 | 1 (100%) | 1 (100%) | |
| IgM, IgG & NS1 positive | 2 (4%) | 2 | 1 (50%) | 2 (100%) | |
| TOTAL Convalescence follow-up | 49 5 (10%) | 14/32 (43.8%) | 28/32 (87.5%) | ||
| Initial result | Follow up result | Days post test | |||
| NS1 positive | IgM positive | 6 | |||
| IgM positive | IgM positive | 15 | |||
| NS1 positive | IgM positive | 19 | |||
| IgM positive | IgM positive | 20 | |||
| NS1 positive | IgG & IgM | 22 | |||
| DENV Seropositive | DENV Seronegative | p-Value | |
|---|---|---|---|
| Number of Patients | 49 | 51 | |
| Age (Years), median (Range) | 40 (27.75–51.5) | 33.5 (22.75–55.5) | |
| Gender | |||
| Male | 24 (48.98%) | 37 (60.66%) | 0.2505 |
| Female | 25 (51.02%) | 24 (39.34%) | |
| Centre of care | |||
| Hospital | 27 (55.10%) | 39 (64.93%) | 0.4340 |
| Community | 22 (44.90%) | 22 (36.07%) |
| Travel Destination | DENV Seropositive | DENV Seronegative |
|---|---|---|
| Number of patients | 49 | 51 |
| Unrecorded travel history | 12 (24.5%) | 10 (19.6%) |
| Nil recent travel history | 0 | 2 (3.9%) |
| Southeast Asia | 36 (73.5%) | 31 (60.8%) |
| Indonesia | 21 (42.9%) | 12 (23.5%) |
| Thailand | 6 (12.2%) | 6 (11.8%) |
| Vietnam | 1 (2.0%) | 2 (3.9%) |
| Singapore/Malaysia | 4 (8.2%) | 2 (3.9%) |
| Philippines | 1 (2.0%) | 1 (2.0%) |
| Borneo | 1 (2.0%) | 0 |
| Cambodia | 0 | 4 (7.8%) |
| Myanmar | 1 (2.0%) | 1 (2.0%) |
| Other (unspecified) | 1 (2.0%) | 3 (5.9%) |
| Australia | 0 | 3 (5.9%) |
| North Queensland | 0 | 3 (5.9%) |
| Pacific Islands | 1 (2.0%) | 5 (9.8%) |
| Tonga | 1 (2.0%) | 0 |
| Fiji | 0 | 3 (5.9%) |
| Vanuatu | 0 | 2 (3.9%) |
| Indian subcontinent | 3 (6.1%) | 2 (3.9%) |
| India | 2 (4.1%) | 1 (2.0%) |
| Pakistan | 0 | 1 (2.0%) |
| Bangladesh | 1 (2.0%) | 0 |
| Africa | 0 | 2 (3.9%) |
| Tanzania | 0 | 1 (2.0%) |
| Guinea | 0 | 1 (2.0%) |
| Hong Kong | 0 | 1 (2.0%) |
| Papua New Guinea | 0 | 2 (3.9%) |
| Recent travel declared (destination unspecified) | 0 | 4 (7.8%) |
| Symptom | DENV Seropositive | DENV Seronegative | p-Value |
|---|---|---|---|
| Fever | 37 (95%) | 36 (71%) | 0.0054 |
| Headache | 16 (41%) | 16 (31%) | 0.3801 |
| Retro-orbital pain | 0 (0%) | 1 (2%) | 1 |
| Nausea and/or vomiting | 10 (26%) | 16 (31%) | 0.6418 |
| Swollen glands | 0 (0%) | 0 (0%) | 1 |
| Myalgia and/or arthralgia | 22 (56%) | 19 (37%) | 0.0890 |
| Rash | 14 (36%) | 4 (8%) | 0.0013 |
| Grouping | Specific Diagnosis 35/51, 68.6% |
|---|---|
| Gastroenteritis | Total, n = 5 (13.5%) Shigella spp. Salmonella spp. (n = 2) Salmonella typhi (n = 2) |
| Respiratory | Total, n = 10 (27%) Influenza A virus (n = 5) Pneumonia (n = 2) Viral pharyngitis Parainfluenza 1 with COPD Rhinovirus with adenovirus |
| Parasitic | Total, n = 6 (21.6%) Malaria (n = 3) Cryptosporidium Hydatid disease Giardia |
| Other | Total, n = 11 (29.7%) |
| Infectious | Total, n = 7 Infected lymphocoele Spontaneous peritonitis Streptococcus pyogenes bacteremia Polymicrobial wound infection Measles virus E coli urosepsis Infective endocarditis Febrile or ‘viral’ illness (n = 3) |
| Non-infectious | Total n = 4 Haemophagocytic lymphohistocytosis Phototoxic rash Gastric diffuse large B-cell lymphoma Benign headache |
| No specific diagnosis | Total, n = 16 (31.4%) |
| Laboratory Measure | Reference Range | DENV Seropositive Median (IQR) | DENV Seronegative Median (IQR) | p-Value |
|---|---|---|---|---|
| Haemoglobin | ||||
| Female | (115–155 g/L) | 132.5 (128–143.25) | 140 (137–148) | 0.680 |
| Male | (135–175 g/L) | 149 (134–155.5) | 140.5 (135.25–153) | 0.316 |
| Platelets | (150–450 ×109/L) | 140 (106.5–183.8) | 231 (165.5–277.5) | <0.0005 |
| Haematocrit | (0.35–0.45) | 0.42 (0.39–0.43) | 0.41 (0.393–0.43) | 0.520 |
| White cell count | (4.00–11.0 ×109/L) | 2.8 (2.1–4.8) | 7.41 (5.295–9.8) | <0.0005 |
| Lymphocytes | (1.50–3.50 ×109/L) | 0.79 (0.54–1.32) | 1.28 (0.98–2.045) | 0.0218 |
| Neutrophils | (1.80–7.50 ×109/L) | 1.67 (1.15–2.71) | 4.91 (3.595–8.36) | <0.0005 |
| CRP | (<8.0 mg/L) | 7.25 (4.5–16) | 51.5 (15.75-91.5) | <0.0005 |
| Pan-DENV | No Serotype Determined | Mixed Serotype Result | DENV-1 | DENV-2 | DENV-3 | DENV-4 | |
|---|---|---|---|---|---|---|---|
| Pan-DENV positive | 14 | 4 | 5 | 1 | 4 | 0 | 0 |
| Pan-DENV negative | 18 | 4 | 2 | 4 | 7 | 1 | 0 |
| DENV-1 | - | 5 | 5 | 0 | 0 | ||
| DENV-2 | - | 5 | 11 | 2 | 0 | ||
| DENV-3 | - | 0 | 2 | 1 | 0 | ||
| DENV-4 | - | 0 | 0 | 0 | 0 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Quinn, E.J.; Cheong, A.H.-C.; Calvert, J.K.; Higgins, G.; Hahesy, T.; Gordon, D.L.; Carr, J.M. Clinical Features and Laboratory Findings of Travelers Returning to South Australia with Dengue Virus Infection. Trop. Med. Infect. Dis. 2018, 3, 6. https://doi.org/10.3390/tropicalmed3010006
Quinn EJ, Cheong AH-C, Calvert JK, Higgins G, Hahesy T, Gordon DL, Carr JM. Clinical Features and Laboratory Findings of Travelers Returning to South Australia with Dengue Virus Infection. Tropical Medicine and Infectious Disease. 2018; 3(1):6. https://doi.org/10.3390/tropicalmed3010006
Chicago/Turabian StyleQuinn, Emma J., Allena H.-C. Cheong, Julie K. Calvert, Geoffrey Higgins, Trish Hahesy, David L. Gordon, and Jillian M. Carr. 2018. "Clinical Features and Laboratory Findings of Travelers Returning to South Australia with Dengue Virus Infection" Tropical Medicine and Infectious Disease 3, no. 1: 6. https://doi.org/10.3390/tropicalmed3010006
APA StyleQuinn, E. J., Cheong, A. H.-C., Calvert, J. K., Higgins, G., Hahesy, T., Gordon, D. L., & Carr, J. M. (2018). Clinical Features and Laboratory Findings of Travelers Returning to South Australia with Dengue Virus Infection. Tropical Medicine and Infectious Disease, 3(1), 6. https://doi.org/10.3390/tropicalmed3010006




