In Vitro Effects of Aminopyridyl Ligands Complexed to Copper(II) on the Physiology and Interaction Process of Trypanosoma cruzi
Abstract
:1. Introduction
2. Materials and Methods
2.1. Mammalian Cells and Parasite Cultivation
2.2. Aminopyridines-Derived Metallodrugs
2.3. Effects on Plasma Membrane Integrity
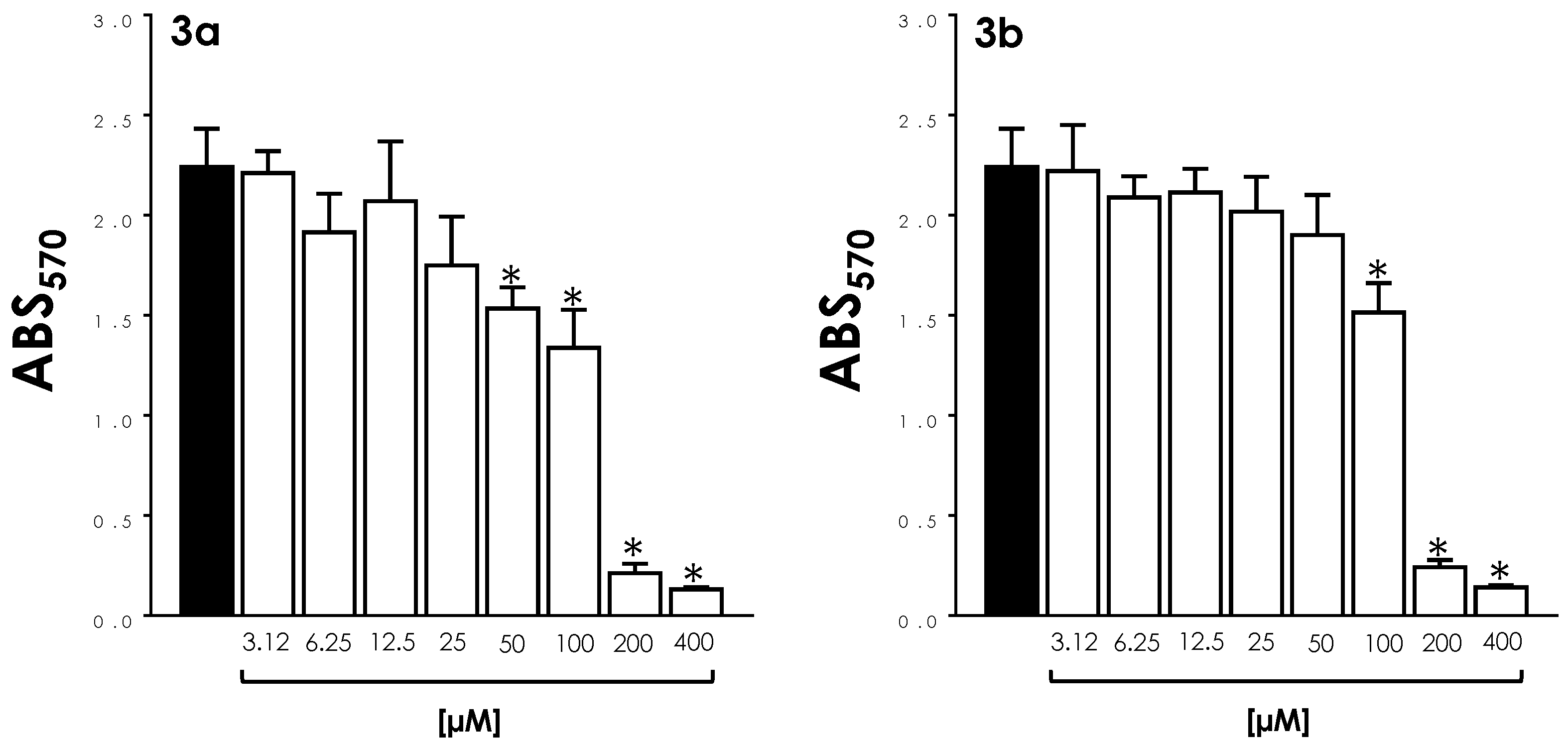
2.4. Effects on Mitochondrial Metabolism
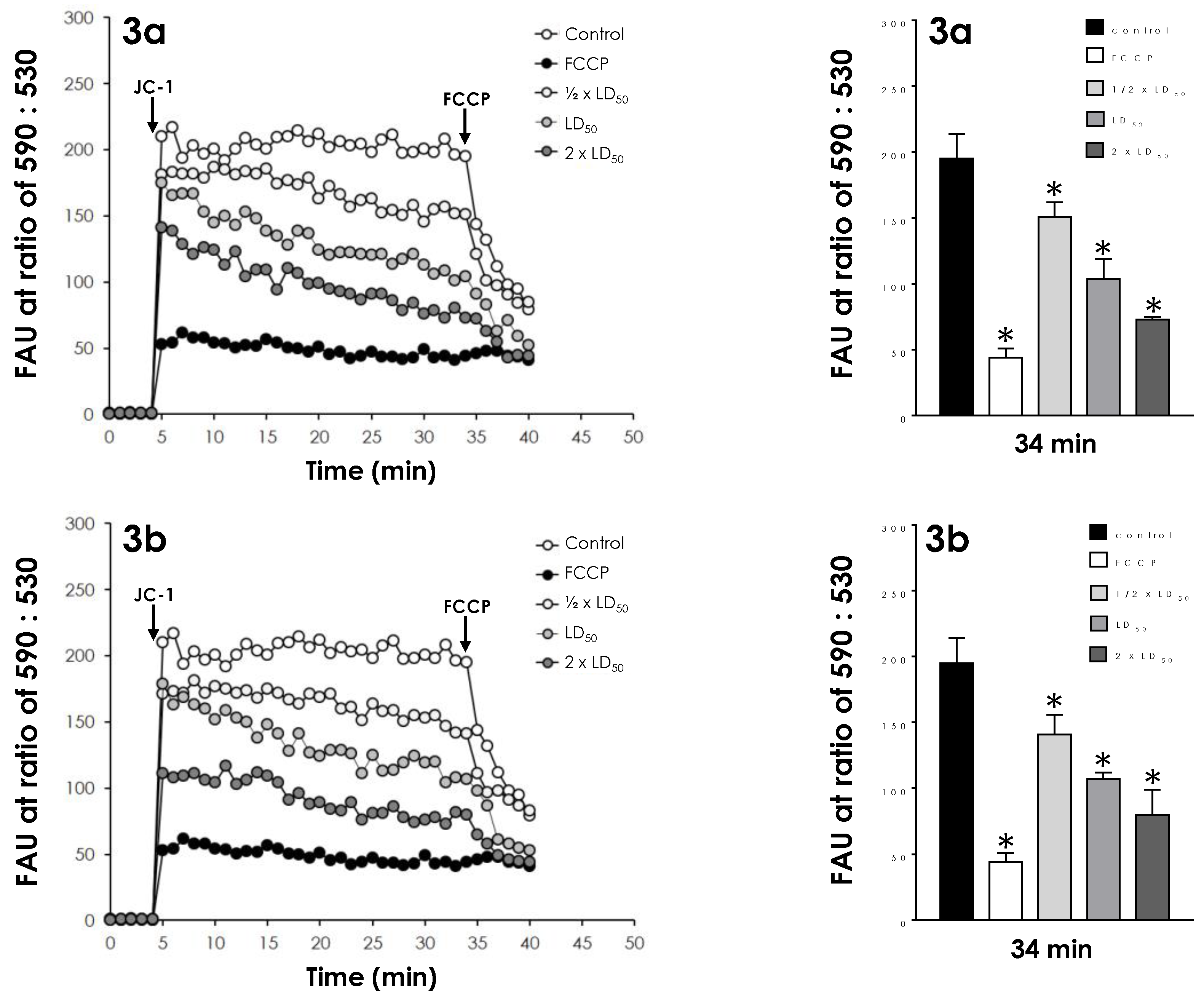
2.5. Effects on Mitochondrial Transmembrane Potential
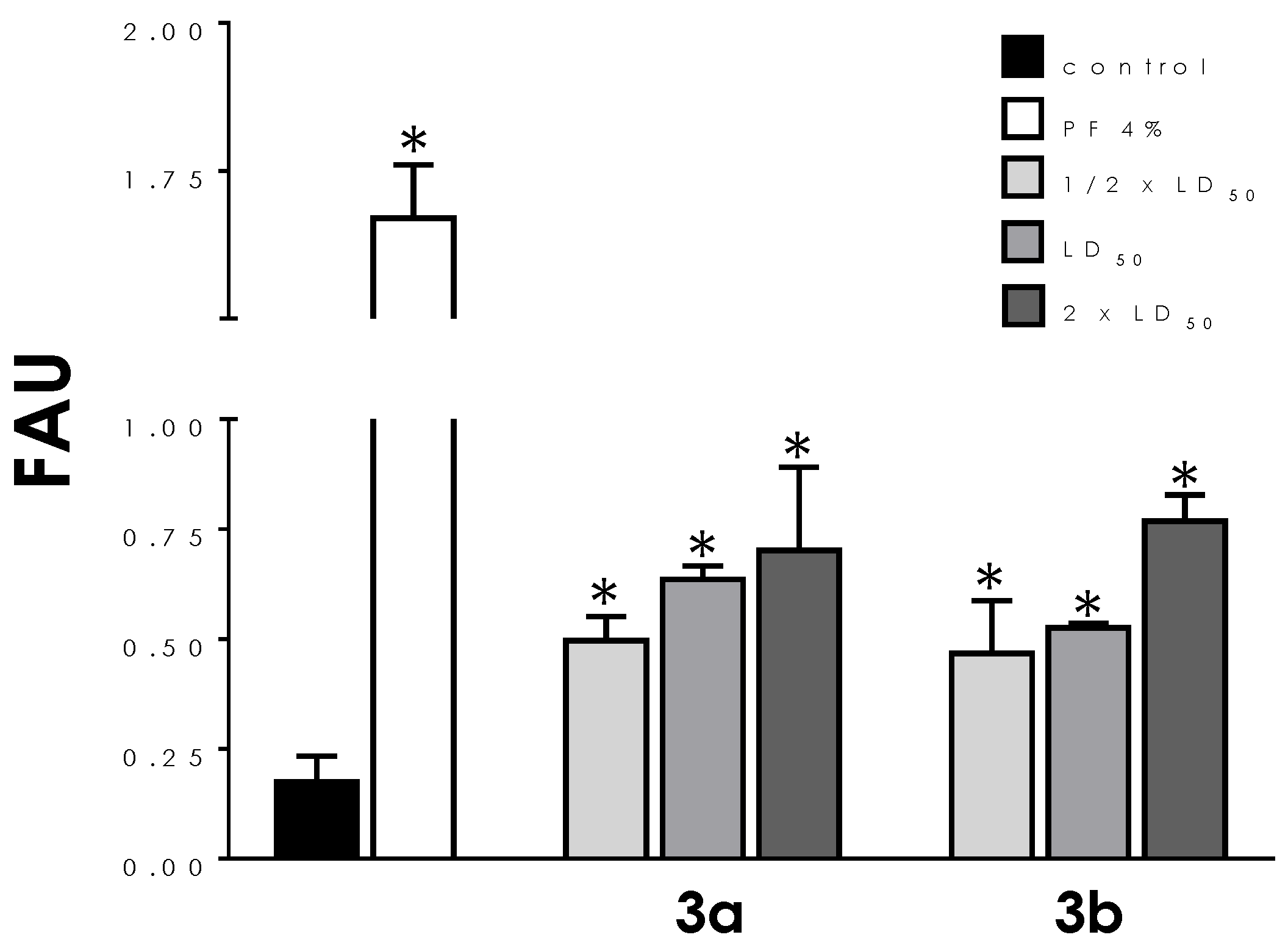
2.6. Effects on Reactive Oxygen Species (ROS) Production
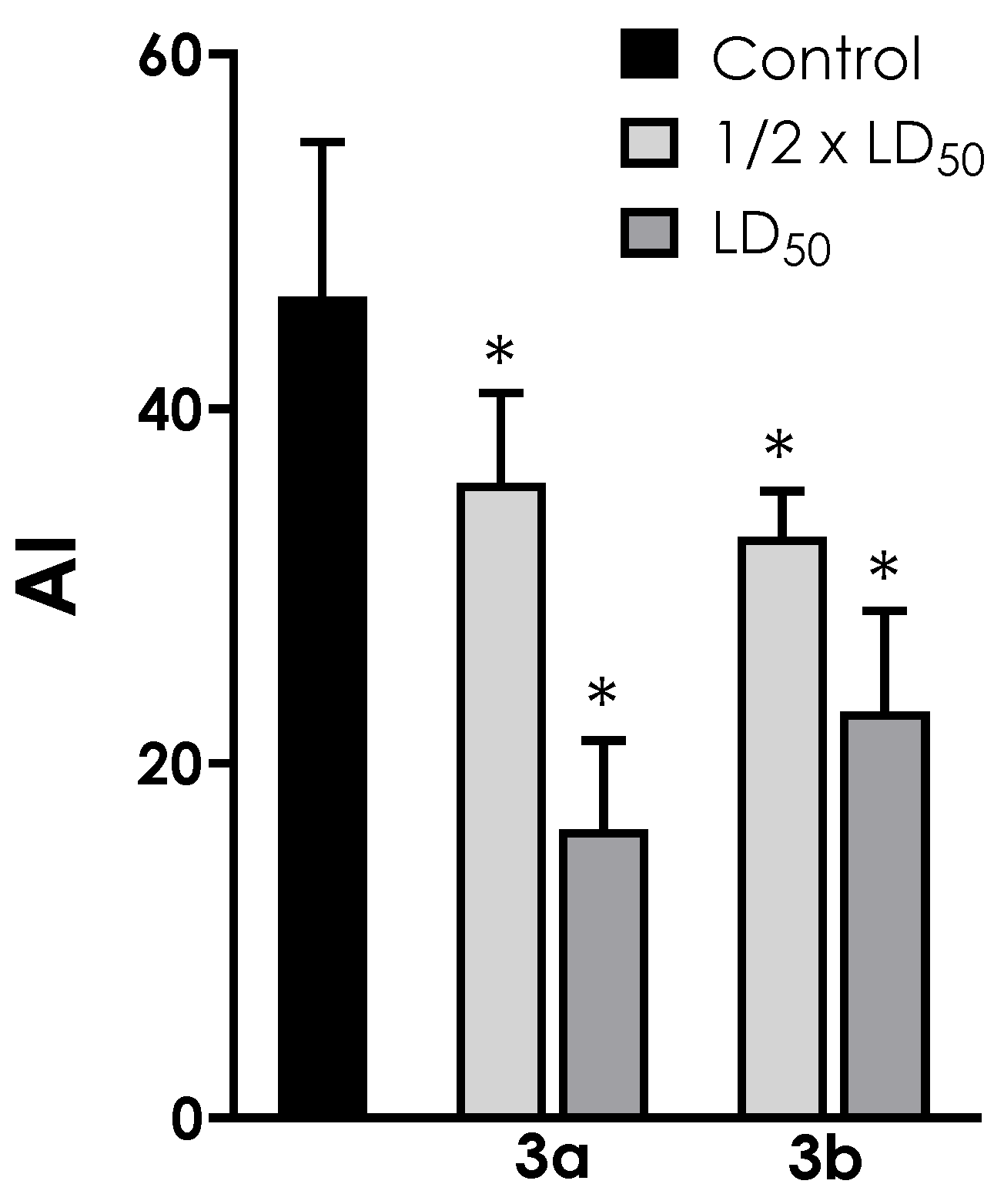
2.7. Effects on Neutral Lipids Storage
2.8. Effects on Interaction Process of Trypomastigotes with Host Cells
2.9. Effects on Epithelial Cell Viability
2.10. Effects on Intracellular Amastigotes
2.11. Statistics
3. Results
3.1. Cell Viability
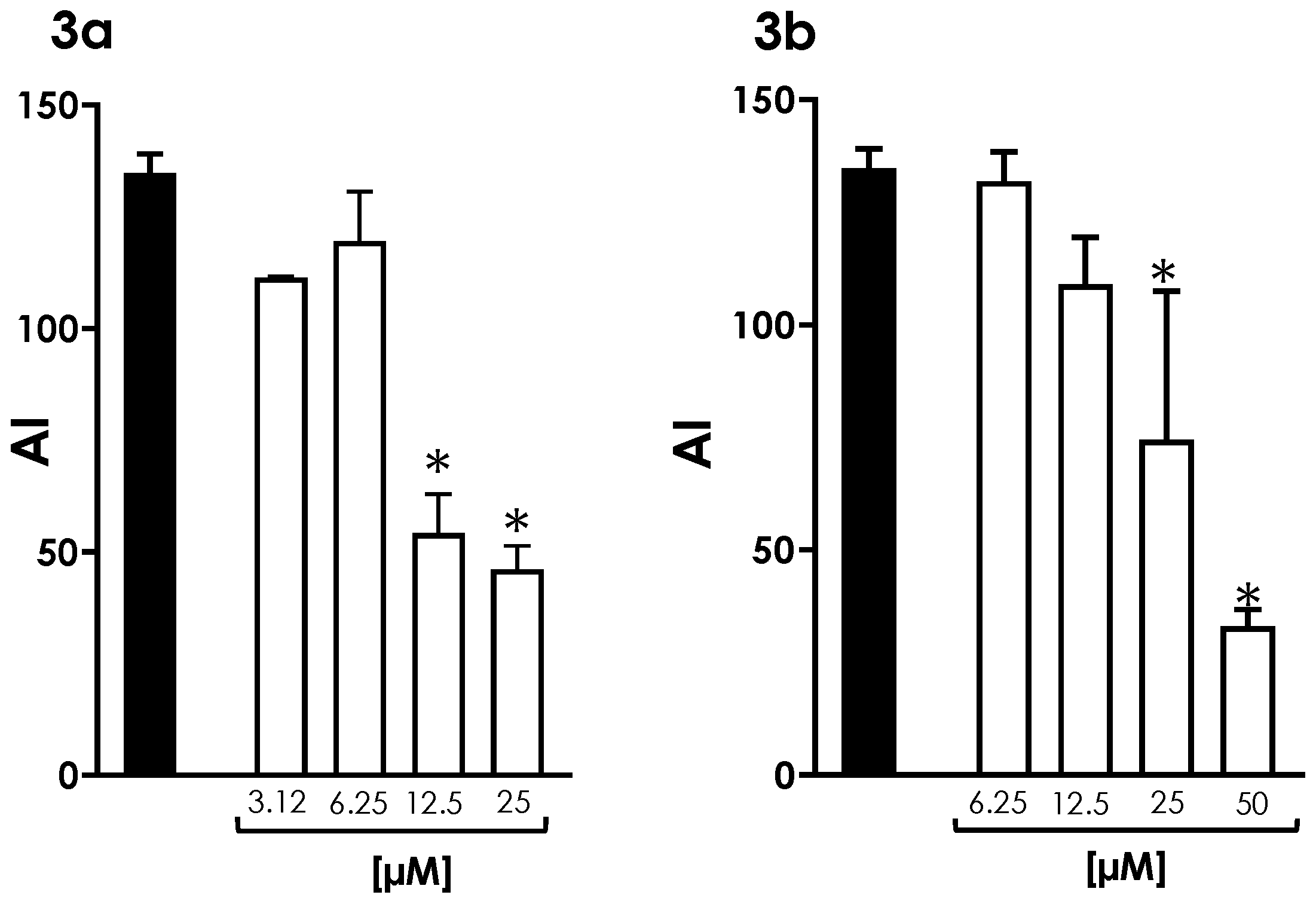
3.2. Mitochondrial Activity
3.3. ROS Production
3.4. Neutral Lipid Accumulation
3.5. Interaction of Trypomastigotes with LLC-MK2 Epithelial Cells
3.6. Survival of Intracellular Amastigotes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pan American Health Organization (PAHO). Chagas Disease. Available online: https://www.paho.org/en/topics/chagas-disease (accessed on 22 March 2023).
- Lidani, K.C.F.; Andrade, F.A.; Bavia, L.; Damasceno, F.S.; Beltrame, M.H.; Messias-Reason, I.J.; Sandri, T.S. Chagas disease: From discovery to a worldwide health problem. Front. Public Health 2019, 7, 166. [Google Scholar] [CrossRef] [PubMed]
- Echavarría, N.G.; Echeverría, L.E.; Stewart, M.; Gallego, C.; Saldarriaga, C. Chagas disease: Chronic chagas cardiomyopathy. Curr. Probl. Cardiol. 2021, 46, 100507. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Chagas Disease (American Trypanosomiasis). Available online: https://www.who.int/health-topics/chagas-disease (accessed on 22 March 2023).
- World Health Organization. Ending the Neglect to Attain the Sustainable Development Goals: A Road Map for Neglected Tropical Diseases 2021–2030: Overview. Available online: https://www.who.int/publications/i/item/9789240010352 (accessed on 22 March 2023).
- Ribeiro, V.; Dias, N.; Paiva, T.; Hagström-Bex, L.; Nitz, N.; Pratesi, R.; Hecht, M. Current trends in the pharmacological management of Chagas disease. Int. J. Parasitol. Drugs Drug Resist. 2020, 12, 7–17. [Google Scholar] [CrossRef] [PubMed]
- Zuma, A.A.; de Souza, W. Chagas Disease chemotherapy: What do we know so far? Curr. Pharm. Des. 2021, 27, 3963–3995. [Google Scholar] [CrossRef]
- Viotti, R.; Vigliano, C.; Lococo, B.; Alvarez, M.G.; Petti, M.; Bertocchi, G.; Armenti, A. Side effects of benznidazole as treatment in chronic Chagas disease: Fears and realities. Expert Rev. Anti Infect. Ther. 2009, 7, 157–163. [Google Scholar] [CrossRef] [PubMed]
- Nogueira, S.S.; Santos, E.C.; Silva, R.O.; Gonçalves, R.V.; Lima, G.D.A.; Novaes, R.D. Monotherapy and combination chemotherapy for Chagas disease treatment: A systematic review of clinical efficacy and safety based on randomized controlled trials. Parasitology 2022, 149, 1679–1694. [Google Scholar] [CrossRef]
- Echeverria, L.E.; Morillo, C.A. American trypanosomiasis (Chagas disease). Infect. Dis. Clin. N. Am. 2019, 33, 119–134. [Google Scholar] [CrossRef] [PubMed]
- Guarner, J. Chagas disease as example of a reemerging parasite. Semin. Diagn. Pathol. 2019, 36, 164–169. [Google Scholar] [CrossRef]
- Molina, I.; Perin, L.; Aviles, A.S.; Vieira, P.M.A.; Fonseca, K.S.; Cunha, L.M.; Carneiro, C.M. The effect of benznidazole dose among the efficacy outcome in the murine animal model. A quantitative integration of the literature. Acta Trop. 2020, 201, 105218. [Google Scholar] [CrossRef]
- Gandra, R.M.; Mc Carron, P.; Fernandes, M.F.; Ramos, L.S.; Mello, T.P.; Aor, A.C.; Branquinha, M.H.; McCann, M.; Devereux, M.; Santos, A.L.S. Antifungal potential of copper(II), manganese(II) and silver(I) 1,10-phenanthroline chelates against multidrug-resistant fungal species forming the Candida haemulonii complex: Impact on the planktonic and biofilm lifestyles. Front. Microbiol. 2017, 8, 1257. [Google Scholar] [CrossRef] [PubMed]
- Paixão, D.A.; Lopes, C.D.; Carneiro, Z.A.; Sousa, L.M.; de Oliveira, L.P.; Lopes, N.P.; Pivatto, M.; Chaves, J.D.S.; de Almeida, M.V.; Ellena, J.; et al. In vitro anti-Trypanosoma cruzi activity of ternary copper(II) complexes and in vivo evaluation of the most promising complex. Biomed. Pharmacother. 2019, 109, 157–166. [Google Scholar] [CrossRef]
- Galdino, A.C.M.; Viganor, L.; Pereira, M.M.; Devereux, M.; McCann, M.; Branquinha, M.H.; Molphy, Z.; O’Carroll, S.; Bain, C.; Menounou, G.; et al. Copper(II) and silver(I)-1,10-phenanthroline-5,6-dione complexes interact with double-stranded DNA: Further evidence of their apparent multi-modal activity towards Pseudomonas aeruginosa. J. Biol. Inorg. Chem. 2022, 27, 201–213. [Google Scholar] [CrossRef]
- De Azevedo-França, J.A.; Barrias, E.; Franco, C.H.J.; Villarreal, W.; Vieira, E.G.; Ferreira, A.M.D.C.; Souza, W.; Navarro, M. Promising fluconazole based zinc(II) and copper(II) coordination polymers against Chagas disease. J. Inorg. Biochem. 2022, 233, 111834. [Google Scholar] [CrossRef]
- Souza, C.C.; de Azevedo-França, J.A.; Barrias, E.; Cavalcante, S.C.F.; Vieira, E.G.; Ferreira, A.M.D.C.; de Souza, W.; Navarro, M. Silver and copper-benznidazole derivatives as potential antiparasitic metallodrugs: Synthesis, characterization, and biological evaluation. J. Inorg. Biochem. 2023, 239, 112047. [Google Scholar] [CrossRef]
- Navarro, M.; Gabbiani, C.; Messori, L.; Gambino, D. Metal-based drugs for malaria, trypanosomiasis and leishmaniasis: Recent achievements and perspectives. Drug Discov. Today 2010, 15, 1070–1078. [Google Scholar] [CrossRef]
- Reddy, A.; Sangenito, L.S.; Guedes, A.A.; Branquinha, M.H.; Kavanagh, K.; McGinley, J.; Santos, A.L.S.; Velasco-Torrijos, T. Glycosylated metal chelators as anti-parasitic agents with tunable selectivity. Dalton Trans. 2017, 19, 5297–5307. [Google Scholar] [CrossRef] [PubMed]
- Sangenito, L.S.; d’Avila-Levy, C.M.; Branquinha, M.H.; Santos, A.L.S. Nelfinavir and lopinavir impair Trypanosoma cruzi trypomastigote infection in mammalian host cells and show anti-amastigote activity. Int. J. Antimicrob. Agents 2016, 48, 703–711. [Google Scholar] [CrossRef]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Macedo-Silva, S.T.; Oliveira-Silva, T.L.; Urbina, J.A.; de Souza, W.; Rodrigues, J.C. Antiproliferative, ultrastructural, and physiological effects of amiodarone on promastigote and amastigote forms of Leishmania amazonensis. Mol. Biol. Int. 2011, 2011, 876021. [Google Scholar] [CrossRef]
- Sangenito, L.S.; Menna-Barreto, R.F.S.; Oliveira, A.C.; d’Avila-Levy, C.M.; Branquinha, M.H.; Santos, A.L.S. Primary evidence of the mechanisms of action of HIV aspartyl peptidase inhibitors on Trypanosoma cruzi trypomastigote forms. Int. J. Antimicrob. Agents 2018, 52, 185–194. [Google Scholar] [CrossRef] [PubMed]
- Chatelain, E. Chagas Disease drug discovery: Toward a new era. J. Biomol. Screen. 2015, 20, 22–35. [Google Scholar] [CrossRef] [PubMed]
- Fidalgo, L.M.; Gille, L. Mitochondria and trypanosomatids: Targets and drugs. Pharm. Res. 2011, 28, 2758–2770. [Google Scholar] [CrossRef]
- Pedra-Rezende, Y.; Bombaça, A.C.S.; Menna-Barreto, R.F.S. Is the mitochondrion a promising drug target in trypanosomatids? Mem. Inst. Oswaldo Cruz 2022, 21, e210379. [Google Scholar] [CrossRef]
- Menna-Barreto, R.F.; Castro, S.L. The double-edged sword in pathogenic trypanosomatids: The pivotal role of mitochondria in oxidative stress and bioenergetics. Biomed. Res. Int. 2014, 2014, 614014. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Bejarano, O.H.; Avendaño, C.; Patarroyo, M.A. Mechanisms associated with Trypanosoma cruzi host target cell adhesion, recognition and internalization. Life 2021, 11, 534. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Círia, M.; Sanz, A.M.; Yunta, M.J.R.; Gómez-Contreras, F.; Navarro, P.; Sánchez-Moreno, M.; Boutaleb-Charki, S.; Osuna, A.; Castiñeiras, A.; Pardo, M.; et al. 1,4-Bis(alkylamino)benzo[g]phthalazines able to form dinuclear complexes of Cu(II) which as free ligands behave as SOD inhibitors and show efficient in vitro activity against Trypanosoma cruzi. Bioorg. Med. Chem. 2007, 15, 2081–2091. [Google Scholar] [CrossRef]
- Pérez-Rebolledo, A.; Teixeira, L.R.; Batista, A.A.; Mangrich, A.S.; Aguirre, G.; Cerecetto, H.; González, M.; Hernández, P.; Ferreira, A.M.; Speziali, N.L.; et al. 4-nitroacetophenone-derived thiosemicarbazones and their copper(II) complexes with significant in vitro anti-trypanosomal activity. Eur. J. Med. Chem. 2008, 43, 939–948. [Google Scholar] [CrossRef]
- Becco, L.; Rodríguez, A.; Bravo, M.E.; Prieto, M.J.; Ruiz-Azuara, L.; Garat, B.; Moreno, V.; Gambino, D. New achievements on biological aspects of copper complexes Casiopeínas®: Interaction with DNA and proteins and anti-Trypanosoma cruzi activity. J. Inorg. Biochem. 2012, 109, 49–56. [Google Scholar] [CrossRef]
- Martins, D.A.; Gouvea, L.R.; Muniz, G.S.; Louro, S.R.; Batista, D.G.; Soeiro, M.N.; Teixeira, L.R. Norfloxacin and N-donor mixed-ligand copper(II) complexes: Synthesis, albumin interaction, and anti-Trypanosoma cruzi activity. Bioinorg. Chem. Appl. 2016, 2016, 5027404. [Google Scholar] [CrossRef]
- Méndez-Arriaga, J.M.; Oyarzabal, I.; Escolano, G.; Rodríguez-Diéguez, A.; Sánchez-Moreno, M.; Salas, J.M. In vitro leishmanicidal and trypanocidal evaluation and magnetic properties of 7-amino-1,2,4-triazolo[1,5-a]pyrimidine Cu(II) complexes. J. Inorg. Biochem. 2018, 180, 26–32. [Google Scholar] [CrossRef]
- Krasnovskaya, O.; Naumov, A.; Guk, D.; Gorelkin, P.; Erofeev, A.; Beloglazkina, E.; Majouga, A. Copper coordination compounds as biologically active agents. Int. J. Mol. Sci. 2020, 21, 3965. [Google Scholar] [CrossRef]
- Mjos, K.D.; Orvig, C. Metallodrugs in medicinal inorganic chemistry. Chem. Rev. 2014, 114, 4540–4563. [Google Scholar] [CrossRef] [PubMed]
- Gottschaldt, M.; Schubert, U.S. Prospects of metal complexes peripherally substituted with sugars in biomedicinal applications. Chemistry 2009, 15, 1548–1557. [Google Scholar] [CrossRef] [PubMed]
- Ong, Y.C.; Roy, S.; Andrews, P.C.; Gasser, G. Metal compounds against neglected tropical diseases. Chem. Rev. 2019, 119, 730–796. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silva-Oliveira, R.; Sangenito, L.S.; Reddy, A.; Velasco-Torrijos, T.; Santos, A.L.S.; Branquinha, M.H. In Vitro Effects of Aminopyridyl Ligands Complexed to Copper(II) on the Physiology and Interaction Process of Trypanosoma cruzi. Trop. Med. Infect. Dis. 2023, 8, 288. https://doi.org/10.3390/tropicalmed8050288
Silva-Oliveira R, Sangenito LS, Reddy A, Velasco-Torrijos T, Santos ALS, Branquinha MH. In Vitro Effects of Aminopyridyl Ligands Complexed to Copper(II) on the Physiology and Interaction Process of Trypanosoma cruzi. Tropical Medicine and Infectious Disease. 2023; 8(5):288. https://doi.org/10.3390/tropicalmed8050288
Chicago/Turabian StyleSilva-Oliveira, Rafaela, Leandro S. Sangenito, Andrew Reddy, Trinidad Velasco-Torrijos, André L. S. Santos, and Marta H. Branquinha. 2023. "In Vitro Effects of Aminopyridyl Ligands Complexed to Copper(II) on the Physiology and Interaction Process of Trypanosoma cruzi" Tropical Medicine and Infectious Disease 8, no. 5: 288. https://doi.org/10.3390/tropicalmed8050288





