In Vitro Corrosion and Biological Assessment of Bioabsorbable WE43 Mg Alloy Specimens
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Specimens and Mechanical Testing
2.2. Immersion Testing
2.3. Cell Viability
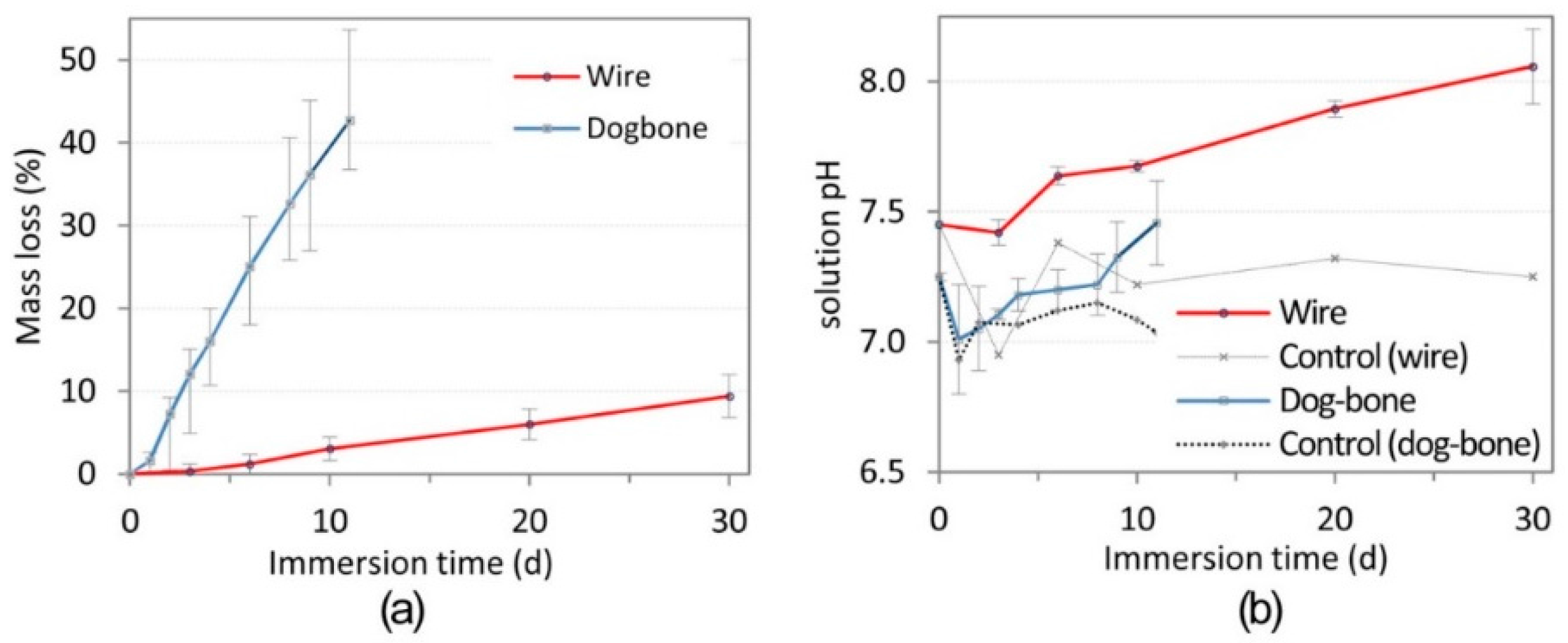
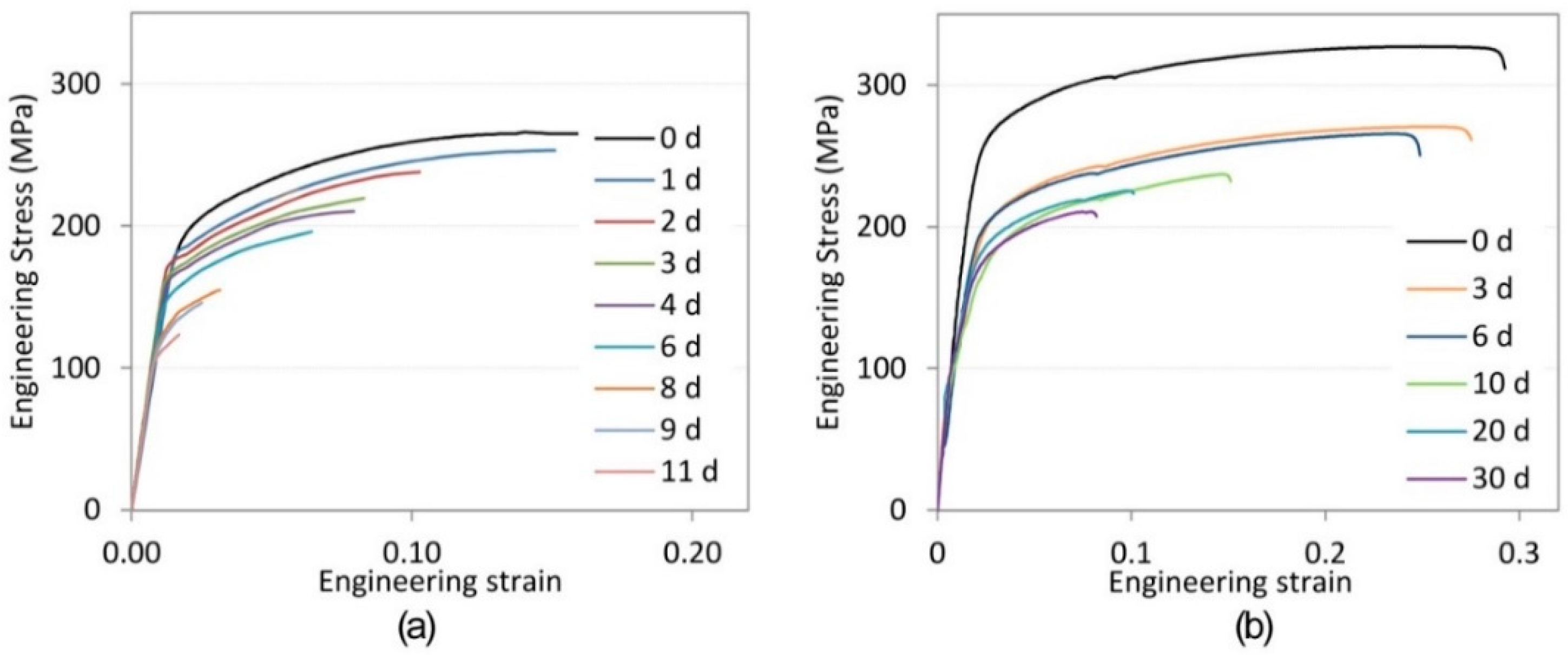
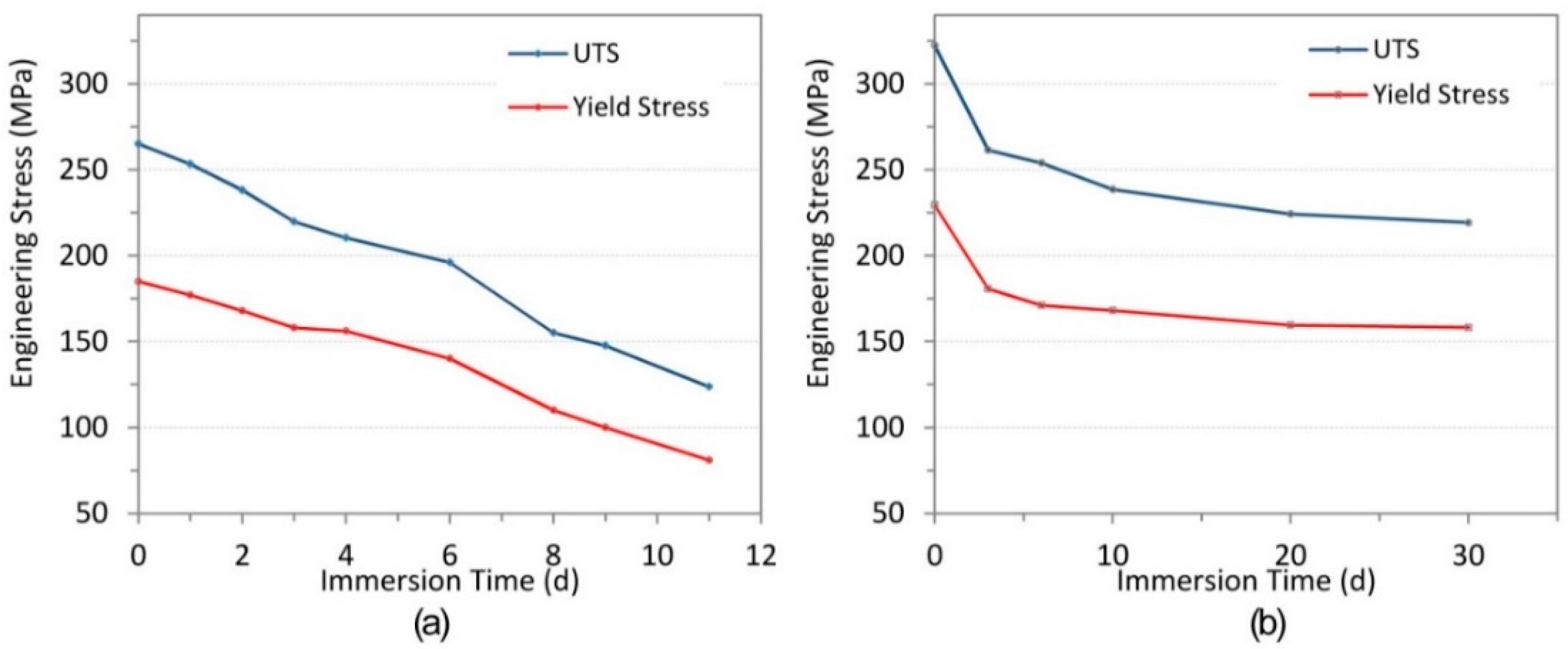
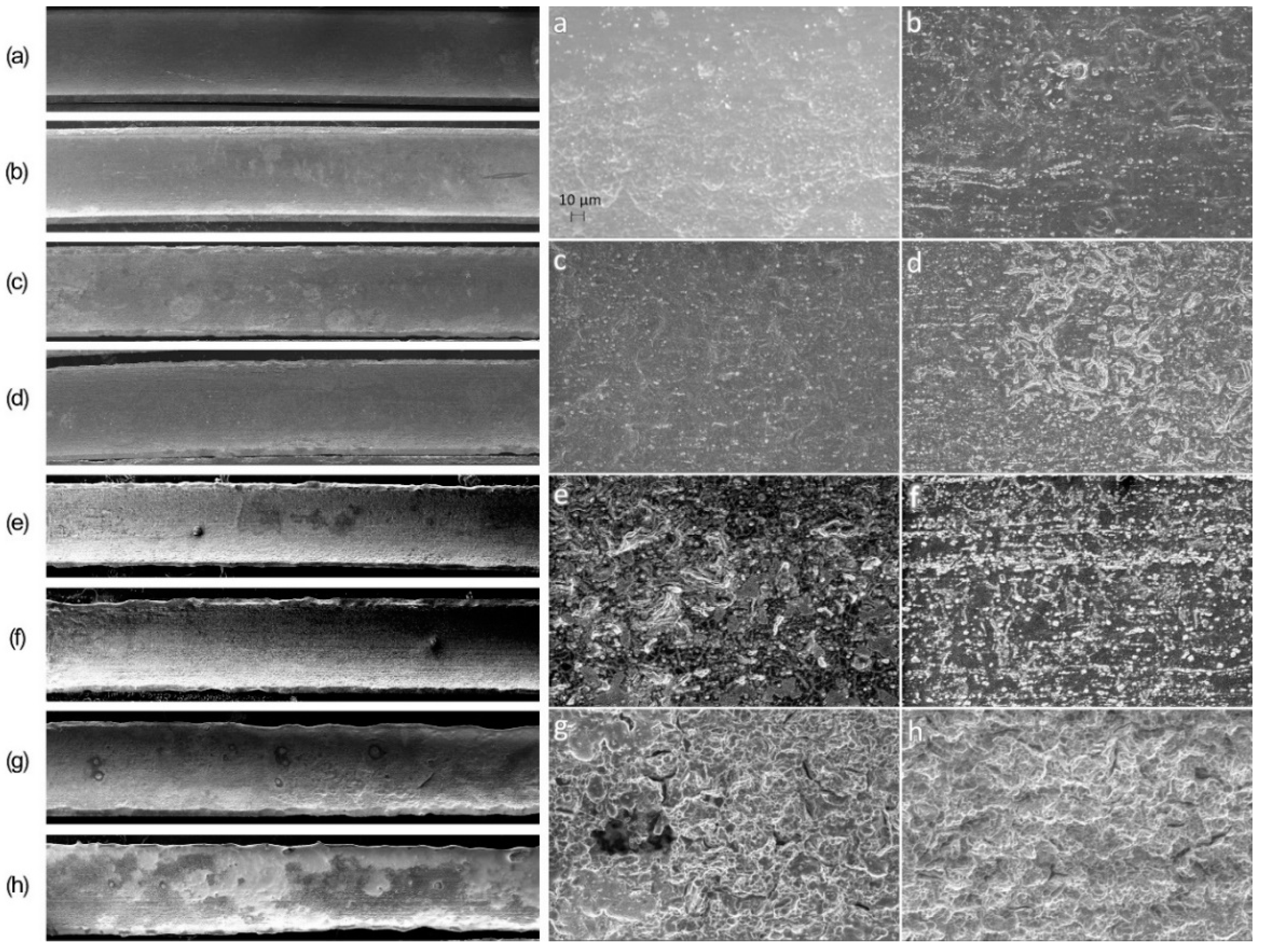
3. Results
3.1. Mechanical Testing
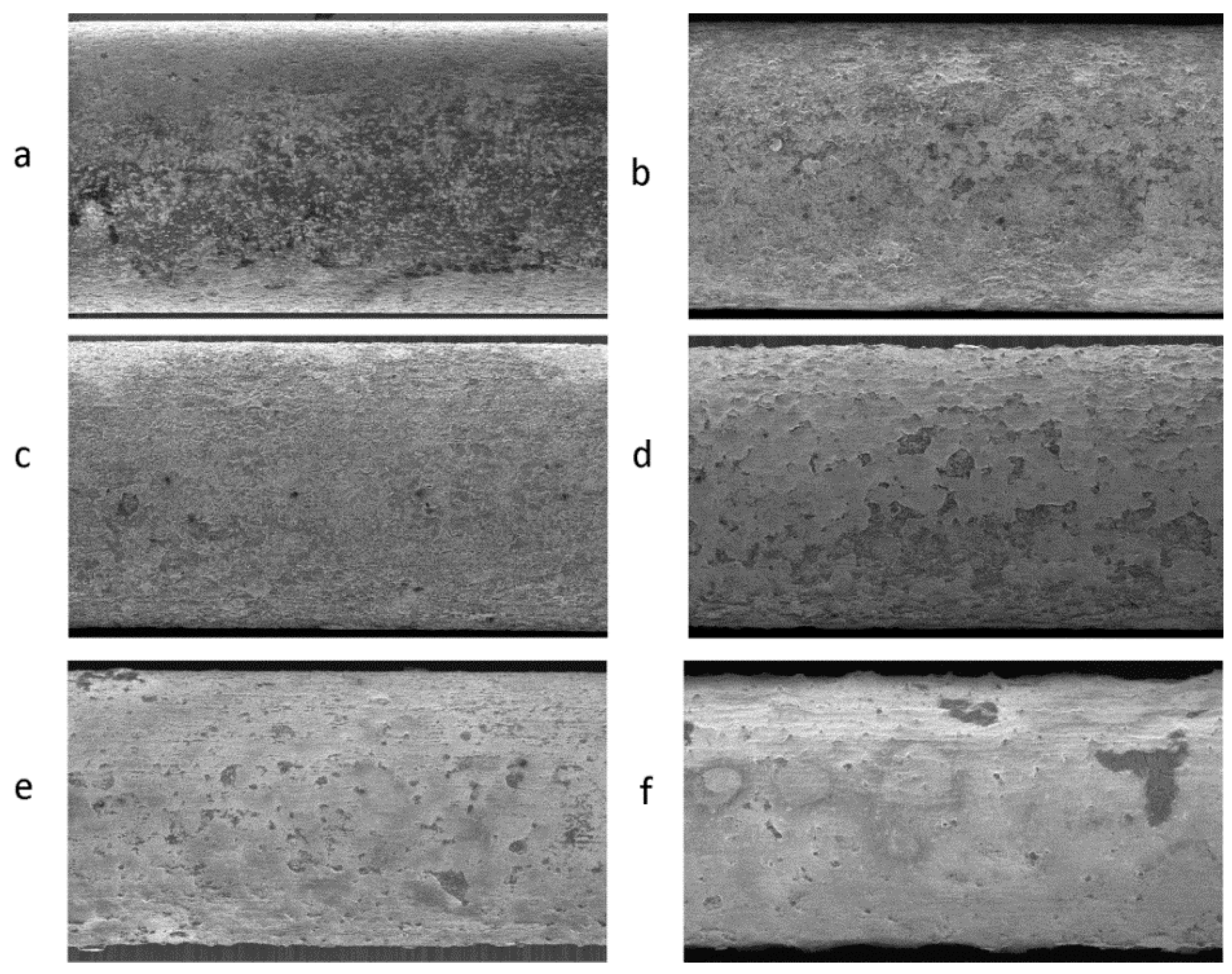
3.2. Surface Morphology Studies
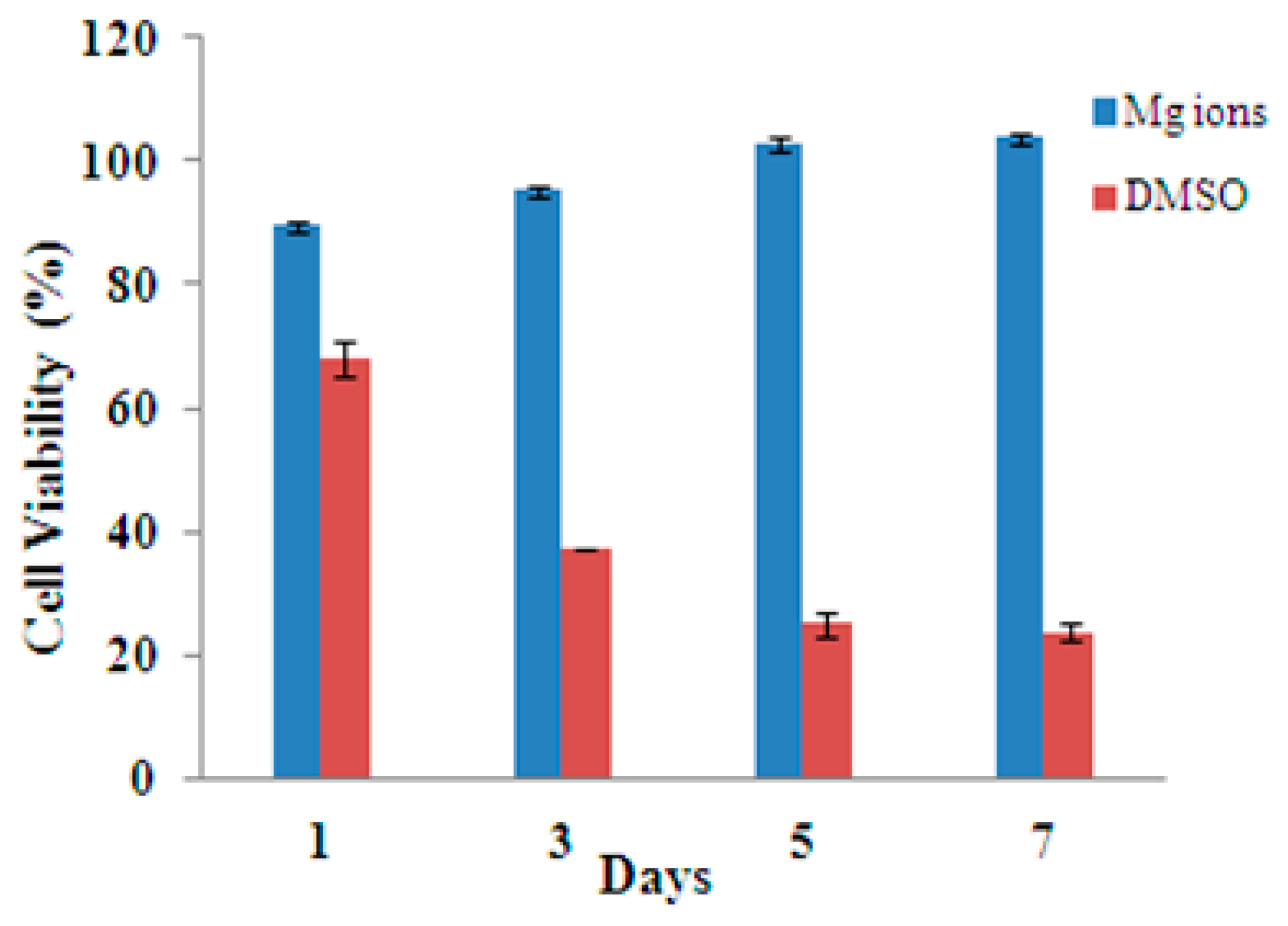
3.3. Indirect Cytotoxicity Evaluation of Mg Ions on HUVECs
4. Discussion
5. Conclusions
Acknowledgement
Author Contributions
Conflicts of Interest
References
- Haude, M.; Erbel, R.; Erne, P.; Verheye, S.; Degen, H.; Böse, D.; Vermeersch, P.; Wijnbergen, I.; Weissman, N.; Prati, F.; et al. Safety and performance of the drug-eluting absorbable metal scaffold (DREAMS) in patients with de-novo coronary lesions: 12 month results of the prospective, multicentre, first-in-man BIOSOLVE-I trial. Lancet 2013, 381, 836–844. [Google Scholar] [CrossRef]
- Erbel, R.; Di Mario, C.; Bartunek, J.; Bonnier, J.; de Bruyne, B.; Eberli, F.R.; Erne, P.; Haude, M.; Heublein, B.; Horrigan, M.; et al. Temporary scaffolding of coronary arteries with bioabsorbable magnesium stents: A prospective, non-randomised multicentre trial. Lancet 2007, 369, 1869–1875. [Google Scholar] [CrossRef]
- Farraro, K.F.; Kim, K.E.; Woo, S.L.; Flowers, J.R.; McCullough, M.B. Revolutionizing orthopaedic biomaterials: The potential of biodegradable and bioresorbable magnesium-based materials for functional tissue engineering. J. Biomech. 2014, 47, 1979–1986. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, S.; Curtin, J.; Duffy, B.; Jaiswal, S. Biodegradable magnesium alloys for orthopaedic applications: A review on corrosion, biocompatibility and surface modifications. Mater. Sci. Eng. C 2016, 68, 948–963. [Google Scholar] [CrossRef] [PubMed]
- Zeng, R.; Dietzel, W.; Witte, F.; Hort, N.; Blawert, C. Progress and challenge for magnesium alloys as biomaterials. Adv. Eng. Mater. 2008, 10, B3–B14. [Google Scholar] [CrossRef]
- Huan, Z.G.; Leeflang, M.A.; Zhou, J.; Fratila-Apachitei, L.E.; Duszczyk, J. In vitro degradation behavior and cytocompatibility of Mg-Zn-Zr alloys. J. Mater. Sci. Mater. Med. 2010, 21, 2623–2635. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Liu, L.; Liu, J.; Pan, F. Microstructure, electromagnetic shielding effectiveness and mechanical properties of Mg–Zn-Y-Zr alloys. Mater. Des. 2015, 65, 360–369. [Google Scholar] [CrossRef]
- Connolley, T.; McHugh, P.E.; Bruzzi, M. A review of deformation and fatigue of metals at small size scales. Fatigue Fract. Eng. Mater. Struct. 2005, 28, 1119–1152. [Google Scholar] [CrossRef]
- Maier, P.; Zimmermann, F.; Rinne, M.; Szakács, G.; Hort, N.; Vogt, C. Solid solution treatment on strength and corrosion of biodegradable Mg6Ag wires. Mater. Corros. 2017. [Google Scholar] [CrossRef]
- Witte, F.; Hort, N.; Vogt, C.; Cohen, S.; Kainer, K.U.; Willumeit, R.; Feyerabend, F. Degradable biomaterials based on magnesium corrosion. Curr. Opin. Solid State Mater. Sci. 2008, 12, 63–72. [Google Scholar] [CrossRef]
- Galvin, E.; O’Brien, D.; Cummins, C.; Mac Donald, B.J.; Lally, C. A strain-mediated corrosion model for bioabsorbable metallic stents. Acta Biomater. 2017, 55, 505–517. [Google Scholar] [CrossRef] [PubMed]
- Galvin, E.; Cummins, C.; Yoshihara, S.; Mac Donald, B.J.; Lally, C. Plastic strains during stent deployment have a critical influence on the rate of corrosion in absorbable magnesium stents. Med. Biol. Eng. Comput. 2017, 55, 1261–1275. [Google Scholar] [CrossRef] [PubMed]
- Griebel, A.; Schaffer, J. Cold-drawn ZM21 and WE43 wires exhibit exceptional strength and ductility. Eur. Cell. Mater. 2014, 28. [Google Scholar] [CrossRef]
- Maier, P.; Szakács, G.; Wala, M.; Hort, N. Mechanical and Corrosive Properties of Two Magnesium Wires: Mg4Gd and Mg6Ag. In Magnesium Technology; Manuel, M.V., Singh, A., Alderman, M., Neelameggham, N.R., Eds.; Springer Science & Business Media: Berlin, Germany, 2015; pp. 393–398. [Google Scholar]
- Bai, J.; Yin, L.; Lu, Y.; Gan, Y.; Xue, F.; Chu, C.; Yan, J.; Yan, K.; Wan, X.; Tang, Z. Preparation, microstructure and degradation performance of biomedical magnesium alloy fine wires. Prog. Nat. Sci. Mater. Int. 2014, 24, 523–530. [Google Scholar] [CrossRef]
- McCartney, W.T.; Galvin, E.; Cummins, C.; Lally, C.; MacDonald, B.J. Use of a biodegradable magnesium implant for osseous fixation in four cases. Int. J. Appl. Res. Vet. Med. 2015, 13, 171–174. [Google Scholar]
- Galvin, E.; Morshed, M.M.; Cummins, C.; Daniels, S.; Lally, C.; MacDonald, B. Surface modification of absorbable magnesium stents by reactive ion etching. J. Plasma Chem. Process. 2013, 33, 1137–1152. [Google Scholar] [CrossRef]
- Bowen, P.K.; Gelbaugh, J.A.; Mercier, P.J.; Goldman, J.; Drelich, J. Tensile testing as a novel method for quantitatively evaluating bioabsorbable material degradation. J. Biomed. Mater. Res. B 2012, 100, 2101–2113. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Zhang, E. Biocorrosion behavior of magnesium alloy in different simulated fluids for biomedical application. Mater. Sci. Eng. C 2009, 29, 1691–1696. [Google Scholar] [CrossRef]
- Bornapour, M.; Muja, N.; Shum-Tim, D.; Cerruti, M.; Pekguleryuz, M. Biocompatibility and biodegradability of Mg-Sr alloys: The formation of Sr-substituted hydroxyapatite. Acta Biomater. 2013, 9, 5319–5330. [Google Scholar] [CrossRef] [PubMed]
- Jaiswal, S.; Bhattacharya, K.; McHale, P.; Duffy, B. Dual effects of β-cyclodextrin-stabilised silver nanoparticles: Enhanced biofilm inhibition and reduced cytotoxicity. J. Mater. Sci. Mater. Med. 2015, 26, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, S.; Morshed, M.; Labour, M.N.; Hoey, D.; Duffy, B.; Curtin, J.; Jaiswal, S. Enhanced corrosion protection and biocompatibility of a PLGA-silane coating on AZ31 Mg alloy for orthopaedic applications. RSC Adv. 2016, 6, 113871–113883. [Google Scholar] [CrossRef]
- Gu, X.; Zheng, Y.; Zhong, S.; Xi, T.; Wang, J.; Wang, W. Corrosion of, and cellular responses to Mg-Zn-Ca bulk metallic glasses. Biomaterials 2010, 31, 1093–1103. [Google Scholar] [CrossRef] [PubMed]
- Xin, Y.; Chu, P.K. Influence of Tris in simulated body fluid on degradation behavior of pure magnesium. Mater. Chem. Phys. 2010, 124, 33–35. [Google Scholar] [CrossRef]
- Kalb, H.; Rzany, A.; Hensel, B. Impact of microgalvanic corrosion on the degradation morphology of WE43 and pure magnesium under exposure to simulated body fluid. Corros. Sci. 2012, 57, 122–130. [Google Scholar] [CrossRef]
- Song, G.; Atrens, A. Recent insights into the mechanism of magnesium corrosion and research suggestions. Adv. Eng. Mater. 2007, 9, 177–183. [Google Scholar] [CrossRef]
- Zhang, X.; Yuan, G.; Mao, L.; Niu, J.; Ding, W. Biocorrosion properties of as-extruded Mg-Nd-Zn-Zr alloy compared with commercial AZ31 and WE43 alloys. Mater. Lett. 2012, 66, 209–211. [Google Scholar] [CrossRef]
- Ge, Q.; Dellasega, D.; Demir, A.G.; Vedani, M. The processing of ultrafine-grained Mg tubes for biodegradable stents. Acta Biomater. 2013, 9, 8604–8610. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Gu, X.; Lou, S.; Zheng, Y. The development of binary Mg-Ca alloys for use as biodegradable materials within bone. Biomaterials 2008, 29, 1329–1344. [Google Scholar] [CrossRef] [PubMed]
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Galvin, E.; Jaiswal, S.; Lally, C.; MacDonald, B.; Duffy, B. In Vitro Corrosion and Biological Assessment of Bioabsorbable WE43 Mg Alloy Specimens. J. Manuf. Mater. Process. 2017, 1, 8. https://doi.org/10.3390/jmmp1010008
Galvin E, Jaiswal S, Lally C, MacDonald B, Duffy B. In Vitro Corrosion and Biological Assessment of Bioabsorbable WE43 Mg Alloy Specimens. Journal of Manufacturing and Materials Processing. 2017; 1(1):8. https://doi.org/10.3390/jmmp1010008
Chicago/Turabian StyleGalvin, Emmet, Swarna Jaiswal, Caitríona Lally, Bryan MacDonald, and Brendan Duffy. 2017. "In Vitro Corrosion and Biological Assessment of Bioabsorbable WE43 Mg Alloy Specimens" Journal of Manufacturing and Materials Processing 1, no. 1: 8. https://doi.org/10.3390/jmmp1010008




