Corrosion Behaviour of Dual-Phase High Carbon Steel—Microstructure Influence
Abstract
:1. Introduction
2. Experimental
2.1. Material Preparation
2.2. Analytical Methods
3. Results and Discussions
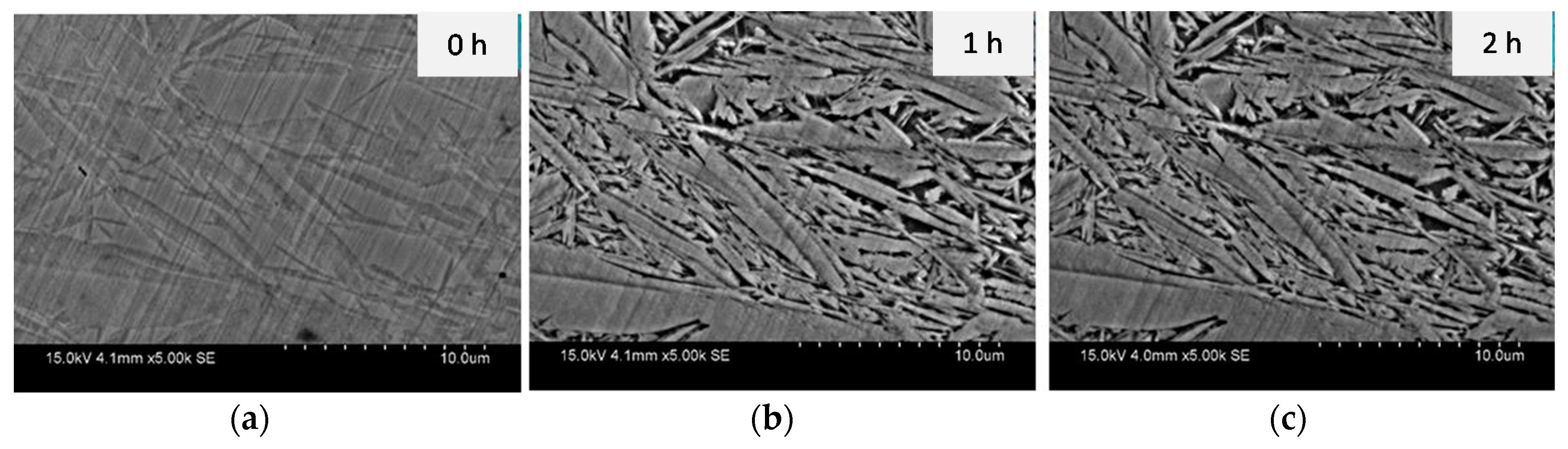
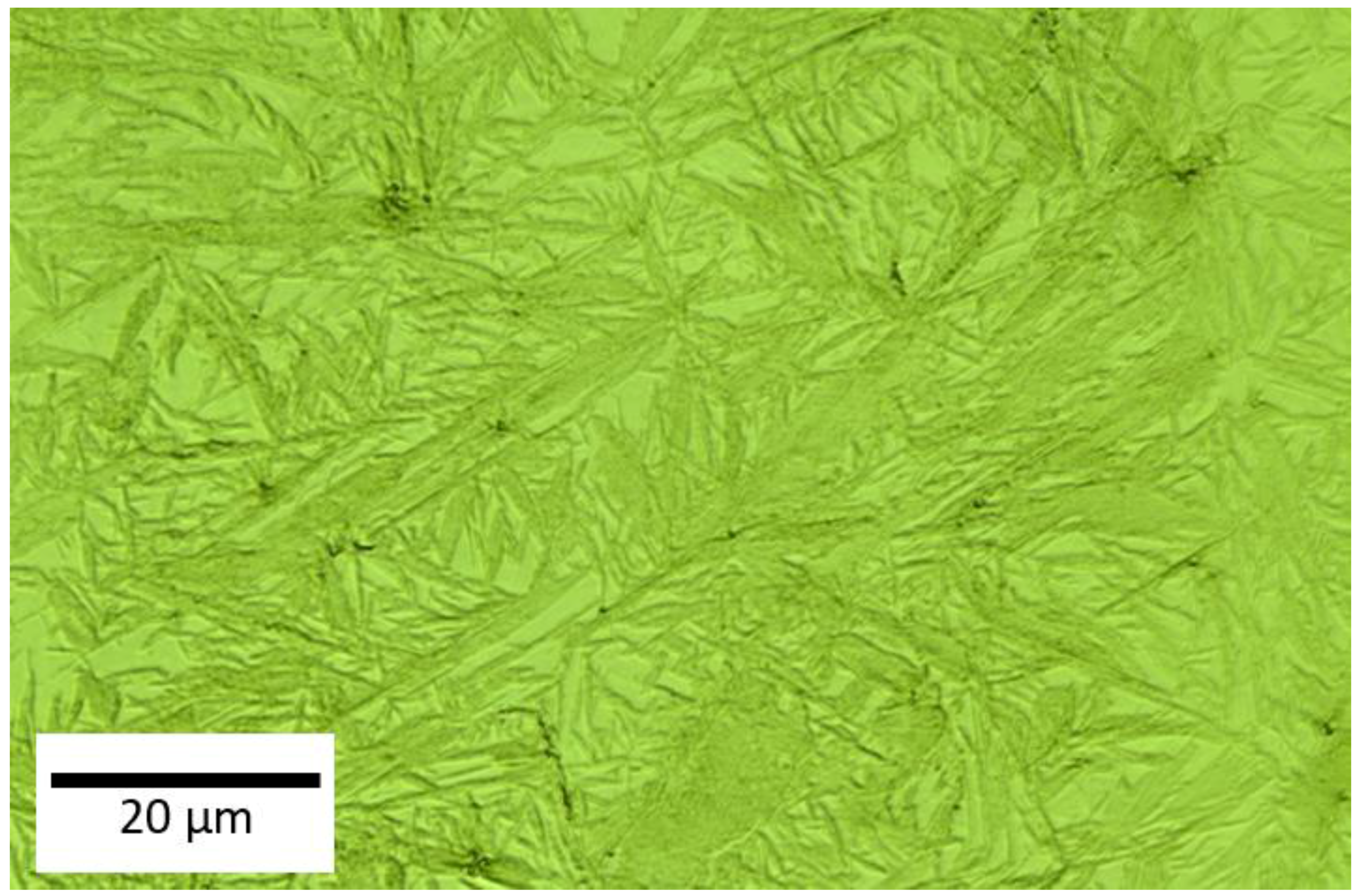
3.1. Dual-Phase Microstructures
3.2. Corrosion Behaviour
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Hossain, R.; Pahlevani, F.; Quadir, M.Z.; Sahajwalla, V. Stability of retained austenite in high carbon steel under compressive stress: An investigation from macro to nano scale. Sci. Rep. 2016, 6, 34958. [Google Scholar] [CrossRef] [PubMed]
- Nili-Ahmadabadi, M.; Pahlevani, F.; Babaghorbani, P. Effect of Slope Plate Variable and Reheating on the Semi-Solid Structure of Ductile Cast Iron. Tsinghua Sci. Technol. 2008, 13, 147–151. [Google Scholar] [CrossRef]
- Pahlevani, F.; Endo, Y.; Yaokawa, J.; Itamura, M.; Kikuchi, M.; Nagasawa, O.; Anzai, K. Development of cup-cast method; semi-solid slurry preparation without external stirring force. Solid State Phenom. 2006, 116–117, 358–361. [Google Scholar] [CrossRef]
- Ikechukwu, A.S.; Obioma, E.; Ugochukwu, N.H. Studies on Corrosion Characteristics of Carbon Steel Exposed to Na2CO3, Na2SO4 and NaCl Solution of Different Concentrations. Int. J. Eng. Sci. 2014, 3, 48–60. [Google Scholar]
- Rani, B.E.A.; Basu, B.B.J. Green Inhibitors for Corrosion Protection of Metals and Alloys: An Overview. Int. J. Corros. 2012, 1–15. [Google Scholar] [CrossRef]
- Makarenko, N.; Kharchenko, U.; Zemnukhova, L. Effect of amino acids on corrosion of copper and steel in acid medium. Russ. J. Appl. Chem. 2011, 84, 1362–1365. [Google Scholar] [CrossRef]
- ASTM International. Austenitic Stainless Steel. In Stainless Steels for Design Engineers; ASTM International: West Conshohocken, PA, USA, 2008; Chapter 6; pp. 69–78. [Google Scholar]
- Hossain, R.; Pahlevani, F.; Sahajwalla, V. Effect of small addition of Cr on stability of retained austenite in high carbon steel. Mater. Charact. 2017, 125, 114–122. [Google Scholar] [CrossRef]
- Hossain, R.; Pahlevani, F.; Witteveen, E.; Banerjee, A.; Joe, B.; Prusty, B.G.; Dippenaar, R.; Sahajwalla, V. Hybrid structure of white layer in high carbon steel—Formation mechanism and its properties. Sci. Rep. 2017, 7, 13288. [Google Scholar] [CrossRef] [PubMed]
- Corle, T.R.; Kino, G.S. Confocal Scanning Optical Microscopy and Related Imaging Systems; Academic Press: New York, NY, USA, 1996. [Google Scholar]
- Altshuler, T.L. Examination of plain carbon steels using an atomic force microscope. In Atomic Force Microscope/Scanning Tunnelling Microscopy; Cohen, S.C., Bray, M.T., Lightbody, M.L., Eds.; Plenum: New York, NY, USA, 1994; pp. 167–180. [Google Scholar]
- Bhushan, B. Nanotribology and Nanomechanics, an Introduction; Springer: Berlin, Germany, 2005. [Google Scholar]
- Wiesendanger, R. Scanning Probe Microscope and Spectroscopy; Cambridge University Press: Cambridge, UK, 1994. [Google Scholar]
- Satapathy, A.K.; Gunasekaran, G.; Sahoo, S.C.; Amit, K.; Rodrigues, P.V. Corrosion inhibition by Justicia gendarussa plant extract in hydrochloric acid solution. Corros. Sci. 2009, 51, 2848–2856. [Google Scholar] [CrossRef]
- Andrade, C.; Alonso, C. Corrosion rate monitoring in the laboratory and on-site. Constr. Build. Mater. 1996, 10, 315–328. [Google Scholar] [CrossRef]
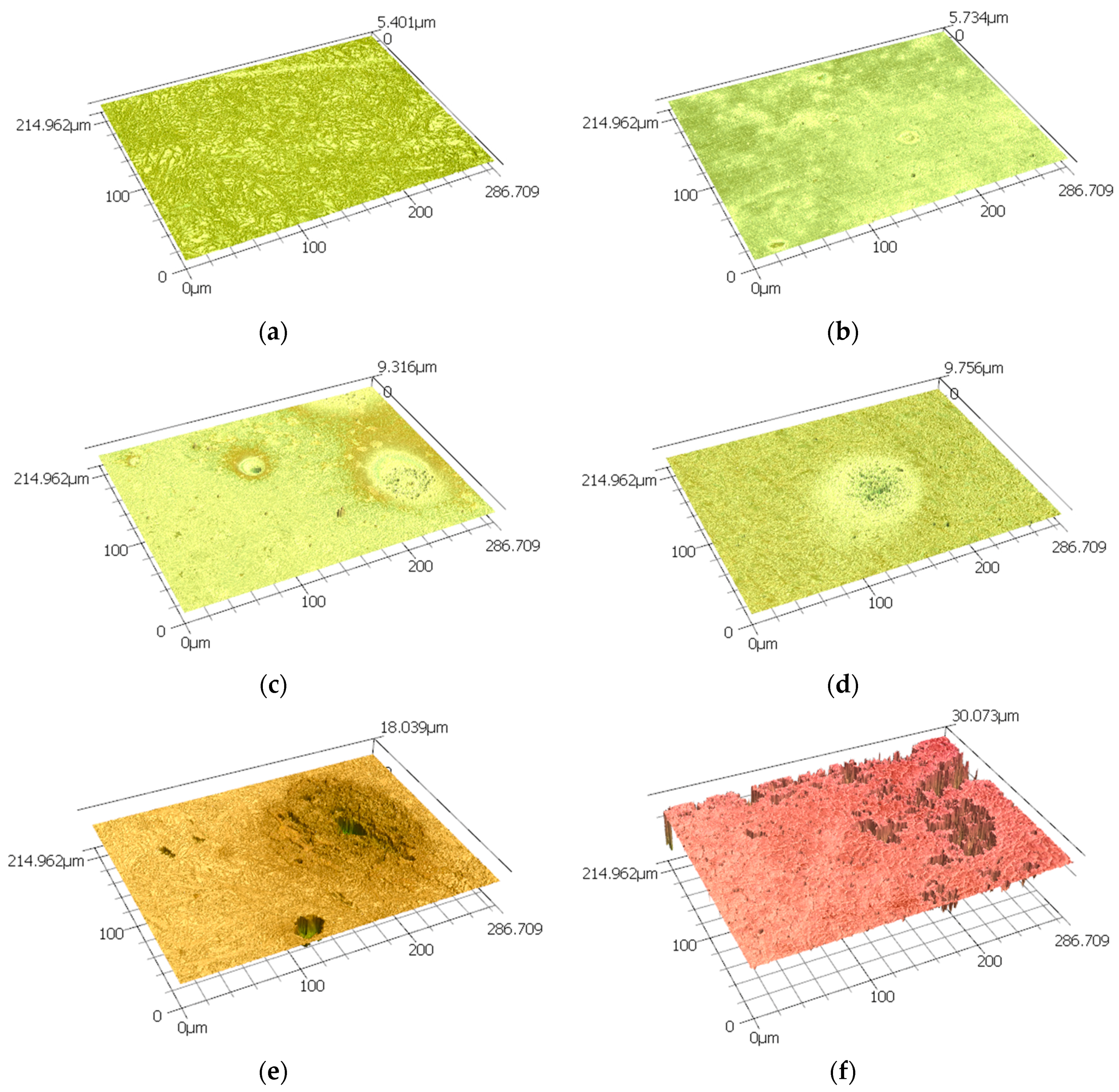
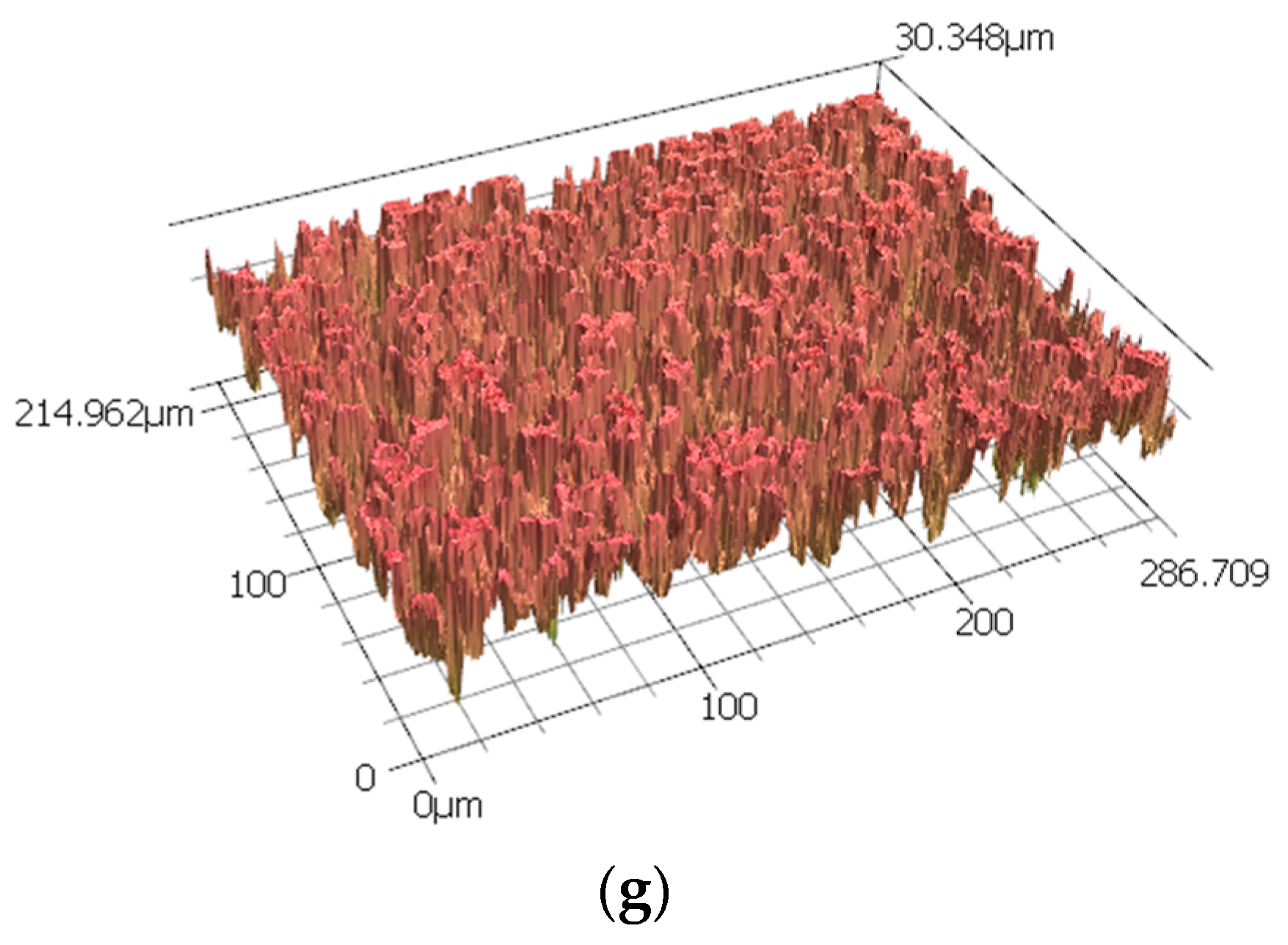
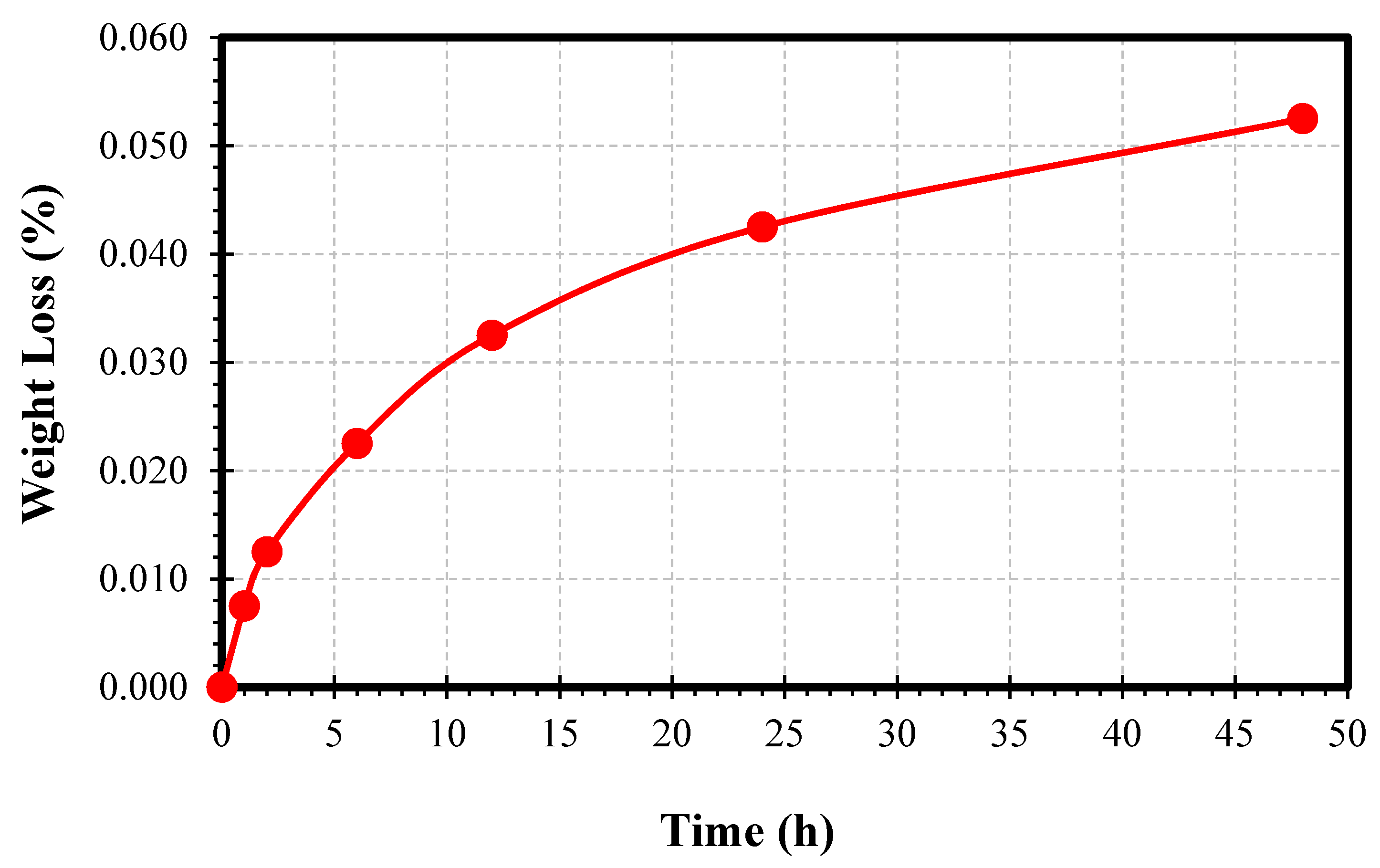
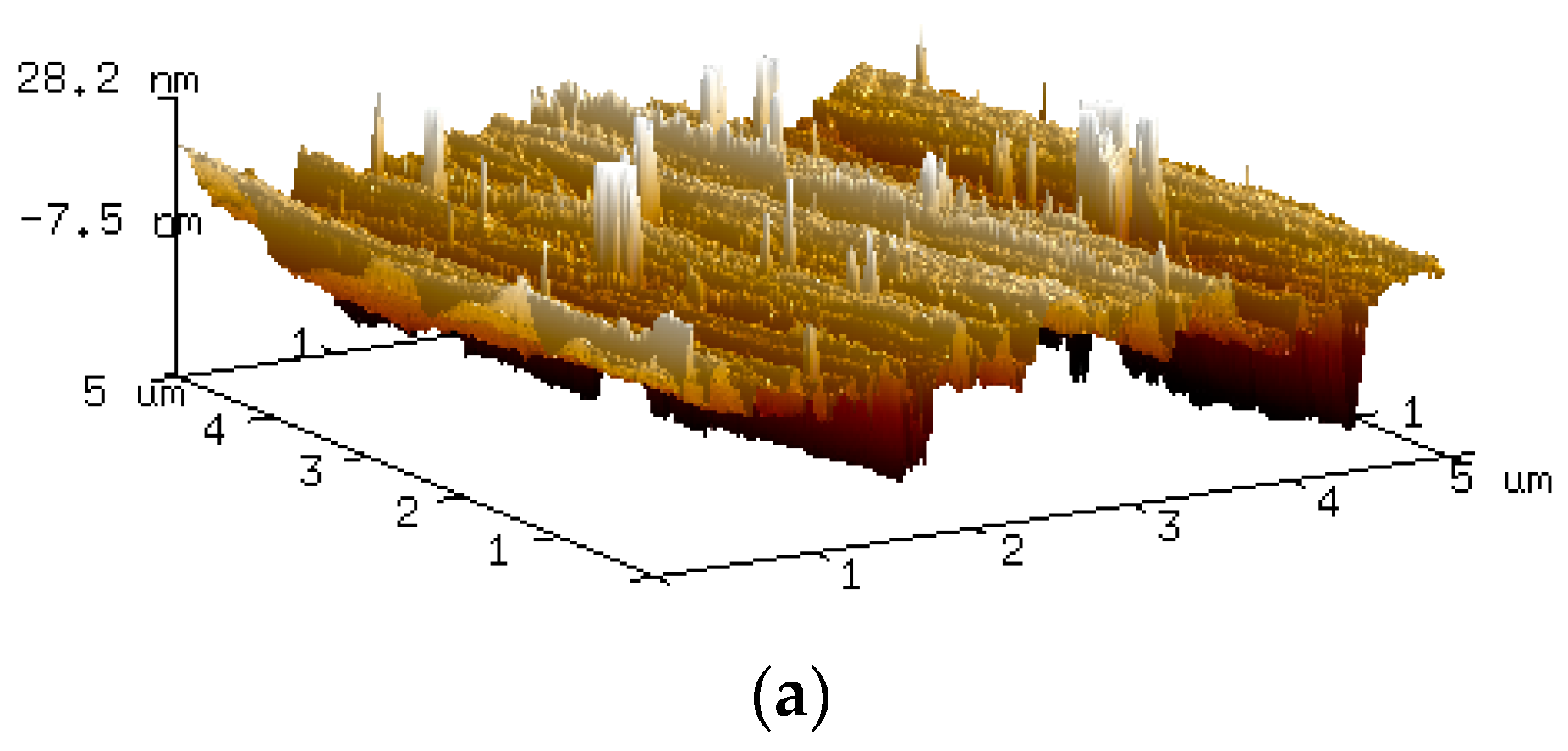
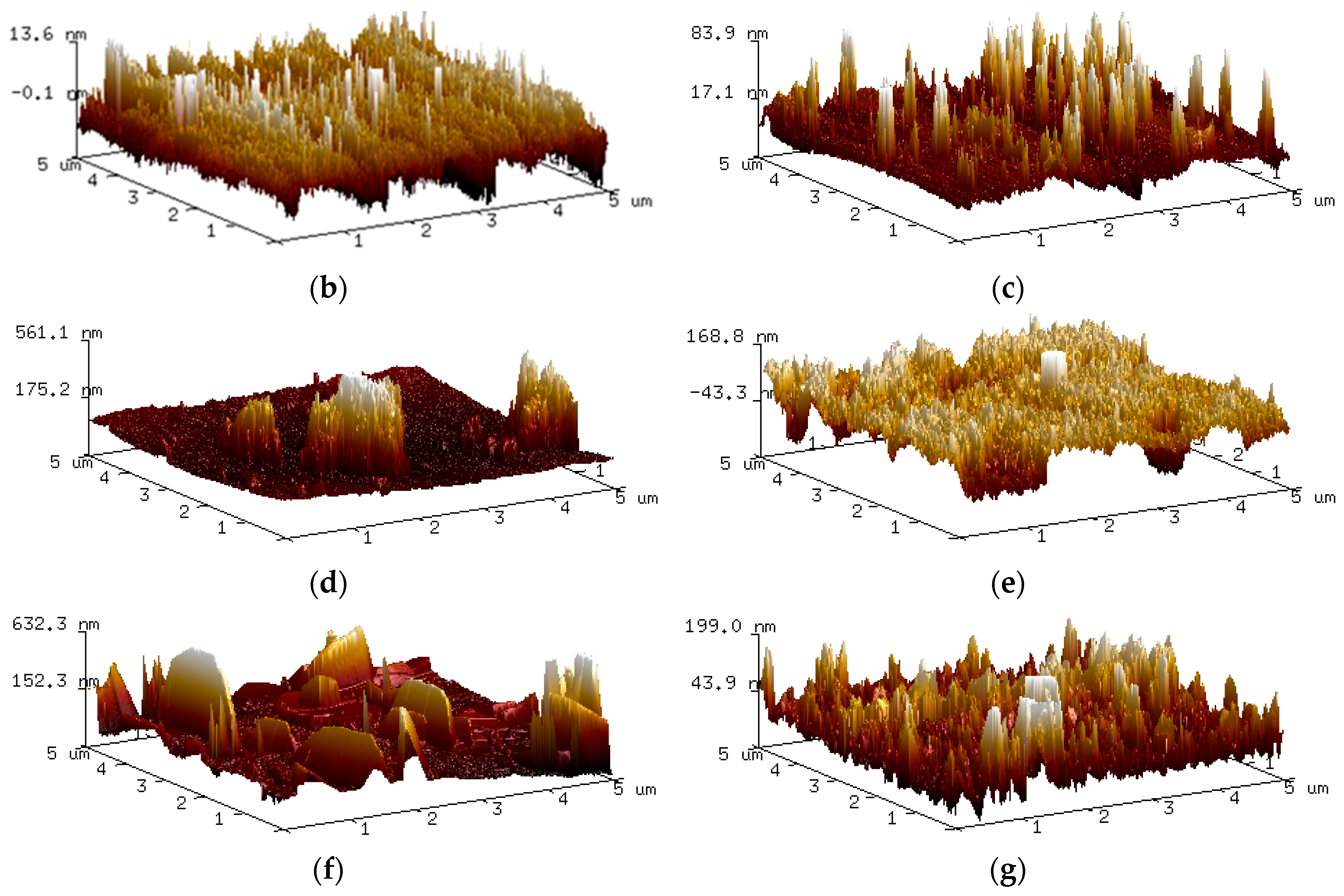

| Time (h) | Ra (µm) | Rz (µm) |
|---|---|---|
| 0 | 0.072 | 5.401 |
| 1 | 0.028 | 5.734 |
| 2 | 0.065 | 9.106 |
| 6 | 0.153 | 9.756 |
| 12 | 0.496 | 17.754 |
| 24 | 1.646 | 28.295 |
| 48 | 3.996 | 30.339 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Handoko, W.; Pahlevani, F.; Sahajwalla, V. Corrosion Behaviour of Dual-Phase High Carbon Steel—Microstructure Influence. J. Manuf. Mater. Process. 2017, 1, 21. https://doi.org/10.3390/jmmp1020021
Handoko W, Pahlevani F, Sahajwalla V. Corrosion Behaviour of Dual-Phase High Carbon Steel—Microstructure Influence. Journal of Manufacturing and Materials Processing. 2017; 1(2):21. https://doi.org/10.3390/jmmp1020021
Chicago/Turabian StyleHandoko, Wilson, Farshid Pahlevani, and Veena Sahajwalla. 2017. "Corrosion Behaviour of Dual-Phase High Carbon Steel—Microstructure Influence" Journal of Manufacturing and Materials Processing 1, no. 2: 21. https://doi.org/10.3390/jmmp1020021






