Potent In Vitro and In Vivo Effects of Stachys lavandulifolia Methanolic Extract against Toxoplasma gondii Infection
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Collection
2.2. Ethics
2.3. Preparing of Extract
2.3.1. Phytochemical Analysis
2.3.2. Quantity Analysis of the Secondary Metabolites
2.4. Parasites
2.5. Animals
2.6. In Vitro Anti-Toxoplasma Effects
2.6.1. Flow Cytometry Analysis
2.6.2. Effect of Intracellular Parasites and Infectivity Rate
2.6.3. Cytotoxicity Effects of SLME on Normal Cells
2.7. In Vivo Anti-Toxoplasma Effects
2.7.1. Evaluation of Liver Lipid Peroxidation (LPO) and Nitric Oxide (NO)
2.7.2. Proinflammatory Cytokines mRNA Expression
2.7.3. Toxicity Effects on Liver and Kidney Functions
2.8. Statistical Analysis
3. Results
3.1. Phytochemical Analysis
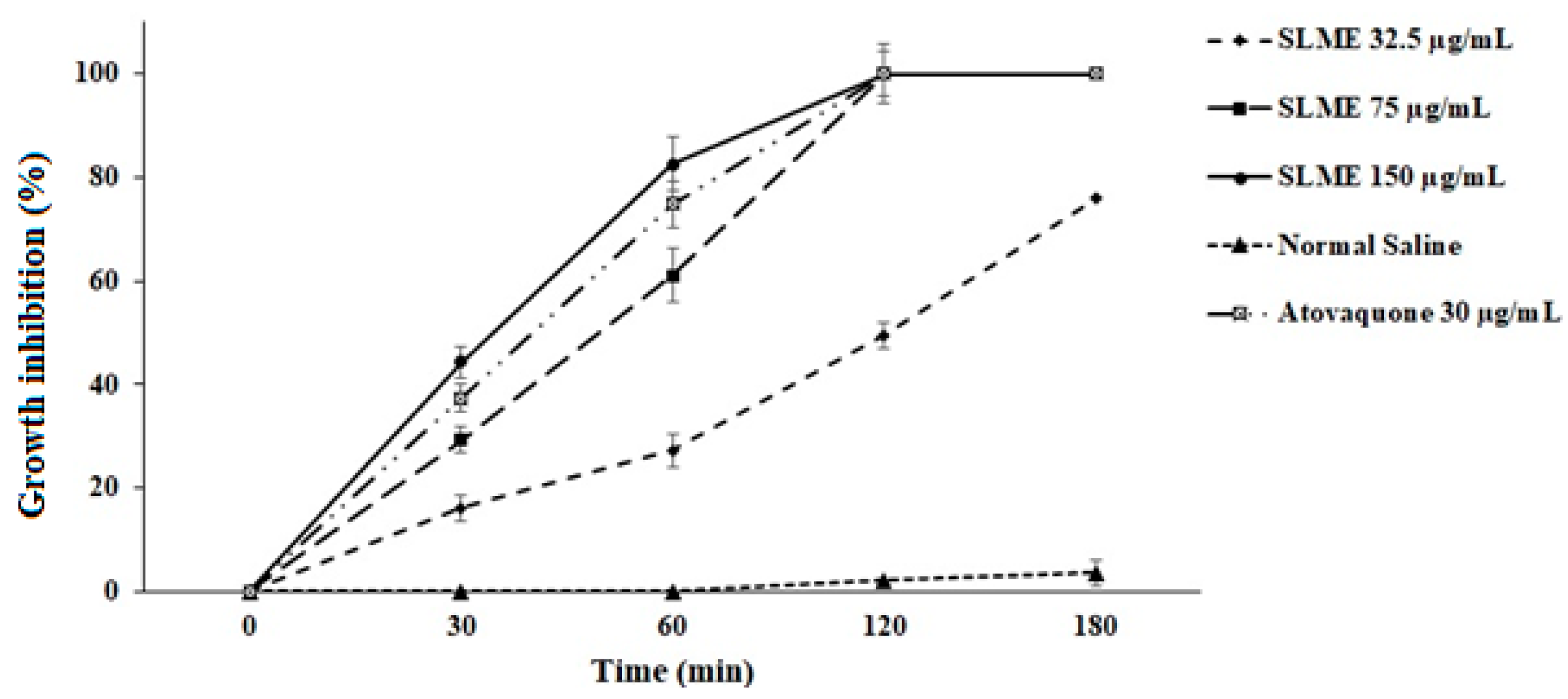
3.2. In Vitro Anti-Toxoplasma Effects
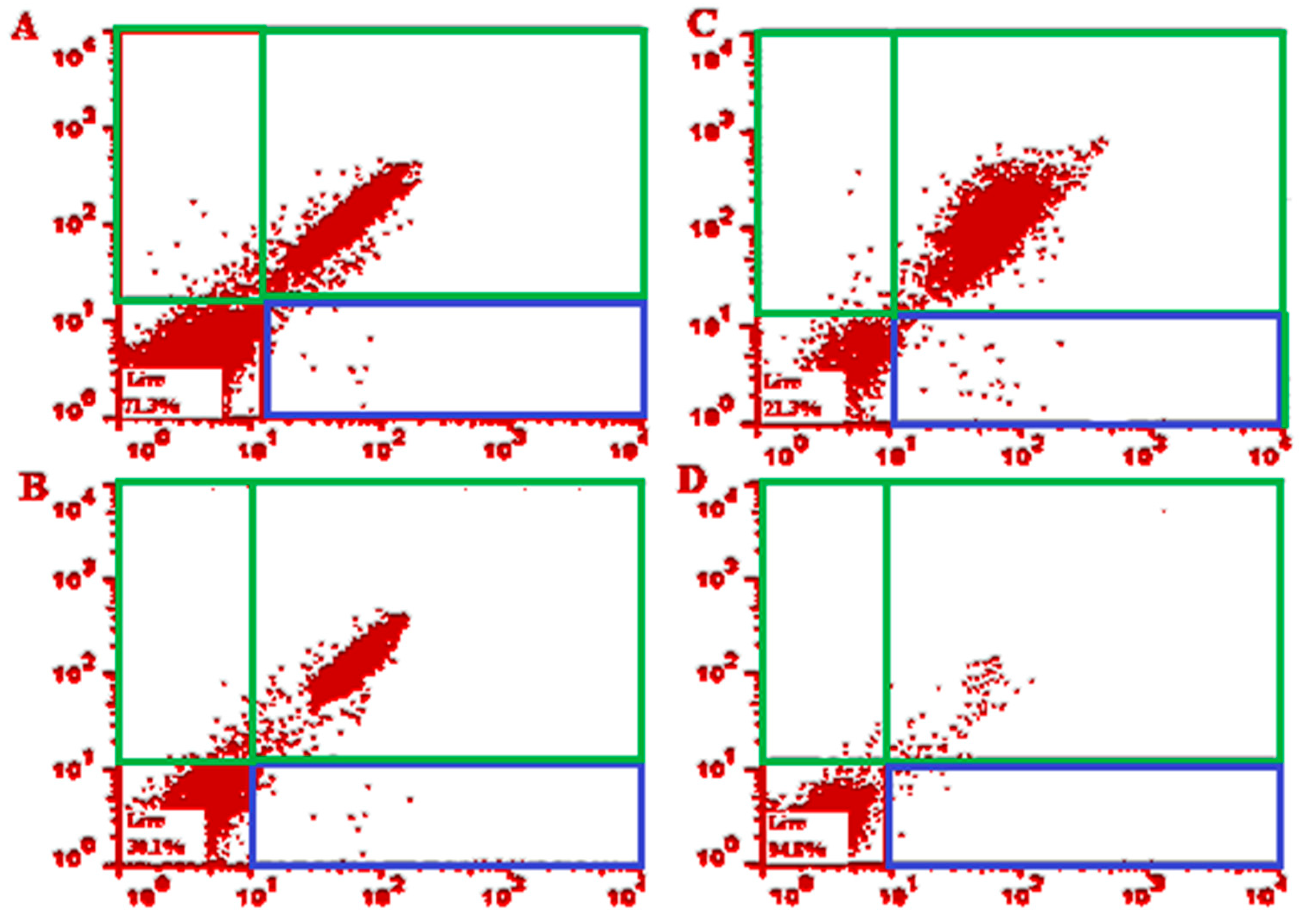
3.3. Flow Cytometry Analysis
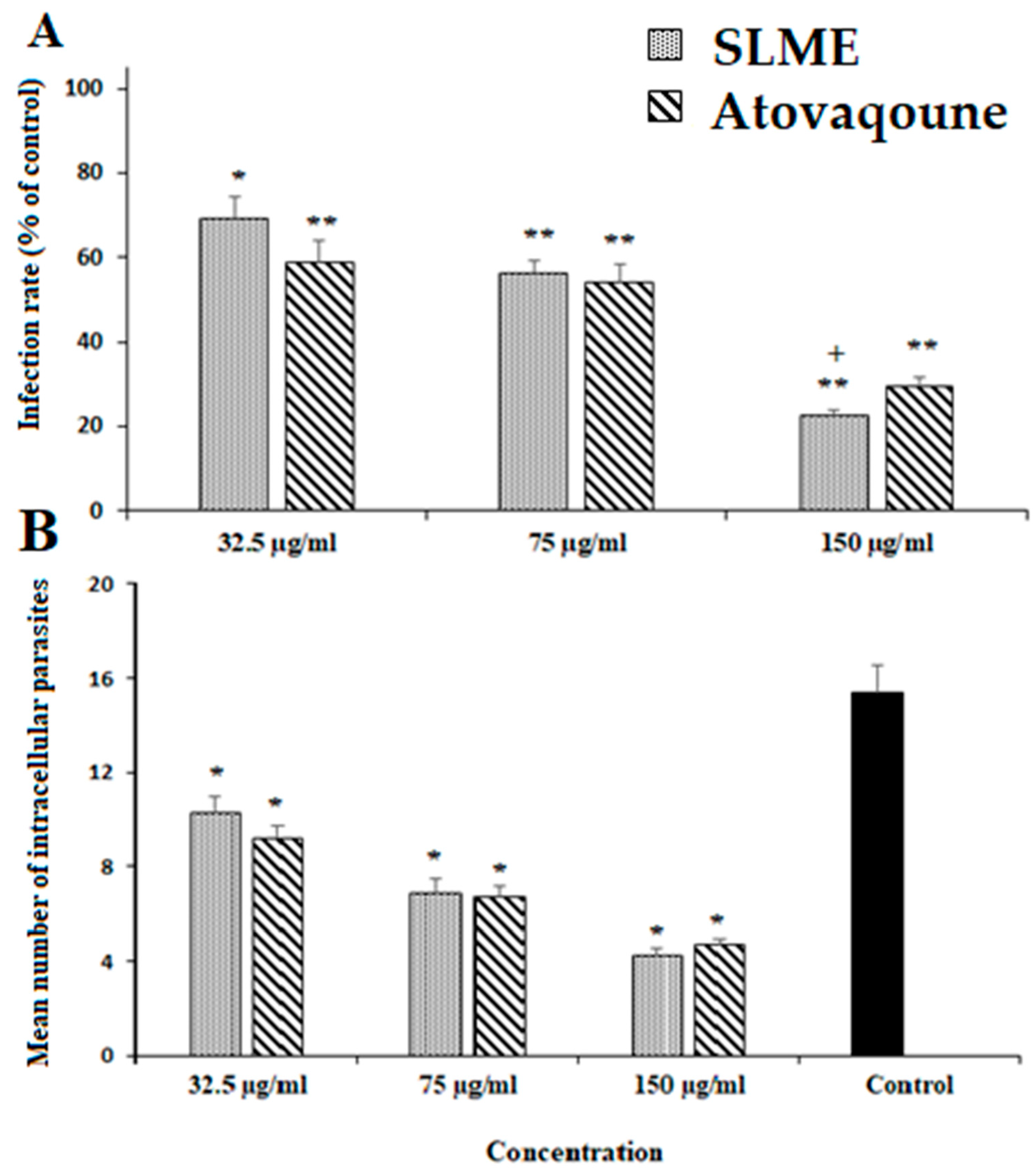
3.4. Effect of SLME on Intracellular Parasites, Infectivity Rate, and Cytotoxicity
3.5. In Vivo Anti-Toxoplasma Effects
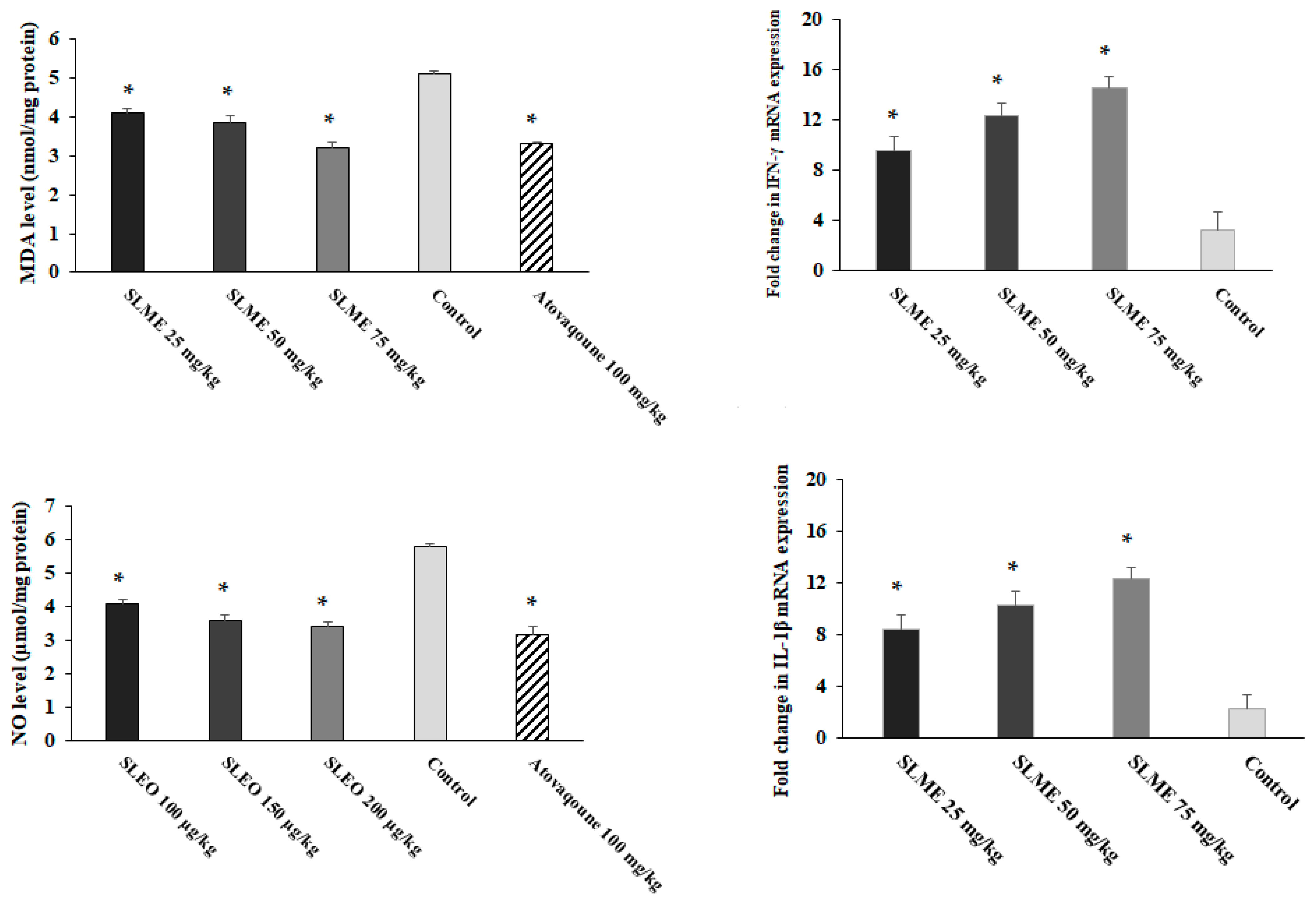
3.6. Effect on Oxidative Stress and Proinflammatory Cytokines
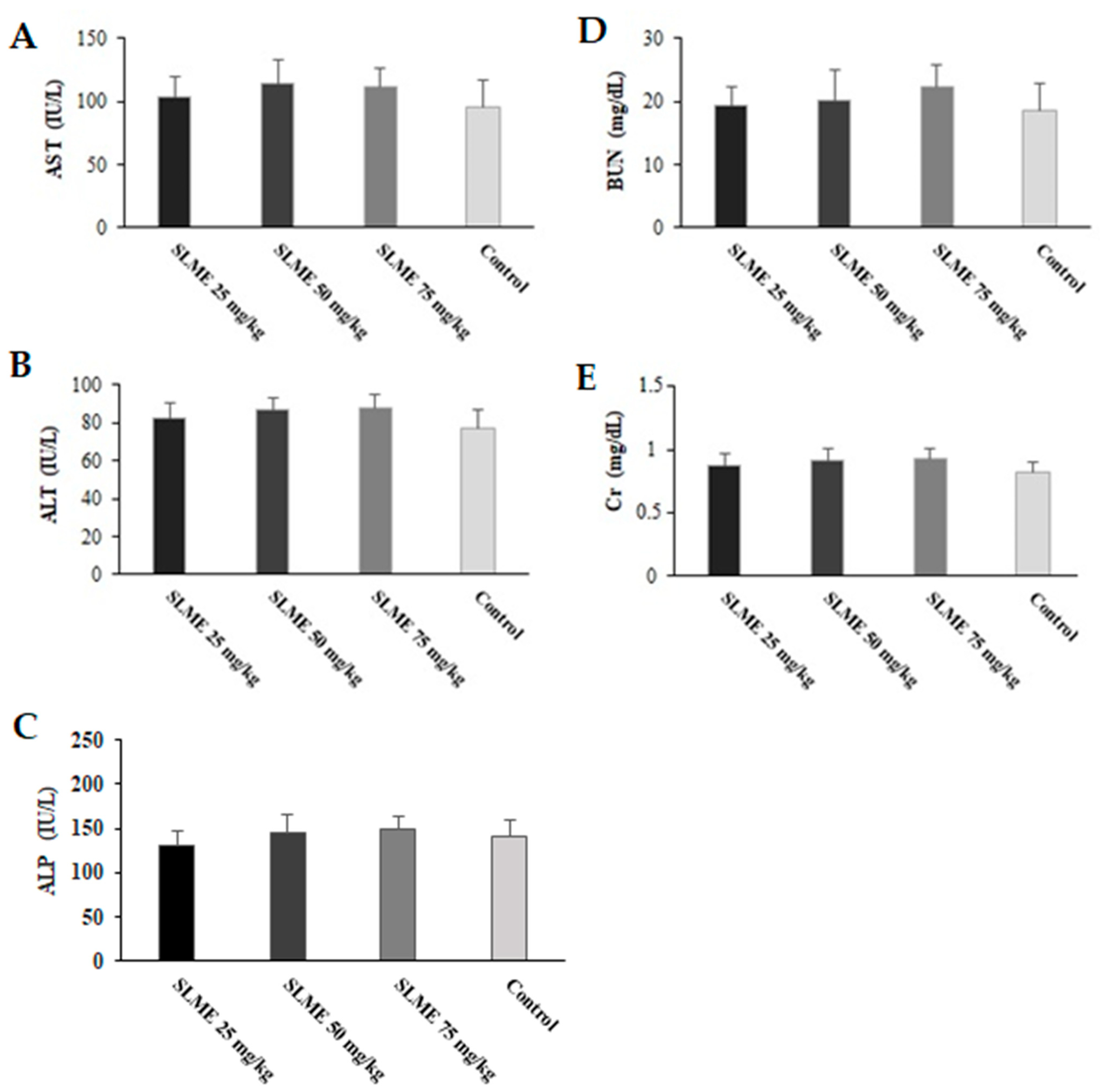
3.7. Toxicity Effects of SLME on Liver and Kidney Functions in Healthy Mice
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Saadatnia, G.; Golkar, M. A review on human toxoplasmosis. Scand. J. Infect. Dis. 2012, 44, 805–814. [Google Scholar] [CrossRef] [PubMed]
- Molan, A.; Nosaka, K.; Hunter, M.; Wang, W. Global status of Toxoplasma gondii infection: Systematic review and prevalence snapshots. Trop. Biomed. 2019, 36, 898–925. [Google Scholar] [PubMed]
- Martinez, V.O.; de Mendonça Lima, F.W.; De Carvalho, C.F.; Menezes-Filho, J.A. Toxoplasma gondii infection and behavioral outcomes in humans: A systematic review. Parasitol. Res. 2018, 117, 3059–3065. [Google Scholar] [CrossRef] [PubMed]
- Hampton, M.M. Congenital toxoplasmosis: A review. Neonatal Netw. 2015, 34, 274–278. [Google Scholar] [CrossRef]
- Dubey, J.P.; Tiao, N.; Gebreyes, W.A.; Jones, J.L. A review of toxoplasmosis in humans and animals in Ethiopia. Epidemiol. Infect. 2012, 140, 1935–1938. [Google Scholar] [CrossRef] [Green Version]
- Melo, M.B.; Jensen, K.D.; Saeij, J.P. Toxoplasma gondii effectors are master regulators of the inflammatory response. Trends Parasitol. 2011, 27, 487–495. [Google Scholar] [CrossRef] [Green Version]
- McCabe, R.E. Antitoxoplasma chemotherapy. In Toxoplasmosis: A Comprehensive Clinical Guide; Cambridge University Press: Cambridge, UK, 2001; pp. 319–359. [Google Scholar]
- Cheraghipour, K.; Masoori, L.; Ezzatpour, B.; Roozbehani, M.; Sheikhian, A.; Malekara, V.; Niazi, M.; Mardanshah, O.; Moradpour, K.; Mahmoudvand, H. The experimental role of medicinal plants in treatment of Toxoplasma gondii infection: A systematic review. Acta Parasitol. 2021, 66, 303–328. [Google Scholar] [CrossRef]
- Tundis, R.; Peruzzi, L.; Menichini, F. Phytochemical and biological studies of Stachys species in relation to chemotaxonomy: A review. Phytochemistry 2014, 102, 7–39. [Google Scholar] [CrossRef]
- Minae, B.; Sardari, M.; Sharifi, H.; Sedigh Rahim Abadi, M.S.; Sadeghpour, O. Stachys lavandulifolia Vahl. and its Relation With Marmazad Activities in Traditional Manuscripts. Iran. Red Crescent Med. J. 2015, 17, e19932. [Google Scholar] [CrossRef] [Green Version]
- Tomou, E.M.; Barda, C.; Skaltsa, H. Genus Stachys: A review of traditional uses, phytochemistry and bioactivity. Medicines 2020, 7, 63. [Google Scholar] [CrossRef]
- Sepulveda-Arias, J.C.; Veloza, L.A.; Mantilla-Muriel, L.E. Anti-Toxoplasma activity of natural products: A review. Recent Pat. Anti-Infect. Drug Discov. 2014, 9, 186–194. [Google Scholar] [CrossRef]
- Sharif, M.; Sarvi, S.; Pagheh, A.S.; Asfaram, S.; Rahimi, M.T.; Mehrzadi, S.; Ahmadpour, E.; Gholami, S.; Daryani, A. The efficacy of herbal medicines against Toxoplasma gondii during the last 3 decades: A systematic review. Can. J. Physiol. Pharmacol. 2016, 94, 1237–1248. [Google Scholar] [CrossRef] [PubMed]
- Masoumi-Ardakani, Y.; Mahmoudvand, H.; Mirzaei, A.; Esmaeilpour, K.; Ghazvini, H.; Khalifeh, S.; Sepehri, G. The effect of Elettaria cardamomum extract on anxiety-like behavior in a rat model of post-traumatic stress disorder. Biomed. Pharmacother. 2017, 87, 489–495. [Google Scholar] [CrossRef]
- Ebrahimi, K.; Shiravand, S.; Mahmoudvand, H. Biosynthesis of copper nanoparticles using aqueous extract of Capparis spinosa fruit and investigation of its antibacterial activity. Marmara Pharm. J. 2017, 21, 866–871. [Google Scholar] [CrossRef]
- Ezatpour, B.; Saedi Dezaki, E.; Mahmoudvand, H.; Azadpour, M.; Ezzatkhah, F. In vitro and in vivo antileishmanial effects of Pistaciakhinjuk against Leishmania tropica and Leishmania major. Evid. Based Complement. Altern. Med. 2015, 2015, 149707. [Google Scholar] [CrossRef] [Green Version]
- Mahmoudvand, H.; Kheirandish, F.; Ghasemi Kia, M.; Tavakoli Kareshk, A.; Yarahmadi, M. Chemical composition, protoscolicidal effects and acute toxicity of Pistacia atlantica Desf. fruit extract. Nat. Prod. Res. 2016, 30, 1208–1211. [Google Scholar] [CrossRef]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of folin-ciocalteu reagent. Methods Enzymol. 1999, 299, 152–178. [Google Scholar]
- Phuyal, N.; Jha, P.K.; Raturi, P.P.; Rajbhandary, S. Total phenolic, flavonoid contents, and antioxidant activities of fruit, seed, and bark extracts of Zanthoxylum armatum DC. Sci. World J. 2020, 2020, 8780704. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alnomasy, S.F. In vitro and in vivo Anti-Toxoplasma Effects of Allium sativum Essential Oil Against Toxoplasma gondii RH Strain. Infect. Drug Resist. 2021, 14, 5057–5068. [Google Scholar] [CrossRef] [PubMed]
- de Campos, G.B.; Franco, R.F.F.; Souza, J.Y.; Mendonça, N.S.D.; Vinaud, M.C.; de Castro, A.M. In vitro evaluation of atovaquone on the replication of Toxoplasma gondii tachyzoites strains Rh and Me49. Sci. Res. Rev. 2020, 13, 114. [Google Scholar] [CrossRef]
- Albalawi, A.E.; Khalaf, A.K.; Alyousif, M.S.; Alanazi, A.D.; Baharvand, P.; Shakibaie, M.; Mahmoudvand, H. Fe3O4@ piroctone olamine magnetic nanoparticles: Synthesize and therapeutic potential in cutaneous leishmaniasis. Biomed. Pharmacother. 2021, 139, 111566. [Google Scholar] [CrossRef] [PubMed]
- Albalawi, A.E.; Abdel-Shafy, S.; Khudair Khalaf, A.; Alanazi, A.D.; Baharvand, P.; Ebrahimi, K.; Mahmoudvand, H. Therapeutic potential of green synthesized copper nanoparticles alone or combined with meglumine antimoniate (glucantime®) in cutaneous leishmaniasis. Nanomaterials 2021, 11, 891. [Google Scholar] [CrossRef] [PubMed]
- Ezzatkhah, F.; Mahmoudvand, H.; Raziani, Y. The role of Curcuma longa essential oil in controlling acute toxoplasmosis by improving the immune system and reducing inflammation and oxidative stress. Front. Cell Infect. Microbiol. 2023, 13, 1161133. [Google Scholar] [CrossRef] [PubMed]
- Kharazmkia, A.; Al-Abodi, H.R.; Yadegari, J.G.; Vahidi, A.; Mahmoudvand, H. Potential effects of alpha-pinene, a monoterpene commonly found in essential oils against Toxoplasma gondii infection; an in vitro and in vivo study. J Parasitic Dis 2022, 46, 1055–1061. [Google Scholar] [CrossRef]
- Shakibaie, M.; Ezzatkhah, F.; Gabal, E.; Badparva, E.; Jahanbakhsh, S.; Mahmoudvand, H. Prophylactic effects of biogenic selenium nanoparticles on acute toxoplasmosis: An in vivo study. Ann. Med. Surg. (Lond.) 2020, 54, 85–88. [Google Scholar] [CrossRef]
- Keyhani, A.; Ziaali, N.; Shakibaie, M.; Kareshk, A.T.; Shojaee, S.; Asadi-Shekaari, M.; Sepahvand, M.; Mahmoudvand, H. Biogenic selenium nanoparticles target chronic toxoplasmosis with minimal cytotoxicity in a mouse model. J. Med. Microbiol. 2020, 69, 104–110. [Google Scholar] [CrossRef]
- Alnomasy, S.; Al-Awsi, G.R.L.; Raziani, Y.; Albalawi, A.E.; Alanazi, A.D.; Niazi, M.; Mahmoudvand, H. Systematic review on medicinal plants used for the treatment of Giardia infection. Saudi J. Biol. Sci. 2021, 28, 5391–5402. [Google Scholar] [CrossRef]
- Kareshk, A.T.; Keyhani, A.; Mahmoudvand, H.; Oliaei, R.T.; Asadi, A.; Andishmand, M.; Azzizian, H.; Babaei, Z.; Naser, Z.A. Efficacy of the Bunium persicum (Boiss) essential oil against acute toxoplasmosis in mice model. Iran. J. Parasitol. 2015, 10, 625. [Google Scholar]
- Mahmoudvand, H.; Tavakoli Kareshk, A.; Nabi Moradi, M.; Monzote Fidalgo, L.; Mirbadie, S.R.; Niazi, M.; Khatami, M. Efficacy and safety of Zataria multiflora Boiss essential oil against acute toxoplasmosis in mice. Iran. J. Parasitol. 2020, 15, 22–30. [Google Scholar] [CrossRef] [Green Version]
- Mahmoudvand, H.; Kareshk, A.T.; Keyhani, A.; Zia-Ali, N.; Aflatoonian, M.R. In vivo evaluation of Berberis vulgaris extract on acute toxoplasmosis in mice. Marmara Pharm. J. 2017, 21, 558–563. [Google Scholar] [CrossRef] [Green Version]
- Borges, A.; José, H.; Homem, V.; Simões, M. Comparison of techniques and solvents on the antimicrobial and antioxidant potential of extracts from Acacia dealbata and Olea europaea. Antibiotics 2020, 9, 48. [Google Scholar] [CrossRef] [PubMed]
- Barakat, A.M.; El-Razik, K.A.; El Fadaly, H.A.; Saleh, W.M.; Ali, F.A.; Gouda, A.A.; Sadek, S.A.; Dahran, N.; El-Khadragy, M.F.; Elmahallawy, E.K. Parasitological, Molecular, and Histopathological Investigation of the Potential Activity of Propolis and Wheat Germ Oil against Acute Toxoplasmosis in Mice. Pharmaceutics 2023, 15, 478. [Google Scholar] [CrossRef] [PubMed]
- Barati, M.; Fakhar, M.; Gholami, S.; Rahimi Esboei, B.; Elmi, T. The evaluation of Stachys lavandulifolia leave extracts on cysts of G. lamblia, in vitro. J. Arch. Mil. Med. 2017, 5, e59529. [Google Scholar] [CrossRef] [Green Version]
- Sereshti, M.; Yousofi Darani, H.; Zebardast, N.; Rafieian-Kopaei, M.; Naeini, M.; Yousofi, H.A. Effect of ethanolic and watery extract of aerial parts of Stachys lavandulifolia on Trichomonas vaginalis, in vitro. J. Med. Plants 2012, 11 (Suppl. S8), 159–165. [Google Scholar]
- Asadi, M.; Bahrami, S.; AnsariSamani, R.; Pakniat, N. Effect of hydroalcoholic extracts of Stachys lavandulifolia Vahl and Mespilus germanica leaves on Leishmania major. Hormozgan Med. J. 2012, 15, 279–284. [Google Scholar]
- Mead, J.; McNair, N. Antiparasitic activity of flavonoids and isoflavones against Cryptosporidium parvum and Encephalitozoon intestinalis. FEMS Microbiol. Lett. 2006, 259, 153–157. [Google Scholar] [CrossRef] [Green Version]
- Lehane, A.M.; Saliba, K.J. Common dietary flavonoids inhibit the growth of the intraerythrocytic malaria parasite. BMC Res. Notes 2008, 1, 26. [Google Scholar] [CrossRef] [Green Version]
- Ghasemian Yadegari, J.; Khudair Khalaf, A.; Darabi, R.; Mahmoudvand, H. Antiparasitic effects and cellular mechanism of Astragalus maximus chloroform extract against clinical isolates of Giardia lamblia. Res. J. Pharmacogn. 2022, 9, 5–13. [Google Scholar] [CrossRef]
- Mendes, L.F.; Gaspar, V.M.; Conde, T.A.; Mano, J.F.; Duarte, I.F. Flavonoid-mediated immunomodulation of human macrophages involves key metabolites and metabolic pathways. Sci. Rep. 2019, 9, 14906. [Google Scholar] [CrossRef] [Green Version]
- Grigore, A. Plant Phenolic Compounds as Immunomodulatory Agents. In Phenolic Compounds; IntechOpen: London, UK, 2017; pp. 75–98. [Google Scholar]
- Nolan, S.J.; Romano, J.D.; Kline, J.T.; Coppens, I. Novel approaches to kill Toxoplasma gondii by exploiting the uncontrolled uptake of unsaturated fatty acids and vulnerability to lipid storage inhibition of the parasite. Antimicrob. Agents Chemother. 2018, 62, e00347-18. [Google Scholar] [CrossRef] [Green Version]
- Souza, M.C.; Fonseca, D.M.; Kanashiro, A.; Benevides, L.; Medina, T.S.; Dias, M.S.; Andrade, W.A.; Bonfá, G.; Silva, M.A.B.; Gozzi, A.; et al. Chronic toxoplasma gondii infection exacerbates secondary polymicrobial sepsis. Front. Cell Infect. Microbiol. 2017, 7, 116. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Al Nasr, I.; Ahmed, F.; Pullishery, F.; El-Ashram, S.; Ramaiah, V.V. Toxoplasmosis and anti-Toxoplasma effects of medicinal plant extracts-A mini-review. Asian Pac. J. Trop. Med. 2016, 9, 730–734. [Google Scholar] [CrossRef] [Green Version]
- Besteiro, S. Toxoplasma control of host apoptosis: The art of not biting too hard the hand that feeds you. Microb. Cell 2015, 2, 178–181. [Google Scholar] [CrossRef] [PubMed]
- Balahoroğlu, R.; Dülger, H.; Özbek, H.; Bayram, İ.; Şekeroğlu, M.R. Protective effects of antioxidants on the experimental liver and kidney toxicity in mice. Eur. J. Gen. Med. 2008, 5, 157–164. [Google Scholar]

| Phytochemical | Test | Presence |
|---|---|---|
| Flavonoids | Ammonia test, alkaline reagent test | + |
| Saponins | Frothing test | - |
| Terpenoids | Salkowski test | + |
| Tannins | 1% gelatin and 10% NaCl solutions | + |
| Alkaloids | Mayer and Dragendorff’s reagents | + |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alanazi, A.D.; Majeed, Q.A.H.; Alnomasy, S.F.; Almohammed, H.I. Potent In Vitro and In Vivo Effects of Stachys lavandulifolia Methanolic Extract against Toxoplasma gondii Infection. Trop. Med. Infect. Dis. 2023, 8, 355. https://doi.org/10.3390/tropicalmed8070355
Alanazi AD, Majeed QAH, Alnomasy SF, Almohammed HI. Potent In Vitro and In Vivo Effects of Stachys lavandulifolia Methanolic Extract against Toxoplasma gondii Infection. Tropical Medicine and Infectious Disease. 2023; 8(7):355. https://doi.org/10.3390/tropicalmed8070355
Chicago/Turabian StyleAlanazi, Abdullah D., Qais A. H. Majeed, Sultan F. Alnomasy, and Hamdan I. Almohammed. 2023. "Potent In Vitro and In Vivo Effects of Stachys lavandulifolia Methanolic Extract against Toxoplasma gondii Infection" Tropical Medicine and Infectious Disease 8, no. 7: 355. https://doi.org/10.3390/tropicalmed8070355






