Patterns of Animal Rabies Prevalence in Northern South Africa between 1998 and 2022
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area Background
2.2. Data Collection
2.2.1. Rabies Data
2.2.2. Diagnoses
2.2.3. Land Use/Land Cover Trends and Human Population
2.3. Data Analysis
2.3.1. Descriptive Analysis
2.3.2. Temporal and Spatial Analysis
2.3.3. Land Use/Land Cover Trends and Human Population
3. Results
3.1. Summary Results of Rabies Animal Cases
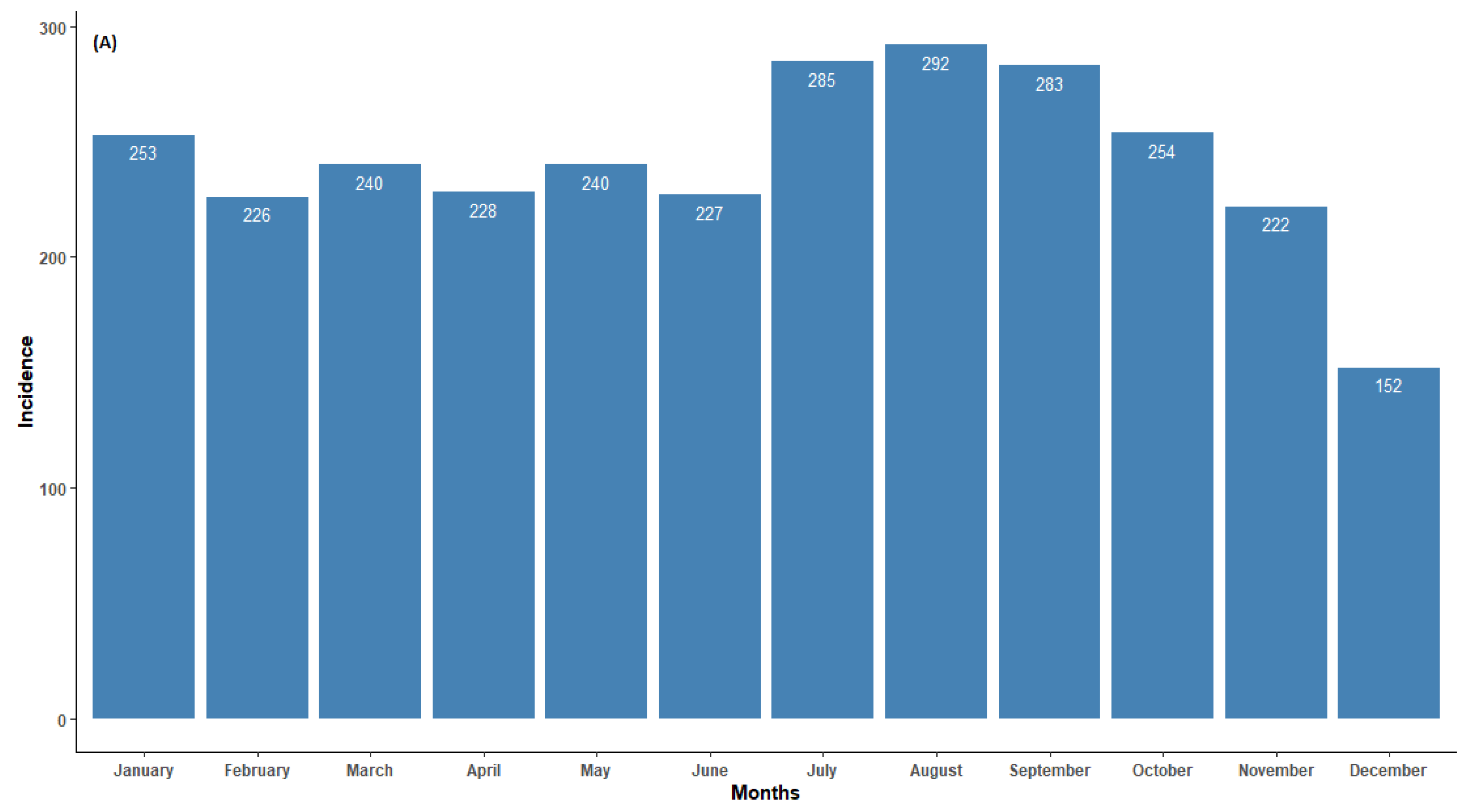
3.2. Temporal Patterns
3.3. Spatial Patterns
3.4. Land Use/Land Cover Trends and Human Population
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Maes, P.; Amarasinghe, G.K.; Ayllón, M.A.; Basler, C.F.; Bavari, S.; Blasdell, K.R.; Briese, T.; Brown, P.A.; Bukreyev, A.; Balkema-Buschmann, A.; et al. Taxonomy of the order Mononegavirales: Second update 2018. Arch. Virol. 2019, 164, 1233–1244. [Google Scholar] [CrossRef] [PubMed]
- Swanepoel, R.; Barnard, B.J.; Meredith, C.D.; Bishop, G.C.; Brückner, G.K.; Foggin, C.M.; Hübschle, O.J. Rabies in southern Africa. Onderstepoort J. Vet. Res. 1993, 60, 325–346. [Google Scholar] [PubMed]
- Singh, R.; Singh, K.P.; Cherian, S.; Saminathan, M.; Kapoor, S.; Manjunatha Reddy, G.B.; Panda, S.; Dhama, K. Rabies—Epidemiology, pathogenesis, public health concerns and advances in diagnosis and control: A comprehensive review. Vet. Q. 2017, 37, 212–251. [Google Scholar] [CrossRef]
- Hampson, K.; Coudeville, L.; Lembo, T.; Sambo, M.; Kieffer, A.; Attlan, M.; Barrat, J.; Blanton, J.D.; Briggs, D.J.; Cleaveland, S.; et al. Estimating the global burden of endemic canine rabies. PLoS Negl. Trop. Dis. 2015, 9, e0003709. [Google Scholar] [CrossRef]
- Knobel, D.L.; Cleaveland, S.; Coleman, P.G.; Fèvre, E.M.; Meltzer, M.I.; Miranda, M.E.G.; Shaw, A.; Zinsstag, J.; Meslin, F.-X. Re-evaluating the burden of rabies in Africa and Asia. Bull. World Health Organ. 2005, 83, 360–368. [Google Scholar] [PubMed]
- Stöhr, K.; Meslin, F.X. The role of veterinary public health in the prevention of zoonoses. Arch. Virol. Suppl. 1997, 13, 207–218. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Investing To Overcome The Global Impact of Neglected Tropical Diseases: Third WHO Report on Neglected Tropical Diseases; WHO: Geneva, Switerzland, 2015; ISBN 978-92-4-156486-1. Available online: https://www.google.co.za/books/edition/Investing_to_Overcome_the_Global_Impact/mV00DgAAQBAJ?hl=en&gbpv=1&printsec=frontcover (accessed on 23 March 2022).
- Sabeta, C.T.; Mansfield, K.L.; McElhinney, L.M.; Fooks, A.R.; Nel, L.H. Molecular epidemiology of rabies in bat-eared foxes (Otocyon megalotis) in South Africa. Virus Res. 2007, 129, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Nel, L.H.; Le Roux, K.; Atlas, R.M. Meeting the Rabies Control Challenge in South Africa. Microbe 2009, 4, 61. [Google Scholar]
- Zulu, G.C.; Sabeta, C.T.; Nel, L.H. Molecular epidemiology of rabies: Focus on domestic dogs (Canis familiaris) and black-backed jackals (Canis mesomelas) from northern South Africa. Virus Res. 2009, 140, 71–78. [Google Scholar] [CrossRef]
- Sabeta, C.T.; Janse van Rensburg, D.; Phahladira, B.; Mohale, D.; Harrison-White, R.F.; Esterhuyzen, C.; Williams, J.H. Rabies of canid biotype in wild dog (Lycaon pictus) and spotted hyaena (Crocuta crocuta) in Madikwe Game Reserve, South Africa in 2014-2015: Diagnosis, possible origins and implications for control. J. S. Afr. Vet. Assoc. 2018, 89, e1–e13. [Google Scholar] [CrossRef]
- Malan, A.J.; Coetzer, A.; Sabeta, C.T.; Nel, L.H. Epidemiological Interface of Sylvatic and Dog Rabies in the North West Province of South Africa. Tropical. Med. 2022, 7, 90. [Google Scholar] [CrossRef]
- von Teichman, B.F.; Thomson, G.R.; Meredith, C.D.; Nel, L.H. Molecular epidemiology of rabies virus in South Africa: Evidence for two distinct virus groups. J. Gen. Virol. 1995, 76 Pt 1, 73–82. [Google Scholar] [CrossRef]
- Ngoepe, C.E.; Sabeta, C.; Nel, L. The spread of canine rabies into Free State province of South Africa: A molecular epidemiological characterization. Virus Res. 2009, 142, 175–180. [Google Scholar] [CrossRef] [PubMed]
- Weyer, J. Rabies in South Africa: Where Do We Stand in 2015? S. Afr. J. Infect. Dis. 2015, 30, 40–41. [Google Scholar] [CrossRef]
- Hergert, M.; Nel, L.H. Dog Bite Histories and Response to Incidents in Canine Rabies-Enzootic KwaZulu-Natal, South Africa. PLoS Negl. Trop. Dis. 2013, 7, e2059. [Google Scholar] [CrossRef] [PubMed]
- Morters, M.K.; Restif, O.; Hampson, K.; Cleaveland, S.; Wood, J.L.N.; Conlan, A.J.K. Evidence-based control of canine rabies: A critical review of population density reduction. J. Anim. Ecol. 2013, 82, 6–14. [Google Scholar] [CrossRef] [PubMed]
- Sabeta, C.; Phahladira, B.; Marston, D.A.; Wise, E.L.; Ellis, R.J.; Fooks, A.R. Complete Genome Sequences of Six South African Rabies Viruses. Genome Announc. 2015, 3, e01085-15. [Google Scholar] [CrossRef]
- Hergert, M.; Le Roux, K.; Nel, L.H. Characteristics of owned dogs in rabies endemic KwaZulu-Natal province, South Africa. BMC Vet. Res. 2018, 14, 278. [Google Scholar] [CrossRef]
- Brown, K. Rabid epidemiologies: The emergence and resurgence of rabies in twentieth century South Africa. J. Hist. Biol. 2011, 44, 81–101. [Google Scholar] [CrossRef] [PubMed]
- Weyer, J.; Szmyd-Potapczuk, A.V.; Blumberg, L.H.; Leman, P.A.; Markotter, W.; Swanepoel, R.; Paweska, J.T.; Nel, L.H. Epidemiology of human rabies in South Africa, 1983–2007. Virus Res 2011, 155, 283–290. [Google Scholar] [CrossRef]
- Weyer, J.; Dermaux-Msimang, V.; Grobbelaar, A.; Le Roux, C.; Moolla, N.; Paweska, J.; Blumberg, L. Epidemiology of human rabies in South Africa, 2008–2018. S. Afr. Med. J. 2020, 110, 877–881. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization; Food and Agriculture Organization; World Organisation for Animal Health. The Global Strategic Plan to End Human Deaths from Dog-Mediated Rabies by 2030; World Health Organizaton (WHO): Geneva, Switzerland; World Organisation for Animal Health (OIE): Paris, France; Food and Agriculture Organization of the United Nations (FAO): Rome, Italy, 2018; p. 55, Report No.: 978-92-5-130461–7; Available online: https://www.fao.org/documents/card/en?details=I8956EN (accessed on 8 January 2024).
- Mogano, K.; Suzuki, T.; Mohale, D.; Phahladira, B.; Ngoepe, E.; Kamata, Y.; Chirima, G.; Sabeta, C.; Makita, K. Spatio-temporal epidemiology of animal and human rabies in northern South Africa between 1998 and 2017. PLoS Negl. Trop. Dis. 2022, 16, e0010464. [Google Scholar] [CrossRef] [PubMed]
- Koeppel, K.N. Spatiotemporal Analysis of Rabies in South Africa, the Role of Black-Backed Jackals (Canis mesomelas) and Aspects of Its Control by Oral Rabies Vaccination. Ph.D. Thesis, University of Pretoria, Pretoria, South Africa, 2020. [Google Scholar]
- McKenzie, A.A. Biology of the black-backed jackal Canis mesomelas with reference to rabies. Onderstepoort J. Vet. Res. 1993, 60, 367–371. [Google Scholar]
- Grover, M.; Bessell, P.R.; Conan, A.; Polak, P.; Sabeta, C.T.; Reininghaus, B.; Knobel, D.L. Spatiotemporal epidemiology of rabies at an interface between domestic dogs and wildlife in South Africa. Sci. Rep. 2018, 8, 10864. [Google Scholar] [CrossRef] [PubMed]
- Thorn, M.; Green, M.; Dalerum, F.; Bateman, P.W.; Scott, D.M. What drives human–carnivore conflict in the North West Province of South Africa? Biol. Conserv. 2012, 150, 23–32. [Google Scholar] [CrossRef]
- McCrindle, C.M.E.; Gallant, J.; Cornelius, S.T.; Schoeman, H.S. Changing Roles of Dogs in Urban African Society: A South African Perspective. Anthrozoös 1999, 12, 157–161. [Google Scholar] [CrossRef]
- Bishop, G.C.; Durrheim, D.N.; Kloeck, P.E.; Godlonton, J.D. Rabies: Guide for the Medical, Veterinary and Allied Professions, 2nd ed.; Department of Agriculture, Forestry and Fisheries: Pretoria, South Africa, 2010. [Google Scholar]
- Grover, M. Spatio-Temporal Analysis of Dog Ecology and Rabies Epidemiology at a Wildlife Interface in the Lowveld Region of South Africa. Master’s Thesis, University of Pretoria, Pretoria, South Africa, 2015. [Google Scholar]
- Meslin, F.X.; Kaplan, M.M.; Koprowski, H. Laboratory Techniques in Rabies, 4th ed.; World Health Organization: Geneva, Switzerland, 1996. [Google Scholar]
- Glass, G.E.; Schwartz, B.S.; Morgan, J.M.; Johnson, D.T.; Noy, P.M.; Israel, E. Environmental risk factors for Lyme disease identified with geographic information systems. Am. J. Public. Health 1995, 85, 944–948. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.; Tong, S.; Mengersen, K.; Oldenburg, B. Exploratory spatial analysis of social and environmental factors associated with the incidence of Ross River virus in Brisbane, Australia. Am. J. Trop. Med. Hyg. 2007, 76, 814–819. [Google Scholar] [CrossRef]
- Ward, M.P.; Wittich, C.A.; Fosgate, G.; Srinivasan, R. Environmental risk factors for equine West Nile virus disease cases in Texas. Vet. Res. Commun. 2009, 33, 461–471. [Google Scholar] [CrossRef]
- Weiss, R.A.; McMichael, A.J. Social and environmental risk factors in the emergence of infectious diseases. Nat. Med. 2004, 10, S70–S76. [Google Scholar] [CrossRef]
- Jones, M.E.; Curns, A.T.; Krebs, J.W.; Childs, J.E. Environmental and human demographic features associated with epizootic raccoon rabies in Maryland, Pennsylvania, and Virginia. J. Wildl. Dis. 2003, 39, 869–874. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.-H.; Lee, C.-G.; Yoon, H.-C.; Nam, H.-M.; Park, C.-K.; Lee, J.-C.; Kang, M.-I.; Wee, S.-H. Rabies, an emerging disease in Korea. J. Vet. Med. B Infect. Dis. Vet. Public. Health 2006, 53, 111–115. [Google Scholar] [CrossRef] [PubMed]
- Tenzin; Dhand, N.K.; Ward, M.P. Human rabies post exposure prophylaxis in Bhutan, 2005–2008: Trends and risk factors. Vaccine 2011, 29, 4094–4101. [Google Scholar] [CrossRef]
- Tenzin; Dhand, N.K.; Ward, M.P. Patterns of Rabies Occurrence in Bhutan between 1996 and 2009. Zoonoses Public Health 2011, 58, 463–471. [Google Scholar] [CrossRef] [PubMed]
- Ward, M.P.; Iglesias, R.M.; Brookes, V.J. Autoregressive Models Applied to Time-Series Data in Veterinary Science. Front. Vet. Sci. 2020, 7, 604. [Google Scholar] [CrossRef]
- Ord, J.K.; Getis, A. Local Spatial Autocorrelation Statistics: Distributional Issues and an Application. Geogr. Anal. 1995, 27, 286–306. [Google Scholar] [CrossRef]
- Lembo, T.; Hampson, K.; Haydon, D.T.; Craft, M.; Dobson, A.; Dushoff, J.; Ernest, E.; Hoare, R.; Kaare, M.; Mlengeya, T.; et al. Exploring reservoir dynamics: A case study of rabies in the Serengeti ecosystem. J. Appl. Ecol. 2008, 45, 1246–1257. [Google Scholar] [CrossRef]
- Gummow, B.; Roefs, Y.A.A.; De Klerk, G. Rabies in South Africa between 1993 and 2005—What has been achieved? J. S. Afr. Vet. Assoc. 2010, 81, 16–21. [Google Scholar] [CrossRef]
- Pfukenyi, D.M.; Pawandiwa, D.; Makaya, P.V.; Ushewokunze-Obatolu, U. A retrospective study of wildlife rabies in Zimbabwe, between 1992 and 2003. Trop. Anim. Health Prod. 2009, 41, 565–572. [Google Scholar] [CrossRef]
- Coetzer, A.; Gwenhure, L.; Makaya, P.; Markotter, W.; Nel, L. Epidemiological aspects of the persistent transmission of rabies during an outbreak (2010–2017) in Harare, Zimbabwe. PLoS ONE 2019, 14, e0210018. [Google Scholar] [CrossRef]
- Courtin, F.; Carpenter, T.E.; Paskin, R.D.; Chomel, B.B. Temporal patterns of domestic and wildlife rabies in central Namibia stock-ranching area, 1986–1996. Prev. Vet. Med. 2000, 43, 13–28. [Google Scholar] [CrossRef] [PubMed]
- Hikufe, E.H.; Freuling, C.M.; Athingo, R.; Shilongo, A.; Ndevaetela, E.-E.; Helao, M.; Shiindi, M.; Hassel, R.; Bishi, A.; Khaiseb, S.; et al. Ecology and epidemiology of rabies in humans, domestic animals and wildlife in Namibia, 2011–2017. PLoS Negl. Trop. Dis. 2019, 13, e0007355. [Google Scholar] [CrossRef] [PubMed]
- Bitek, A.O.; Osoro, E.; Munyua, P.M.; Nanyingi, M.; Muthiani, Y.; Kiambi, S.; Muturi, M.; Mwatondo, A.; Muriithi, R.; Cleaveland, S.; et al. A hundred years of rabies in Kenya and the strategy for eliminating dog-mediated rabies by 2030. AAS Open Res. 2019, 1, 23. [Google Scholar] [CrossRef] [PubMed]
- Munang’andu, H.M.; Mweene, A.S.; Siamudaala, V.; Muma, J.B.; Matandiko, W. Rabies Status in Zambia for the Period 1985–2004. Zoonoses Public Health 2011, 58, 21–27. [Google Scholar] [CrossRef]
- Babaniyi, O.; Songolo, P.; Matapo, B.; Masaninga, F.; Mulenga, F.; Michelo, C.; Mubanga, J.; Kazembe, L.N. Epidemiological characteristics of rabies in Zambia: A retrospective study (2004–2013). Clin. Epidemiol. Glob. Health 2016, 4, 83–88. [Google Scholar] [CrossRef]
- Dermaux-Msimang, V.; Blumberg, L.; Paweska, J.; Weyer, J. Epidemiology of Human Rabies in South Africa, 2008–2018. NCID Bull. 2019, 17, 63–72. [Google Scholar]
- Vigilato, M.A.; Belotto, A.J.; Tamayo Silva, H.; Rocha, F.; Molina-Flores, B.; Pompei, J.C.; Zanetti, L.; Cosivi, O. Towards the Elimination of Canine Rabies in the Americas: Governance of a Regional Program. In History of Rabies in the Americas: From the Pre-Columbian to the Present; Springer: Cham, Switzerland, 2023; Volume 1, pp. 293–305. [Google Scholar]
- Escobar, L.E.; Peterson, A.T.; Papeş, M.; Favi, M.; Yung, V.; Restif, O.; Qiao, H.; Medina-Vogel, G. Ecological approaches in veterinary epidemiology: Mapping the risk of bat-borne rabies using vegetation indices and night-time light satellite imagery. Vet. Res. 2015, 46, 92. [Google Scholar] [CrossRef]
- Alegria-Moran, R.; Miranda, D.; Barnard, M.; Parra, A.; Lapierre, L. Characterization of the epidemiology of bat-borne rabies in Chile between 2003 and 2013. Prev. Vet. Med. 2017, 143, 30–38. [Google Scholar] [CrossRef]
- Kamler, J.F.; Stenkewitz, U.; Klare, U.; Jacobsen, N.F.; Macdonald, D.W. Resource partitioning among cape foxes, bat-eared foxes, and black-backed jackals in South Africa. J. Wildl. Manag. 2012, 76, 1241–1253. [Google Scholar] [CrossRef]
- Welch, R.J.; Le Roux, A.; Petelle, M.B.; Périquet, S. The influence of environmental and social factors on high- and low-cost vigilance in bat-eared foxes. Behav. Ecol. Sociobiol. 2018, 72, 29. [Google Scholar] [CrossRef]
- Okell, C.N.; Pinchbeck, G.P.; Stringer, A.P.; Tefera, G.; Christley, R.M. A community-based participatory study investigating the epidemiology and effects of rabies to livestock owners in rural Ethiopia. Prev. Vet. Med. 2013, 108, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Stafford, J.M.; Wendler, G.; Curtis, J. Temperature and precipitation of Alaska: 50 year trend analysis. Theor. Appl. Climatol. 2000, 67, 33–44. [Google Scholar] [CrossRef]
- Lachica, Z.P.T.; Peralta, J.M.; Diamante, E.O.; Murao, L.A.E.; Mata, M.A.E.; Alviola Iv, P.A. A cointegration analysis of rabies cases and weather components in Davao City, Philippines from 2006 to 2017. PLoS ONE 2020, 15, e0236278. [Google Scholar] [CrossRef]
- Haydon, D.T.; Cleaveland, S.; Taylor, L.H.; Laurenson, M.K. Identifying Reservoirs of Infection: A Conceptual and Practical Challenge. Emerg. Infect. Dis. 2002, 8, 1468–1473. [Google Scholar] [CrossRef] [PubMed]
- Karunanayake, D.; Matsumoto, T.; Wimalaratne, O.; Nanayakkara, S.; Perera, D.; Nishizono, A.; Ahmed, K. Twelve Years of Rabies Surveillance in Sri Lanka, 1999–2010. PLoS Negl. Trop. Dis. 2014, 8, e3205. [Google Scholar] [CrossRef]
- Vos, A.; Ün, H.; Hampson, K.; De Balogh, K.; Aylan, O.; Freuling, C.M.; Müller, T.; Fooks, A.R.; Johnson, N. Bovine rabies in Turkey: Patterns of infection and implications for costs and control. Epidemiol. Infect. 2014, 142, 1925–1933. [Google Scholar] [CrossRef] [PubMed]
- Deressa, A.; Ali, A.; Bayene, M.; Selassie, B.; Yimer, E.; Hussen, K. The status of rabies in Ethiopia: A retrospective record review. Ethiop. J. Health Dev. 2010, 24, 127–132. [Google Scholar] [CrossRef]
- Seetahal, J.F.R.; Sanchez-Vazquez, M.J.; Vokaty, A.; Carrington, C.V.F.; Mahabir, R.; Adesiyun, A.A.; Rupprecht, C.E. Of bats and livestock: The epidemiology of rabies in Trinidad, West Indies. Vet. Microbiol. 2019, 228, 93–100. [Google Scholar] [CrossRef] [PubMed]
- Matulis, G.A.; Altantogtokh, D.; Lantos, P.M.; Jones, J.H.; Wofford, R.N.; Janko, M.; Tsogbadrakh, N.; Bayar, T.; Ganzorig, S.; Boldbaatar, B.; et al. Hotspots in a cold land-reported cases of rabies in wildlife and livestock in Mongolia from 2012–2018. Zoonoses Public Health 2022, 69, 655–662. [Google Scholar] [CrossRef]
- Tenzin; Dhand, N.K.; Ward, M.P. Anthropogenic and environmental risk factors for rabies occurrence in Bhutan. Prev. Vet. Med. 2012, 107, 21–26. [Google Scholar] [CrossRef]
- Oliveira, F.A.S.; Castro, R.J.S.; De Oliveira, J.F.; Barreto, F.M.; Farias, M.P.O.; Marinho, G.L.D.O.C.; Soares, M.J.D.S.; Silva-Júnior, A.; Schwarz, D.G.G. Geographical and temporal spread of equine rabies in Brazil. Acta Trop. 2022, 227, 106302. [Google Scholar] [CrossRef]
- Talbi, C.; Lemey, P.; Suchard, M.A.; Abdelatif, E.; Elharrak, M.; Jalal, N.; Faouzi, A.; Echevarría, J.E.; Vazquez Morón, S.; Rambaut, A.; et al. Phylodynamics and Human-Mediated Dispersal of a Zoonotic Virus. PLoS Pathog. 2010, 6, e1001166. [Google Scholar] [CrossRef]
- Choi, B.C.K. The Past, Present, and Future of Public Health Surveillance. Scientifica 2012, 2012, 875253. [Google Scholar] [CrossRef]
- Zhang, L.; Wilson, D.P. Trends in Notifiable Infectious Diseases in China: Implications for Surveillance and Population Health Policy. PLoS ONE 2012, 7, e31076. [Google Scholar] [CrossRef] [PubMed]
- Chirima, J.G.; Owen-Smith, N.; Elbasit, A.M. Expansions and contractions of occupied range by large mammalian herbivores in Kruger Park, South Africa: A response to rainfall variability. Biodivers. Int. J. 2018, 2, 545–557. [Google Scholar] [CrossRef]
- Owen-Smith, N.; Fryxell, J.M.; Merrill, E.H. Foraging theory upscaled: The behavioural ecology of herbivore movement. Phil. Trans. R. Soc. B 2010, 365, 2267–2278. [Google Scholar] [CrossRef]
- Ngoepe, E.; Chirima, J.G.; Mohale, D.; Mogano, K.; Suzuki, T.; Makita, K.; Sabeta, C.T. Rabies outbreak in black-backed jackals (Canis mesomelas), South Africa, 2016. Epidemiol. Infect. 2022, 150, e137. [Google Scholar] [CrossRef]
- Loveridge, A.J.; Macdonald, D.W. Seasonality in spatial organization and dispersal of sympatric jackals (Canis mesomelas and C. adustus): Implications for rabies management. J. Zool. 2001, 253, 101–111. [Google Scholar] [CrossRef]
- Ferguson, J.W.H.; Nel, J.A.J.; Wet, M.J.D. Social organization and movement patterns of Black-backed jackals Canis mesomelas in South Africa. J. Zool. 1983, 199, 487–502. [Google Scholar] [CrossRef]
- Pongsiri, M.J.; Roman, J.; Ezenwa, V.O.; Goldberg, T.L.; Koren, H.S.; Newbold, S.C.; Ostfeld, R.S.; Pattanayak, S.K.; Salkeld, D.J. Biodiversity Loss Affects Global Disease Ecology. BioScience 2009, 59, 945–954. [Google Scholar] [CrossRef]
- Gottdenker, N.L.; Streicker, D.G.; Faust, C.L.; Carroll, C.R. Anthropogenic Land Use Change and Infectious Diseases: A Review of the Evidence. EcoHealth 2014, 11, 619–632. [Google Scholar] [CrossRef] [PubMed]
- Nattrass, N.; Drouilly, M.; O’Riain, M.J. Learning from science and history about black-backed jackals Canis mesomelas and their conflict with sheep farmers in South Africa. Mammal. Rev. 2020, 50, 101–111. [Google Scholar] [CrossRef]
- Foley, J.A.; DeFries, R.; Asner, G.P.; Barford, C.; Bonan, G.; Carpenter, S.R.; Chapin, F.S.; Coe, M.T.; Daily, G.C.; Gibbs, H.K.; et al. Global Consequences of Land Use. Science 2005, 309, 570–574. [Google Scholar] [CrossRef] [PubMed]








| Province | Total Submitted (%) | Negative (%) | Positive (%) | Inconclusive (%) |
|---|---|---|---|---|
| Limpopo | 2835 (29.59) | 1660 (25.26) | 1156 (39.83) | 31 (28.97) |
| Mpumalanga | 4546 (47.45) | 3317 (50.48) | 1173 (40.42) | 56 (52.34) |
| North West | 2199 (22.95) | 1594 (24.26) | 573 (19.75) | 20 (18.69) |
| Total | 9580 | 6571 (100) | 2902 (100) | 107 (100) |
| Categories (n) | Animal Species (n) | Confirmed Cases (%) | |
|---|---|---|---|
| Domestic animals (6866) | Companion animals (5005) | 1733 (59.71) | |
| Dogs (4342) | 1576 (54.31) | ||
| Cats (603) | 48 (1.65) | ||
| Equine (56) | 22 (0.76) | ||
| Livestock animals (1861) | 635 (21.88) | ||
| Cattle (1647) | 537 (18.50) | ||
| Sheep (63) | 17 (0.59) | ||
| Pigs (26) | 8 (0.28) | ||
| Goats (126) | 73 (2.52) | ||
| Wildlife animals (2714) | Wildlife animals (2714) | 621 (21.39) | |
| Mongoose (541) | 259 (8.92) | ||
| Jackals (1121) | 287 (9.89) | ||
| Bat-eared fox (877) | 12 (0.41) | ||
| * Other wildlife (178) | 63 (2.17) | ||
| Total (9580) | 2902 (30.29) |
| Period | Moran’s I | Z-Score | p-Value |
|---|---|---|---|
| 1998–2002 | 0.004479 | 0.267113 | 0.789 |
| 2003–2007 | 0.213372 | 2.980477 | 0.002878 |
| 2008–2012 | 0.127508 | 3.045964 | 0.002319 |
| 2013–2017 | 0.232307 | 3.990029 | 0.000066 |
| 2018–2022 | 0.204465 | 4.508940 | 0.000007 |
| Period | Variables | p-Values | AIC |
|---|---|---|---|
| 1998–2002 | Thicket bush + Grassland | 0.030 * | 83.74 |
| 0.009 ** | |||
| 2008–2012 | Cultivated commercial crops + Waterbodies | 0.023 * | 59.90 |
| 0.035 * | |||
| 2018–2022 | Plantations + Woodlands | 0.046 * | 62.13 |
| 0.294 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mogano, K.; Sabeta, C.T.; Suzuki, T.; Makita, K.; Chirima, G.J. Patterns of Animal Rabies Prevalence in Northern South Africa between 1998 and 2022. Trop. Med. Infect. Dis. 2024, 9, 27. https://doi.org/10.3390/tropicalmed9010027
Mogano K, Sabeta CT, Suzuki T, Makita K, Chirima GJ. Patterns of Animal Rabies Prevalence in Northern South Africa between 1998 and 2022. Tropical Medicine and Infectious Disease. 2024; 9(1):27. https://doi.org/10.3390/tropicalmed9010027
Chicago/Turabian StyleMogano, Kgaogelo, Claude Taurai Sabeta, Toru Suzuki, Kohei Makita, and George Johannes Chirima. 2024. "Patterns of Animal Rabies Prevalence in Northern South Africa between 1998 and 2022" Tropical Medicine and Infectious Disease 9, no. 1: 27. https://doi.org/10.3390/tropicalmed9010027







