Spatiotemporal Analysis of Malaria Transmission in the Autonomous Indigenous Regions of Panama, Central America, 2015–2022
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Data Collection and Sources
2.3. Data Analysis
2.3.1. Descriptive Analysis
2.3.2. Spatial Analysis
Spatial Reference Matrix
Spatial Autocorrelation
Spatial Clusters and Spatiotemporal Clusters Based on the Scan Method
2.4. Software and Packages
3. Results
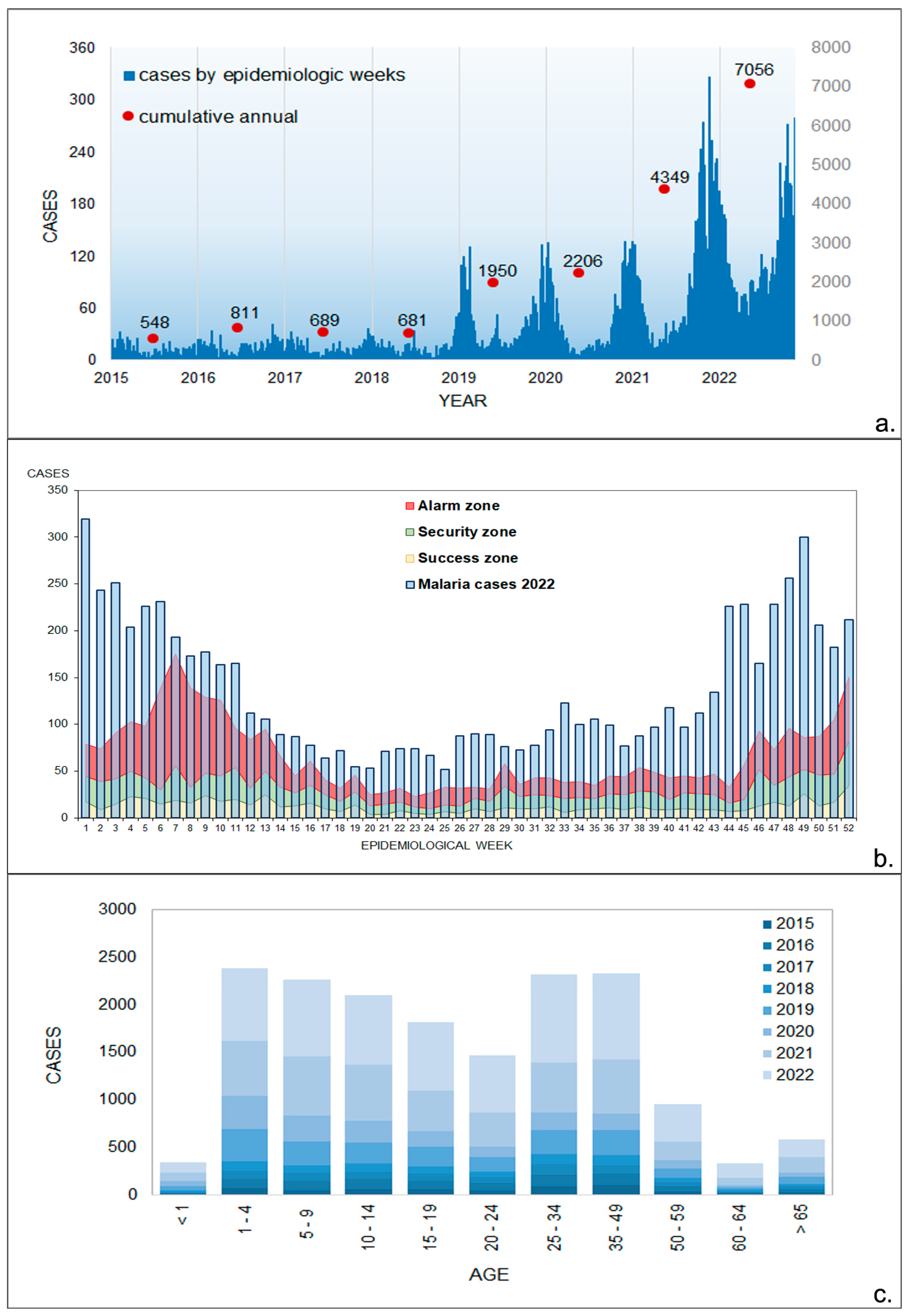
3.1. Epidemiological Data
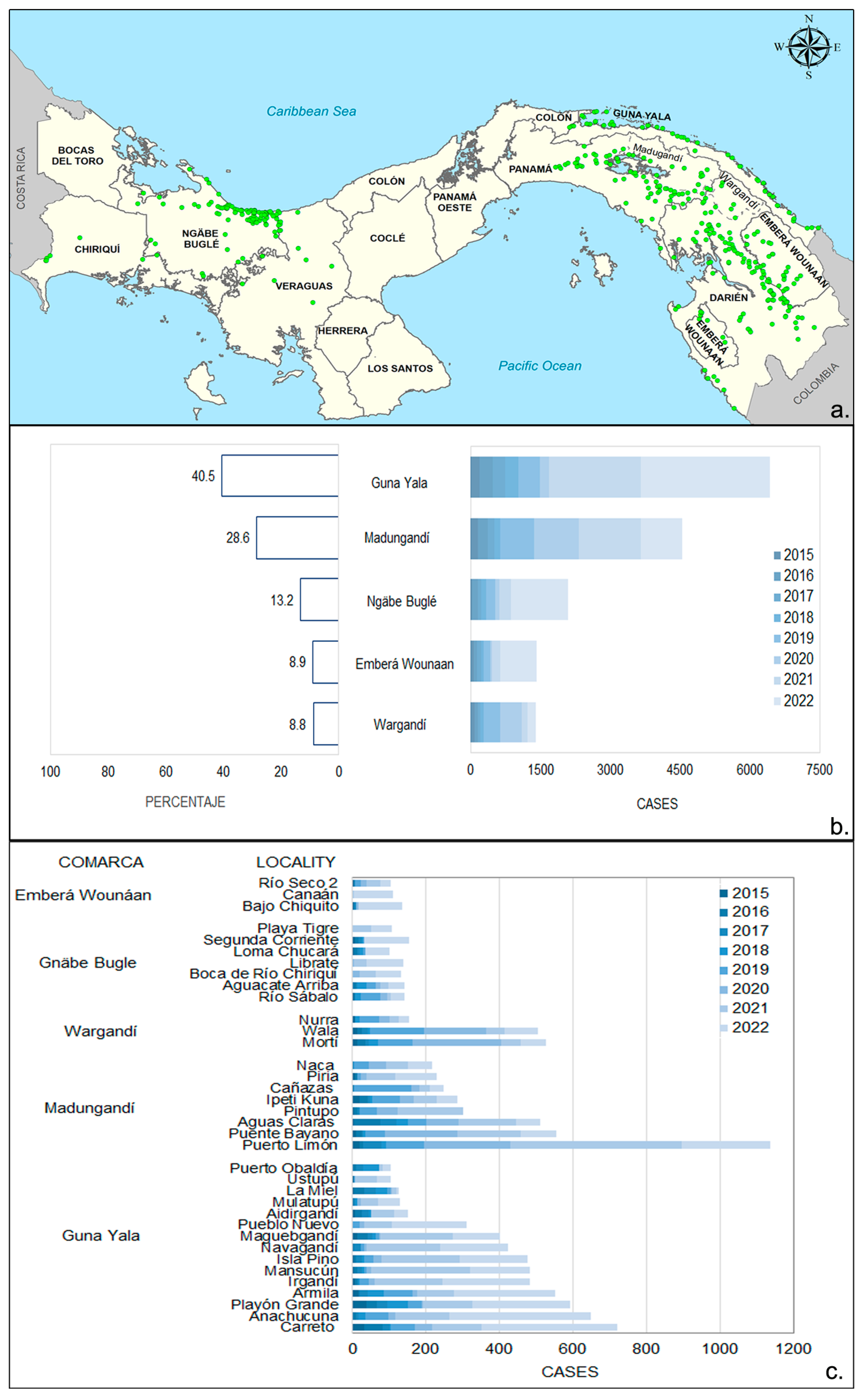
3.2. Spatial Distribution of Malaria Cases in Endemic Areas
3.3. Monthly Malaria Cases
3.4. Trends in Environmental Factors
3.5. Spatial Analysis
3.5.1. LISA Cluster Analysis
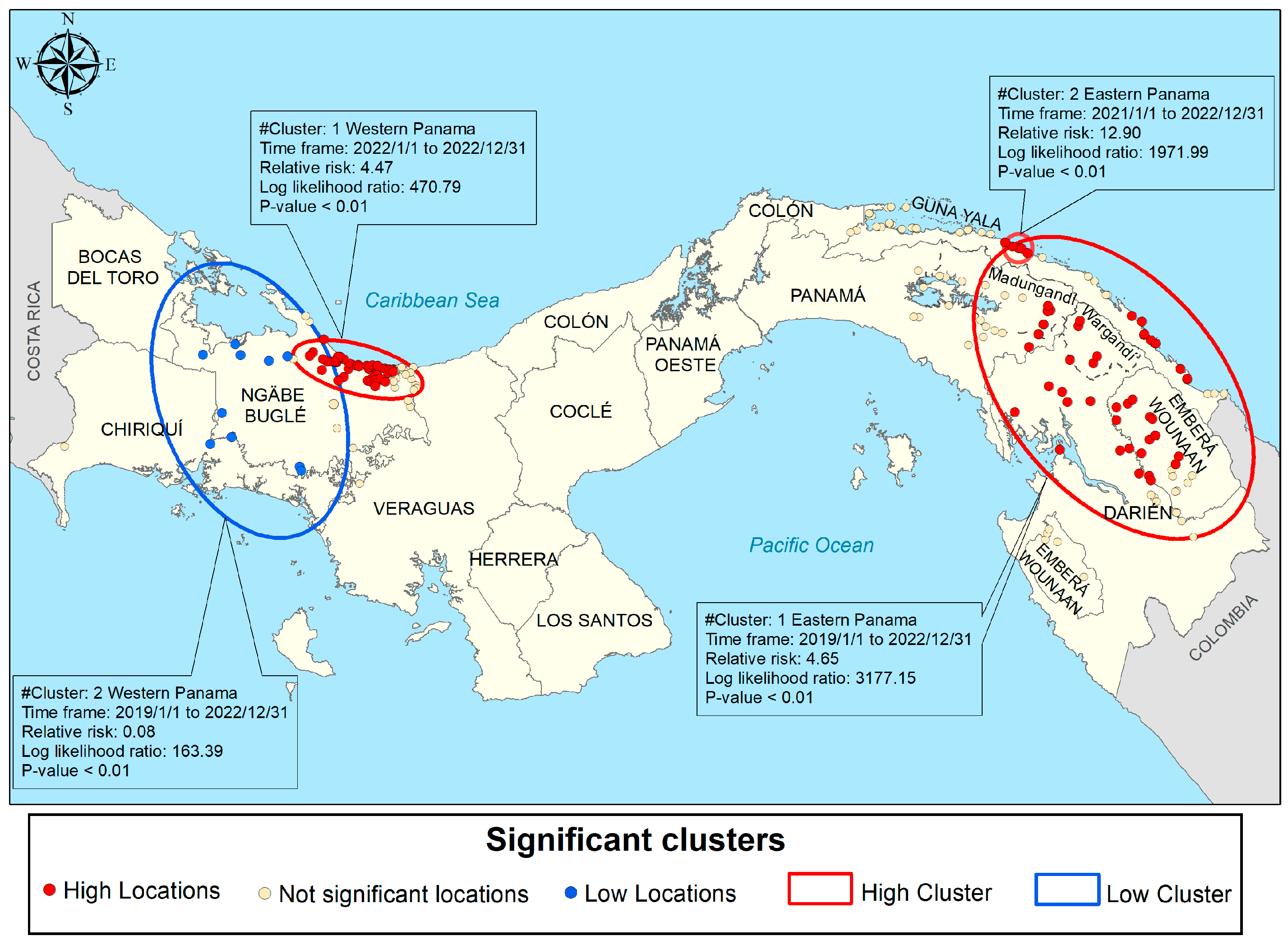
3.5.2. Purely Spatial Scan Cluster Analysis
3.5.3. Spatiotemporal Scan Cluster Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. World Malaria Day: WHO Launches Effort to Stamp out Malaria in 25 More Countries by 2025. Available online: https://www.who.int/news/item/21-04-2021-world-malaria-day-who-launches-effort-to-stamp-out-malaria-in-25-more-countries-by-2025 (accessed on 6 January 2023).
- Ministerio de Salud. Plan Estratégico de Eliminación de la Malaria (PEEM) en Panamá 2018–2022; Ministerio de Salud de Panamá: Panamá, Panama, 2018; pp. 8–39. [Google Scholar]
- Hurtado, L.; Cumbrera, A.; Rigg, C.; Perea, M.; Santamaría, A.M.; Chaves, L.F.; Moreno, D.; Romero, L.; Lasso, J.; Caceres, L.; et al. Long-term transmission patterns and public health policies leading to malaria elimination in Panamá. Malar. J. 2020, 19, 265. [Google Scholar] [CrossRef] [PubMed]
- Ministerio de Salud, Dirección General de Salud Pública, Departamento de Control de Vectores, Departamento de Epidemiología. Plan de Acción Rápida Para el Combate Contra la Malaria en focos Específicos de Áreas de Difícil Acceso; MINSA: Panamá, Panama, 2023. [Google Scholar]
- Loaiza Rodríguez, J.d.R.; Eastwood, G.; Chaves Sanabria, L.F. Planetary Health Approaches to Understand and Control Vector-Borne Diseases; Wageningen Academic: Wageningen, The Netherlands, 2023; pp. 206–225. [Google Scholar]
- World Health Organization. World Malaria Report 2022; World Health Organization: Geneva, Switzerland, 2022. [Google Scholar]
- The Commonwealth Malaria Report 2022. Available online: https://reliefweb.int/report/world/commonwealth-malaria-report-2022 (accessed on 23 February 2023).
- Lainhart, W.; Dutari, L.C.; Rovira, J.R.; Sucupira, I.M.; Póvoa, M.M.; Conn, J.E.; Loaiza, J.R. Epidemic and Non-Epidemic Hot Spots of Malaria Transmission Occur in Indigenous Comarcas of Panama. PLoS Negl. Trop. Dis. 2016, 10, e0004718. [Google Scholar] [CrossRef] [PubMed]
- Instituto de Estadísticas y Censo. Panamá en Cifras: Años 2015–2019; Instituto de Estadísticas y Censo: Panamá, Panama, 2021. [Google Scholar]
- Ministerio de Ambiente y GIZ. Programa de Restauración Forestal; Ministerio de Ambiente y GIZ: Panamá, Panama, 2020. [Google Scholar]
- Cecchini, S.; Mojica, R.H.Y.A.R. La Matriz de Desigualdad Social en Panamá; Serie Políticas Sociales, No. 236 (LC/TS.2020/121); Comisión Económica para América Latina y el Caribe (CEPAL): Santiago, Chile, 2020. [Google Scholar]
- Ministerio de Ambiente. Plan Nacional Contra la Sequía de Panamá; Ministerio de Ambiente: Panamá, Panama, 2020.
- CATHALAC. Una Nueva Regionalización Climática de Panamá Como Aporte a la Seguridad Hídrica, Trabajo de la División de Investigación Aplicada y Desarrollo; CATHALAC: Panamá, Panama, 2016. [Google Scholar]
- Bortman, M. Elaboración de corredores o canales endémicos mediante planillas de cálculo. Rev. Panam. Salud Pública 1999, 5, 1–8, ene. Obtenido de. Available online: http://www.scielosp.org/scielo.php?script=sci_arttext&pid=S1020-49891999000100001&lng=en&nrm=iso&tlng=es (accessed on 20 January 2023). [CrossRef] [PubMed]
- Venables, W.; Ripley, B. Modern Applied Statistics with S, 4th ed.; Springer: New York, NY, USA, 2002. [Google Scholar]
- Borcard, D.; Gillet, F.; Legendre, P. Numerical Ecology with R, 2nd ed.; Springer: New York, NY, USA, 2018; 435p. [Google Scholar]
- Brunsdon, C.; Comber, L. An Introduction to R for Spatial Analysis and Mapping; Sage Publications Ltd.: London, UK, 2015; 343p. [Google Scholar]
- Kulldorff, M. A spatial scan statistic. Commun. Stat. Theory Methods 1997, 26, 1481–1496. [Google Scholar] [CrossRef]
- Kulldorff, M.; Huang, L.; Pickle, L.; Duczmal, L. An elliptic spatial scan statistic. Stat. Med. 2006, 25, 3929–3943. [Google Scholar] [CrossRef] [PubMed]
- Kulldorff, M. SaTScanTM v8.0: Software for the Spatial and Space-Time Scan Statistics; Information Management Services Inc.: Rockville, MD, USA, 2009. [Google Scholar]
- Samudio, F.; Santamaría, A.M.; Obaldía, N., 3rd; Pascale, J.M.; Bayard, V.; Calzada, J.E. Prevalence of Plasmodium falciparum mutations associated with antimalarial drug resistance during an epidemic in Kuna Yala, Panama, Central America. Am. J. Trop. Med. Hyg. 2005, 73, 839–841. [Google Scholar] [CrossRef] [PubMed]
- Calzada, J.E.; Samudio, F.; Bayard, V.; Obaldia, N.; de Mosca, I.B.; Pascale, J.M. Revising antimalarial drug policy in Central America: Experience in Panama. Trans. R. Soc. Trop. Med. Hyg. 2008, 102, 694–698. [Google Scholar] [CrossRef] [PubMed]
- Obaldia, N.; Baro, N.K.; Calzada, J.E.; Santamaria, A.M.; Daniels, R.; Wong, W.; Chang, H.-H.; Hamilton, E.J.; Arevalo-Herrera, M.; Herrera, S.; et al. Clonal Outbreak of Plasmodium falciparum Infection in Eastern Panama. J. Infect. Dis. 2015, 211, 1087–1096. [Google Scholar] [CrossRef] [PubMed]
- Santamaría, A.M.; Vásquez, V.; Rigg, C.; Moreno, D.; Romero, L.; Justo, C.; Chaves, L.F.; Saldaña, A.; Calzada, J.E. Plasmodium falciparum Genetic Diversity in Panamá Based on glurp, msp-1 and msp-2 Genes: Implications for Malaria Elimination in Mesoamerica. Life 2020, 10, 319. [Google Scholar] [CrossRef] [PubMed]
- Santamaría, A.M.; Vásquez, V.; Rigg, C.; Samudio, F.; Moreno, D.; Romero, L.; Saldaña, A.; Chaves, L.F.; Calzada, J.E. Plasmodium vivax Genetic Diversity in Panama: Challenges for Malaria Elimination in Mesoamerica. Pathogens 2021, 10, 989. [Google Scholar] [CrossRef] [PubMed]
- Loaiza, J.R.; Bermingham, E.; Scott, M.E.; Rovira, J.R.; Conn, J.E. Species composition and distribution of adult Anopheles (Diptera: Culicidae) in Panama. J. Med. Entomol. 2008, 45, 841–851. [Google Scholar] [CrossRef] [PubMed]
- Instituto Geográfico Nacional Tommy Guardia. Atlas Nacional de la República de Panamá 2015; Instituto Geográfico Nacional Tommy Guardia: Panamá, Panama, 2016; ISBN 978-9962-11-048-4. [Google Scholar]
- Servicio Nacional de Migración de Panamá. Movimiento Migratorio. 2023. Available online: https://www.migracion.gob.pa/images/img2023/pdf/MOVIMIENTO_MIGRATORIO_ACTUALIZADO_DIC_2023.pdf (accessed on 12 February 2024).
- Naranjo, L.; Williams, Y.; Levy, J.; Obando, R.; González, J.A.; Pachar, M.; Chen, R.; Franco-Paredes, C.; Higuita, N.A.; Henao-Martínez, A.; et al. The Endless Vulnerability of Migrant Children In-Transit across the Darién Gap. Am. J. Trop. Med. Hyg. 2023, 109, 515–519. [Google Scholar] [CrossRef] [PubMed]
- Pacheco, M.A.; Forero-Peña, D.A.; Schneider, K.A.; Chavero, M.; Gamardo, A.; Figuera, L.; Kadakia, E.R.; Grillet, M.E.; Oliveira-Ferreira, J.; Escalante, A.A. Malaria in Venezuela: Changes in the complexity of infection reflects the increment in transmission intensity. Malar. J. 2020, 19, 176. [Google Scholar] [CrossRef] [PubMed]
- Montenegro, Y.A. Sanciones impuestas por Estados Unidos a Venezuela: Consecuencias regionales. Rev. Relac. Int. Estrateg. Y Segur. 2021, 16, 121–140. [Google Scholar] [CrossRef]
- Agudelo Higuita, N.I.; Franco-Paredes, C.; Henao-Martínez, A.F.; Mendez Rojas, B.; Suarez, J.A.; Naranjo, L.; Alger, J. Migrants in transit across Central America and the potential spread of chloroquine resistant malaria-a call for action. Lancet Reg. Health Am. 2023, 22, 100505. [Google Scholar] [CrossRef] [PubMed]
- Blackburn, D.; Drennon, M.; Broussard, K.; Morrison, A.M.; Stanek, D.; Sarney, E.; Ferracci, C.; Huard, S.; Brennan, W.; Eaton, J.; et al. Outbreak of Locally Acquired Mosquito-Transmitted (Autochthonous) Malaria—Florida and Texas, May–July 2023. MMWR. Morb. Mortal. Wkly. Rep. 2023, 72, 973–978. [Google Scholar] [CrossRef] [PubMed]
- International Organization for Migration. The Displacement Tracking Matrix (DTM). Panamá—Monitoreo de Flujo de Población Migrante—Darién y Chiriquí (Agosto 2023). Available online: https://dtm.iom.int/panama (accessed on 19 February 2024).
- Ministerio de Salud, Direccion General de Salud. Panamá. Caracterizacion de focos malaricos. Panama 2018. Available online: https://www3.paho.org/pan/dmdocuments/Anexo%203-CARACTERIZACION-FOCOS%20MALARICOS.2019.pdf (accessed on 19 February 2024).
- Chaves, L.F.; Ramírez Rojas, M.; Prado, M.; Garcés, J.L.; Salas Peraza, D.; Marín Rodríguez, R. Health policy impacts on malaria transmission in Costa Rica. Parasitology 2020, 147, 999–1007. [Google Scholar] [CrossRef] [PubMed]
- Tatem, A.J.; Gething, P.W.; Smith, D.L.; Hay, S.I. Urbanization and the global malaria recession. Malar. J. 2013, 12, 133. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cumbrera, A.; Calzada, J.E.; Chaves, L.F.; Hurtado, L.A. Spatiotemporal Analysis of Malaria Transmission in the Autonomous Indigenous Regions of Panama, Central America, 2015–2022. Trop. Med. Infect. Dis. 2024, 9, 90. https://doi.org/10.3390/tropicalmed9040090
Cumbrera A, Calzada JE, Chaves LF, Hurtado LA. Spatiotemporal Analysis of Malaria Transmission in the Autonomous Indigenous Regions of Panama, Central America, 2015–2022. Tropical Medicine and Infectious Disease. 2024; 9(4):90. https://doi.org/10.3390/tropicalmed9040090
Chicago/Turabian StyleCumbrera, Alberto, José Eduardo Calzada, Luis Fernando Chaves, and Lisbeth Amarilis Hurtado. 2024. "Spatiotemporal Analysis of Malaria Transmission in the Autonomous Indigenous Regions of Panama, Central America, 2015–2022" Tropical Medicine and Infectious Disease 9, no. 4: 90. https://doi.org/10.3390/tropicalmed9040090





