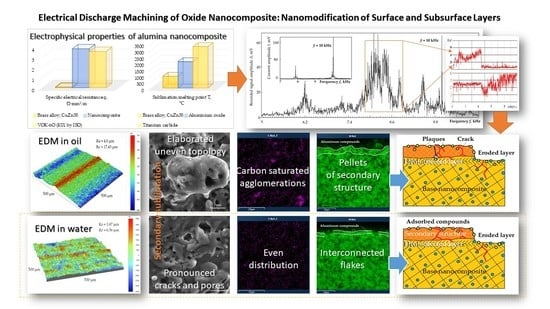
Electrical Discharge Machining of Oxide Nanocomposite: Nanomodification of Surface and Subsurface Layers
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Discharge Gap
2.2. Equipment and Methods
2.3. Monitoring
3. Results
3.1. Monitoring
3.2. Roughness and Surface Topology
3.3. Chemical Content of Eroded Surfaces under Discharge Pulses
3.4. Microstructure and Chemical Content of Cross-Section
3.5. X-Ray Photoelectron Spectroscopy
3.6. Discharge Gap
4. Discussion
5. Conclusions
6. Patents
- Kozochkin, M.P.; Grigoriev, S.N.; Porvatov, A.N., Okunkova, A.A. The method of controlling the electrical discharge machining of parts on an automated cutting machine with a system of CNC; RU 2598022.
- Kozochkin, M.P.; Khoteenkov, K.E.; Porvatov, A.N., Grigoriev, S.N. The method of EDM cutting of products; RU 2638607.
- Grigoriev, S.N.; Kozochkin, M.P.; Okunkova, A.A. The method of positioning the wire electrode on the EDM cutting machines; RU 2572678.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Mouralova, K.; Zahradnicek, R.; Benes, L.; Prokes, T.; Hrdy, R.; Fries, J. Study of Micro Structural Material Changes after WEDM Based on TEM Lamella Analysis. Metals 2020, 10, 949. [Google Scholar] [CrossRef]
- Wu, Y.-Y.; Huang, T.-W.; Sheu, D.-Y. Desktop Micro-EDM System for High-Aspect Ratio Micro-Hole Drilling in Tungsten Cemented Carbide by Cut-Side Micro-Tool. Micromachines 2020, 11, 675. [Google Scholar] [CrossRef] [PubMed]
- Borchers, F.; Clausen, B.; Eckert, S.; Ehle, L.; Epp, J.; Harst, S.; Hettig, M.; Klink, A.; Kohls, E.; Meyer, H.; et al. Comparison of Different Manufacturing Processes of AISI 4140 Steel with Regard to Surface Modification and Its Influencing Depth. Metals 2020, 10, 895. [Google Scholar] [CrossRef]
- Lalwani, V.; Sharma, P.; Pruncu, C.I.; Unune, D.R. Response Surface Methodology and Artificial Neural Network-Based Models for Predicting Performance of Wire Electrical Discharge Machining of Inconel 718 Alloy. J. Manuf. Mater. Process. 2020, 4, 44. [Google Scholar]
- Moudood, M.A.; Sabur, A.; Ali, M.Y.; Jaafar, I.H. Effect of Peak Current on Material Removal Rate for Electrical Discharge Machining of Non-Conductive Al2O3 Ceramic. Adv. Mater. Res. 2014, 845, 730–734. [Google Scholar] [CrossRef]
- Guo, Y.; Hou, P.; Shao, D.; Li, Z.; Wang, L.; Tang, L. High-Speed Wire Electrical Discharge Machining of Insulating Zirconia with a Novel Assisting Electrode. Mater. Manuf. Process. 2014, 29, 526–531. [Google Scholar] [CrossRef]
- Volosova, M.; Okunkova, A.; Peretyagin, P.; Melnik, Y.A.; Kapustina, N. On Electrical Discharge Machining of Non-Conductive Ceramics: A Review. Technologies 2019, 7, 55. [Google Scholar] [CrossRef] [Green Version]
- Volosova, M.A.; Okunkova, A.A.; Fedorov, S.V.; Hamdy, K.; Mikhailova, M.A. Electrical Discharge Machining Non-Conductive Ceramics: Combination of Materials. Technologies 2020, 8, 32. [Google Scholar] [CrossRef]
- Bassoli, E.; Denti, L.; Gatto, A.; Iuliano, L. Influence of electrode size and geometry in electro-discharge drilling of Inconel 718. Int. J. Adv. Manuf. Technol. 2016, 86, 2329–2337. [Google Scholar] [CrossRef]
- Malek, O.; Gonzalez-Julian, J.; Vleugels, J.; Vanderauwera, W.; Lauwers, B.; Belmonte, M. Carbon nanofillers for machining insulating ceramics. Mater. Today 2011, 14, 496–501. [Google Scholar] [CrossRef]
- Movchan, B.A.; Grechanyuk, N.I. Microhardness and microbrittleness of condensates of the TiC-Al2O3 system. Inorg. Mater. 1981, 17, 972–973. [Google Scholar]
- Watanabe, M.; Fukuura, I. Strength of Al2O3 and Al2O3-tic ceramics in relation to their fracture sources. Am. Ceram. Soc. Bull. 1981, 60, 394. [Google Scholar]
- Lee, M.; Borom, M. Rapid rate sintering of Al2O3–TiC composites for cutting-tool application. Adv. Ceram. Mater. 1988, 3, 38–44. [Google Scholar] [CrossRef]
- Cutler, R.A.; Hurford, A.C.; Virkar, A.V. Pressureless-sintered Al2O3-TiC composites. Mater. Sci. Eng. A 1988, 105–106, 183–192. [Google Scholar] [CrossRef]
- Smirnov, B.I.; Nikolaev, V.I.; Burenkov, Y.A.; Routbort, J.L.; Goretta, K.C. Some physical properties of an Al2O3-SiC-TiC composite. Tech. Phys. Lett. 1997, 23, 923–926. [Google Scholar] [CrossRef]
- Cai, K.F.; McLachlan, D.S.; Axen, N.; Manyatsa, R. Preparation, microstructures and properties of Al2O3-TiC composites. Ceram. Int. 2002, 28, 217–222. [Google Scholar] [CrossRef]
- Zhang, Y.F.; Wang, L.J.; Jiang, W.; Bai, G.Z.; Chen, L.D. Effect of fabrication method on microstructure and properties of Al2O3-TiC composites. Mater. Trans. 2005, 46, 2015–2019. [Google Scholar] [CrossRef] [Green Version]
- Lazarenko, B.R.; Mikhailov, V.V.; Gitlevich, A.E.; Verkhoturov, A.D.; Anfimov, I.S. Distribution of elements in surface layers during electric spark alloying. (Raspredelenie Elementov V Poverkhnostnykh Sloyakh Pri Elektroiskrovom Legirovanii). Surf. Eng. Appl. Electrochem. (Elektron. Obrab. Mater.) 1977, 3, 28–33. [Google Scholar]
- Lazarenko, B.R.; Duradzhi, V.N.; Bryantsev, I.V. Effect of Incorporating an additional inductance on the characteristics of anode and cathode processes. (O Vliyanii Vklyucheniya Dopolnitel'noi Induktivnosti Na Kharakteristiki Anodnogo I Katodnogo Protsessov). Surf. Eng. Appl. Electrochem. (Elektron. Obrab. Mater.) 1979, 5, 8–13. [Google Scholar]
- Lazarenko, B.R.; Lazarenko, N.I. Electric spark machining of metals in water and electrolytes. (Elektroiskrovaya Obrabotka Metallov V Vode I Elektrolitakh). Surf. Eng. Appl. Electrochem. (Elektron. Obrab. Mater.) 1980, 1, 5–8. [Google Scholar]
- Zhang, Y.; Liu, Z.; Zhang, M.; Qiu, M.B.; Zhang, H. Study on the influence that hard water had in high-speed wire electrical discharge machining. Int. J. Adv. Manuf. Technol. 2019, 104, 1251–1258. [Google Scholar] [CrossRef]
- Senthilkumar, T.S.; Muralikannan, R. Evaluation of recast layer and parametric optimization of EDM process on aluminium based HMMCs using grey relational analysis. Mater. Res. Express 2019, 6, 1065a6. [Google Scholar] [CrossRef]
- Grigoriev, S.N.; Kozochkin, M.P.; Porvatov, A.N.; Volosova, M.A.; Okunkova, A.A. Electrical discharge machining of ceramic nanocomposites: Sublimation phenomena and adaptive control. Heliyon 2019, 5, e02629. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wei, C.; Zhao, L.; Hu, D.; Ni, J. Electrical discharge machining of ceramic matrix composites with ceramic fiber reinforcements. Int. J. Adv. Manuf. Technol. 2013, 64, 187–194. [Google Scholar] [CrossRef]
- Kong, W.; Guo, C.; Zhu, X. Simulation analysis of bubble motion under ultrasonic assisted Electrical Discharge Machining. In Proceedings of the 2015 3rd International Conference on Machinery, Materials and Information Technology Applications, Qingdao, China, 28–29 November 2015; ACSR-Advances in Comptuer Science Research. Volume 35, pp. 1728–1731. [Google Scholar]
- Liu, Y.; Chang, H.; Zhang, W.; Ma, F.; Sha, Z.; Zhang, S. A Simulation Study of Debris Removal Process in Ultrasonic Vibration Assisted Electrical Discharge Machining (EDM) of Deep Holes. Micromachines 2018, 9, 378. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Chen, S.-Y.; Xiong, W.; Wu, C.Z. Study on workpiece surface forming mechanism by successive discharges during USV-MF complex-assisted WEDM-LS process. Int. J. Adv. Manuf. Technol. 2020, 108, 2985–3000. [Google Scholar] [CrossRef]
- Tzeng, Y.F.; Lee, C.Y. Effects of powder characteristics on electrodischarge machining efficiency. Int. J. Adv. Manuf. Technol. 2001, 17, 586–592. [Google Scholar] [CrossRef]
- Baseri, H.; Sadeghian, S. Effects of nanopowder TiO2-mixed dielectric and rotary tool on EDM. Int. J. Adv. Manuf. Technol. 2016, 83, 519–528. [Google Scholar] [CrossRef]
- Tran, T.-H.; Nguyen, M.-C.; Luu, A.-T.; Do, T.-V.; Le, T.-Q.; Vu, T.-T.; Tran, N.-G.; Do, T.-T.; Vu, N.-P. Electrical Discharge Machining with SiC Powder-Mixed Dielectric: An Effective Application in the Machining Process of Hardened 90CrSi Steel. Machines 2020, 8, 36. [Google Scholar] [CrossRef]
- Rashid, A.; Bilal, A.; Liu, C.; Jahan, M.P.; Talamona, D.; Perveen, A. Effect of Conductive Coatings on Micro-Electro-Discharge Machinability of Aluminum Nitride Ceramic Using On-Machine-Fabricated Microelectrodes. Materials 2019, 12, 3316. [Google Scholar] [CrossRef] [Green Version]
- Kucukturk, G.; Cogun, C. A New Method for Machining of Electrically Nonconductive Workpieces Using Electric Discharge Machining Technique. Mach. Sci. Technol. 2010, 14, 189–207. [Google Scholar] [CrossRef]
- Melnik, Y.A.; Kozochkin, M.P.; Porvatov, A.N.; Okunkova, A.A. On adaptive control for electrical discharge machining using vibroacoustic emission. Technologies 2018, 6, 96. [Google Scholar] [CrossRef] [Green Version]
- Meyer, D.R.; Cavallini, A.; Lusuardi, L. Influence of Impulse Voltage Repetition Frequency on RPDIV in Partial Vacuum. IEEE Trans. Dielectr. Electr. Insul. 2018, 25, 873–882. [Google Scholar] [CrossRef]
- Grigor’ev, S.N.; Kozochkin, M.P.; Fedorov, S.V.; Porvatov, A.N.; Okun’kova, A.A. Study of electroerosion processing by vibroacoustic diagnostic methods. Meas. Tech. 2015, 58, 878–884. [Google Scholar] [CrossRef]
- Kozochkin, M.P.; Porvatov, A.N. Estimation of Uncertainty in Solving Multi-Parameter Diagnostic Problems. Meas. Tech. 2015, 58, 173–178. [Google Scholar] [CrossRef]
- Grigoriev, S.N.; Masterenko, D.A.; Teleshevskii, V.I.; Emelyanov, P.N. Contemporary state and outlook for development of metrological assurance in the machine-building industry. Meas. Tech. 2013, 55, 1311. [Google Scholar] [CrossRef]
- Volosova, M.A.; Grigor’ev, S.N.; Kuzin, V.V. Effect of titanium nitride coating on stress structural inhomogeneity in oxide-carbide ceramic. Part 4. Action of heat flow. Refract. Ind. Ceram. 2015, 56, 91–96. [Google Scholar] [CrossRef]
- Volosova, M.A.; Gurin, V.D. Influence of vacuum-plasma nitride coatings on contact processes and a mechanism of wear of working surfaces of high-speed steel cutting tool at interrupted cutting. J. Frict. Wear 2013, 34, 183–189. [Google Scholar] [CrossRef]
- Fominski, V.Y.; Grigoriev, S.N.; Romanov, R.I.; Volosova, M.A.; Grunin, A.I.; Teterina, G.D. The formation of a hybrid structure from tungsten selenide and oxide plates for a hydrogen-evolution electrocatalyst. Tech. Phys. Lett. 2016, 42, 555–558. [Google Scholar] [CrossRef]
- Volosova, M.A.; Grigoriev, S.N.; Ostrikov, E.A. Use of laser ablation for formation of discontinuous (discrete) wear-resistant coatings formed on solid carbide cutting tool by electron beam alloying and vacuum-arc deposition. Mech. Ind. 2016, 17, 720. [Google Scholar] [CrossRef]
- Lin, Y.-J.; Lin, Y.-C.; Wang, A.-C.; Wang, D.A.; Chow, H.M. Machining characteristics of EDM for non-conductive ceramics using adherent copper foils. In Advanced Materials Research, Materials Processing Technologies, Proceedings of the International Conference on Advances in Materials and Manufacturing Processes, Shenzhen, China, 6–8 November 2010; Jiang, Z.Y., Liu, X.H., Bu, J.L., Eds.; Trans Tech Publications Ltd.: Durnten-Zurich, Switzerland, 2010. [Google Scholar] [CrossRef]
- Popov, M.Y.; Churkin, V.D.; Kulnitskiy, B.A.; Kirichenko, A.N.; Bulatov, K.M.; Bykov, A.A.; Zinin, P.V.; Blank, V. Transformation of diamond to fullerene-type onions at pressure 70 GPa and temperature 2400 K. Nanotechnology 2020, 31, 315602. [Google Scholar] [CrossRef] [PubMed]
- Borzdov, Y.M.; Khokhryakov, A.F.; Kupriyanov, I.N.; Nechaev, D.V.; Palyanov, Y.N. Crystallization of Diamond from Melts of Europium Salts. Crystals 2020, 10, 376. [Google Scholar] [CrossRef]
- Fomin, Y.D.; Brazhkin, V.V. Comparative study of melting of graphite and graphene. Carbon 2020, 157, 767–778. [Google Scholar] [CrossRef]
- Sychev, A.E.; Busurina, M.L.; Sachkova, N.V.; Vrel, D. Interaction of Graphite with a Ti-Al Melt during Self-Propagating High-Temperature Synthesis. Inorg. Mater. 2019, 55, 780–784. [Google Scholar] [CrossRef]
- Niamat, M.; Sarfraz, S.; Shehab, E.; Ismail, S.O.; Khalid, Q.S. Experimental characterization of electrical discharge machining of aluminum 6061 T6 alloy using different dielectrics. Arab. J. Sci. Eng. 2019, 44, 8043–8052. [Google Scholar] [CrossRef] [Green Version]
- Obrosov, A.; Gulyaev, R.; Zak, A.; Ratzke, M.; Naveed, M.; Dudzinski, W.; Weiß, S. Chemical and morphological characterization of magnetron sputtered at different bias voltages Cr-Al-C coatings. Materials 2017, 10, 156. [Google Scholar] [CrossRef] [Green Version]
- Bains, P.S.; Singh, S.; Sidhu, S.S.; Kaur, S.; Ablyaz, T.R. Investigation of surface properties of Al–SiC composites in hybrid electrical discharge machining. In Futuristic Composites, 1st ed.; Sidhu, S., Bains, P., Zitoune, R., Yazdani, M., Eds.; Springer: Singapore, 2018; pp. 181–196. [Google Scholar]
- Vozniakovskii, A.A.; Kidalov, S.V.; Kol’tsova, T.S. Development of composite material aluminum-carbon nanotubes with high hardness and controlled thermal conductivity. J. Compos. Mater. 2019, 53, 2959–2965. [Google Scholar] [CrossRef]
- Mujumdar, S.S.; Curreli, D.; Kapoor, S.G. Effect of dielectric conductivity on micro-electrical discharge machining plasma characteristics using optical emission spectroscopy. J. Micro Nano Manuf. 2018, 6, UNSP 031001. [Google Scholar] [CrossRef]
- Kou, Z.; Han, F. Machining characteristics and removal mechanisms of moving electric arcs in high-speed EDM milling. J. Manuf. Process. 2018, 32, 676. [Google Scholar] [CrossRef]
- Salmanov, V.M.; Guseinov, A.G.; Mamedov, R.M.; Salmanova, A.A.; Gasanova, L.G.; Magomedov, A.Z.; Akhmedova, F.S. Effect of laser radiation on InSe and GaSe thin films grown via laser sublimation and chemical deposition. Russ. J. Phys. Chem. 2018, 92, 1790. [Google Scholar] [CrossRef]
- Grigoriev, S.; Metel, A. Plasma- and Beam-Assisted Deposition Methods. In Nanostructured Thin Films and Nanodispersion Strengthened Coatings, NATO Science Series II: Mathematics, Physics and Chemistry; Voevodin, A.A., Shtansky, D.V., Levashov, E.A., Moore, J.J., Eds.; Springer: Dordrecht, The Netherlands, 2004; Volume 155, pp. 147–154. [Google Scholar] [CrossRef]
- Grigoriev, S.; Melnik, Y.; Metel, A. Broad fast neutral molecule beam sources for industrial-scale beam-assisted deposition. Surf. Coat. Tech. 2002, 156, 44–49. [Google Scholar] [CrossRef]
- Yun, J.D.; Go, C.; Wang, D.H.; Ahn, Y.C. Electrical discharge machining of aluminum oxide matrix composites containing titanium carbide as a conductive second phase. In Processing and Fabrication of Advanced Materials VI, Vols 1 & 2, Proceedings of the International Symposium on Processing and Fabrication of Advanced Materials, Singapore, 24–26 November 1997; Khor, K.A., Srivatsan, T.S., Moore, J.J., Eds.; IOM Communications Ltd.: London, UK, 1998; pp. 1773–1781. [Google Scholar]
- Kumar, R.; Chaubey, A.K.; Maity, T.; Prashanth, K.G. Mechanical and Tribological Properties of Al2O3-TiC Composite Fabricated by Spark Plasma Sintering Process with Metallic (Ni, Nb) Binders. Metals 2018, 8, 50. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.F.; Wang, L.J.; Jiang, W.; Chen, L.D. Microstructure and properties of Al2O3-TiC composites fabricated by combination of high-energy ball milling and spark plasma sintering (SPS). J. Inorg. Mater. 2005, 20, 1445–1449. [Google Scholar]
- Konyashin, I.Y. Toughening of the Al2O3-TiC ceramics by depositing thin coatings. Surf. Coat. Tech. 1996, 79, 192–199. [Google Scholar] [CrossRef]
- Gadalov, V.N.; Romanenko, D.N.; Samoilov, V.V.; Nikolaenko, A.V.; Grigor’ev, S.B. Procedure of evaluating the surface roughness of the electrospark coating after burnishing with mineral ceramics. Russ. J. Non-Ferr. Met. 2012, 53, 348–350. [Google Scholar] [CrossRef]
- Pronin, A.I.; Myl’nikov, V.V.; Shchelkunov, E.B.; Chernyshov, E.A. Investigation of Cutting Ceramic Stability during Sharpening of Hardened Steel Blanks. Glass Ceram. 2018, 74, 364–366. [Google Scholar] [CrossRef]
- Ponomarev, A.N. An investigation of diffusion in droplets and of evaporation of volatile components into vacuum. High. Temp. 2005, 43, 930–936. [Google Scholar] [CrossRef]
- Nikitina, E.V.; Kazakovtseva, N.A. High Temperature Corrosion of 12Kh18N10T Steel in Molten Lithium and Potassium Chlorides with a Cerium Trichloride Addition. Russ. Met. 2018, 8, 747–749. [Google Scholar] [CrossRef]
- Elshina, L.A.; Elshina, V.A. Synthesis of a Nanocrystalline alpha-Al2O3 Powder in Molten Halides in the Temperature Range 700–800 °C. Russ. Met. 2020, 2, 138–141. [Google Scholar] [CrossRef]
- Pang, W.K.; Low, I.M. Understanding and improving the thermal stability of layered ternary carbides in ceramic matrix composites. In Advances in Ceramic Matrix Composites; Woodhead Publishing Series in Composites Science and Engineering; Low, I.M., Ed.; Woodhead Publ Ltd.: Cambs, UK, 2014; Volume 45, pp. 340–368. [Google Scholar] [CrossRef]
- Fedorov, S.V.; Pavlov, M.D.; Okunkova, A.A. Effect of structural and phase transformations in alloyed subsurface layer of hard-alloy tools on their wear resistance during cutting of high-temperature alloys. J. Frict. Wear 2013, 34, 190–198. [Google Scholar] [CrossRef]
- Volosova, M.A.; Okunkova, A.A.; Povolotskiy, D.E.; Podrabinnik, P.A. Study of electrical discharge machining for the parts of nuclear industry usage. Mech. Ind. 2015, 16, 706. [Google Scholar] [CrossRef] [Green Version]
- Gavrin, V.N.; Kozlova, Y.P.; Veretenkin, E.P.; Logachev, A.V.; Logacheva, A.I.; Lednev, I.S.; Okunkova, A.A. Reactor target from metal chromium for “pure” high-intensive artificial neutrino source. Phys. Part. Nucl. Lett. 2016, 13, 267–273. [Google Scholar] [CrossRef]
- Grigoriev, S.N.; Kozochkin, M.P.; Kropotkina, E.Y.; Okunkova, A.A. Study of wire tool-electrode behavior during electrical discharge machining by vibroacoustic monitoring. Mech. Ind. 2016, 17, 717. [Google Scholar] [CrossRef] [Green Version]
- Ay, M.; Etyemez, A. Optimization of the effects of wire EDM parameters on tolerances. Emerg. Mater. Res. 2020, 9, 527–531. [Google Scholar] [CrossRef]
- Markopoulos, A.P.; Papazoglou, E.-L.; Karmiris-Obratański, P. Experimental Study on the Influence of Machining Conditions on the Quality of Electrical Discharge Machined Surfaces of aluminum alloy Al5052. Machines 2020, 8, 12. [Google Scholar] [CrossRef] [Green Version]
- Goswami, K.; Samuel, G.L. Monitoring of material-removal mechanism in micro-electrical discharge machining by pulse classification and acoustic emission signals. Proc. Inst. Mech. Eng. B-J. Eng. 2020. [Google Scholar] [CrossRef]
- Dwaraka, R.; Arunachalam, N. Investigation on the effect of EDM process variables and environments on acoustic emission signals. Mach. Sci. Technol. 2020, 24, 638–662. [Google Scholar] [CrossRef]
- Nemilov, E.F. Spravochnik po Elektroerozionnoy Obrabotke Materialov (Handbook of Electrical Discharge Machining of Materials); Mashinostroenie: Leningrad, Russia, 1989; p. 164. [Google Scholar]
- Rudnev, V.S. Micro- and nano-formations on the surface of plasma electrolytic oxide coatings on aluminum and titanium. Surf. Coat. Tech. 2013, 235, 134–143. [Google Scholar] [CrossRef]
- Shinkevich, E.V.; Root, L.O. Stability of Products of Combusting in Air Aluminum Nanopowder with Titanium and Zirconium Dioxides to Hydrofluoric Acid Action in Reductive Media. Bull. Tomsk Polytech. Univ.-Geo Assets Eng. 2015, 326, 46–53. [Google Scholar]
- Osokin, A.A. Role of the Chemical Activity of Titanium in Welding to Aluminum. Weld. Prod. 1978, 25, 7–8. [Google Scholar]
- Szpyrkowicz, L.; Radaelli, M.; Daniele, S. Electrocatalysis of chlorine evolution on different materials and its influence on the performance of an electrochemical reactor for indirect oxidation of pollutants. Catal. Today 2005, 100, 425–429. [Google Scholar] [CrossRef]
- Ehrburger, P.; Lahaye, J.; Dziedzinl, P.; Fangeat, R. Effect of Aging on the Behavior of Copper-Chromium Compounds Supported on Activated Carbon. Carbon 1991, 29, 297–303. [Google Scholar] [CrossRef]
- Yue, X.; Yang, X.; Tian, J.; He, Z.; Fan, Y. Thermal, mechanical and chemical material removal mechanism of carbon fiber reinforced polymers in electrical discharge machining. Int. J. Mach. Tool. Manuf. 2018, 133, 4–17. [Google Scholar] [CrossRef]
- Abdelmalek, A.; Bedrane, Z.; Amara, E.-H.; Sotillo, B.; Bharadwaj, V.; Ramponi, R.; Eaton, S. Ablation of Copper Metal Films by Femtosecond Laser Multipulse Irradiation. Appl. Sci. 2018, 8, 1826. [Google Scholar] [CrossRef] [Green Version]
- Gutkin, B.G. Avtomatizatsiya Elektroerozionnykh Stankov (Automation of Electrical Discharge Machines); Mashinostroenie: Leningrad, USSR, 1971; pp. 28–29. (In Russian) [Google Scholar]
- Mirifar, S.; Kadivar, M.; Azarhoushang, B. First Steps through Intelligent Grinding Using Machine Learning via Integrated Acoustic Emission Sensors. J. Manuf. Mater. Process. 2020, 4, 35. [Google Scholar] [CrossRef]
- Warmuzek, M.; Polkowska, A. Micromechanism of Damage of the Graphite Spheroid in the Nodular Cast Iron during Static Tensile Test. J. Manuf. Mater. Process. 2020, 4, 22. [Google Scholar] [CrossRef] [Green Version]
- Vereschaka, A.A.; Grigoriev, S.N.; Vereschaka, A.S.; Popov, A.Y.; Batako, A.D. Nano-scale multilayered composite coatings for cutting tools operating under heavy cutting conditions. Proc. CIRP 2014, 14, 239–244. [Google Scholar] [CrossRef] [Green Version]
- Vereschaka, A.A.; Volosova, M.A.; Grigoriev, S.N.; Vereschaka, A.S. Development of wear-resistant complex for high-speed steel tool when using process of combined cathodic vacuum arc deposition. Proc. CIRP 2013, 9, 8–12. [Google Scholar] [CrossRef] [Green Version]
- Gusarov, A.V.; Grigoriev, S.N.; Volosova, M.A.; Melnik, Y.A.; Laskin, A.; Kotoban, D.V.; Okunkova, A.A. On productivity of laser additive manufacturing. J. Mater. Process. Technol. 2018, 261, 213–232. [Google Scholar] [CrossRef]
- Abdulhadi, H.A.; Ahmad, S.N.A.S.; Ismail, I.; Ishak, M.; Mohammed, G.R. Thermally-Induced Crack Evaluation in H13 Tool Steel. Metals 2017, 7, 475. [Google Scholar] [CrossRef] [Green Version]
- Brzezińska, M.; García-Muñoz, P.; Ruppert, A.M.; Keller, N. Photoactive ZnO Materials for Solar Light-Induced CuxO-ZnO Catalyst Preparation. Materials 2018, 11, 2260. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, H.; Zhang, X.; Hwang, J.; Park, J. Morphological Influence of Solution-Processed Zinc Oxide Films on Electrical Characteristics of Thin-Film Transistors. Materials 2016, 9, 851. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Silva, N.; Ramírez, S.; Díaz, I.; Garcia, A.; Hassan, N. Easy, Quick, and Reproducible Sonochemical Synthesis of CuO Nanoparticles. Materials 2019, 12, 804. [Google Scholar] [CrossRef] [Green Version]
- Zheng, W.; Chen, Y.; Peng, X.; Zhong, K.; Lin, Y.; Huang, Z. The Phase Evolution and Physical Properties of Binary Copper Oxide Thin Films Prepared by Reactive Magnetron Sputtering. Materials 2018, 11, 1253. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kshirsagar, R.; Jones, S.; Lawrence, J.; Tabor, J. Optimization of TIG Welding Parameters Using a Hybrid Nelder Mead-Evolutionary Algorithms Method. J. Manuf. Mater. Process. 2020, 4, 10. [Google Scholar] [CrossRef] [Green Version]
- Dahat, S.; Hurtig, K.; Andersson, J.; Scotti, A. A Methodology to Parameterize Wire + Arc Additive Manufacturing: A Case Study for Wall Quality Analysis. J. Manuf. Mater. Process. 2020, 4, 14. [Google Scholar] [CrossRef] [Green Version]
- Kuzin, V.V.; Grigor’ev, S.N.; Volosova, M.A. Effect of a TiC Coating on the Stress-Strain State of a Plate of a High-Density Nitride Ceramic Under Nonsteady Thermoelastic Conditions. Refract. Ind. Ceram. 2014, 54, 376–380. [Google Scholar] [CrossRef]
- Kuzin, V.V.; Grigoriev, S.N.; Fedorov, M.Y. Role of the thermal factor in the wear mechanism of ceramic tools. Part 2: Microlevel. J. Frict. Wear 2015, 36, 40–44. [Google Scholar] [CrossRef]
- Pola, A.; Montesano, L.; Tocci, M.; La Vecchia, G.M. Influence of Ultrasound Treatment on Cavitation Erosion Resistance of AlSi7 Alloy. Materials 2017, 10, 256. [Google Scholar] [CrossRef]
- Baiocco, G.; Rubino, G.; Tagliaferri, V.; Ucciardello, N. Al2O3 Coatings on Magnesium Alloy Deposited by the Fluidized Bed (FB) Technique. Materials 2018, 11, 94. [Google Scholar] [CrossRef] [Green Version]
- Simakova, I.L.; Simakov, A.V.; Murzin, D.Y. Valorization of Biomass Derived Terpene Compounds by Catalytic Amination. Catalysts 2018, 8, 365. [Google Scholar] [CrossRef] [Green Version]
- Guerrero-Torres, A.; Jiménez-Gómez, C.P.; Cecilia, J.A.; García-Sancho, C.; Quirante-Sánchez, J.J.; Mérida-Robles, J.M.; Maireles-Torres, P. Influence of the Incorporation of Basic or Amphoteric Oxides on the Performance of Cu-Based Catalysts Supported on Sepiolite in Furfural Hydrogenation. Catalysts 2019, 9, 315. [Google Scholar] [CrossRef] [Green Version]



















| Material | |
|---|---|
| Brass alloy CuZn35 | 0.065 |
| Al2O3 + 30%TiC ceramic nanocomposite | 3.773 |
| VOK-6O ceramic composite (analogue of K01, standards of ISO) | 3.510 |
| Aluminum oxide 1 | 2.10 × 105 ÷ 106 |
| Titanium carbide 1 | 6.0 × 103 |
| Characteristic | Value and Description |
|---|---|
| CUT 1000 OilTech | |
| Max axis motions along the axes X × Y × Z, mm | 220 × 160 × 100 |
| Max angle of conical machining, degree | ±3° |
| Max weight of workpiece, kg | 35 |
| Accuracy of positioning along the axes, µm | ±0.5 |
| Achievable roughness Ra, µm | 0.05 |
| Dielectric medium | Mineral oil |
| Machine body | Solid |
| Max power consumption, kW | not confined |
| ARTA 123 Pro | |
| Max axis motions along the axes X × Y × Z, mm | 125 × 200 × 80 |
| Max weight of workpiece, kg | not confined |
| Accuracy of positioning along the axes, µm | ±1 |
| Achievable roughness Ra, µm | 0.6 |
| Dielectric medium | Deionized water |
| Machine body | Solid |
| Max power consumption, kW | <6 |
| Factor | Value |
|---|---|
| Operational voltage, Vo | 75, 85, 108 V |
| Pulse frequency, fp | 5 kHz |
| Pulse width, Tp | 1 µs |
| Speed of the tool rewinding, Ws | 7 m/min |
| Feed rate, Rf | 0.4 mm/min |
| Chemical Element | Binding Energy E | Binding Energy Peak, eV | Atomic % |
|---|---|---|---|
| Carbon | C1s Graphite | 283.8 | 16.64 |
| C1s C-C | 284.4 | 17.06 | |
| C1s Carbide | 283.3 | 12.66 | |
| C1s C=O | 287.4 | 8.49 | |
| C1s C-O | 285.1 | 19.68 | |
| Aluminum | Al2p3 Oxide | 74.3 | 2.75 |
| Nitrogen | N1s C-N | 399.3 | 3.61 |
| N1s N-Si, Me-N | 397.6 | 0.23 | |
| Calcium | Ca2p3 Carbonate | 346.8 | 1.58 |
| Oxygen | O1s C=O | 532.5 | 5.16 |
| O1s Scan B | 530.9 | 6.55 | |
| O1s C-O | 531.7 | 3.66 | |
| Copper | Cu2p3 Me | 932.4 | 0.16 |
| Zinc | Zn2p3 ZnO | 1021.7 | 0.11 |
| Silicon | Si2p3 organic, Si3N4, Silicates | 101.8 | 1.39 |
| Titanium | Ti2p3 TiC | 454.4 | 0.09 |
| Ti2p3 TiO2 | 458.3 | 0.15 | |
| Ti2p3 TiN | 455 | 0.02 |
| Chemical Element | Binding Energy E | Binding energy peak, eV | Atomic, % |
|---|---|---|---|
| Carbon | C1s C-O | 285.5 | 30.54 |
| C1s C=O | 288.3 | 4.85 | |
| C1s C-C | 284.6 | 25.42 | |
| Aluminum | Al2p3 Oxide | 73.8 | 10.47 |
| Nitrogen | N1s C-NH2 | 399.6 | 2.67 |
| Calcium | Ca2p3 CaCO3 | 347.4 | 1.18 |
| Oxygen | O1s C=O | 532.8 | 5.77 |
| O1s C-O | 531.7 | 9.49 | |
| O1s MeO | 530.5 | 8.67 | |
| Titanium | Ti2p3 Me | 453.6 | 0.34 |
| Ti2p3 TiO2 | 457.8 | 0.22 | |
| Ti2p3 TiN | 455 | 0.17 | |
| Zinc | Zn2p3 Me | 1022 | 0.11 |
| Copper | Cu2p3 CuO | 932.9 | 0.12 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grigoriev, S.N.; Volosova, M.A.; Okunkova, A.A.; Fedorov, S.V.; Hamdy, K.; Podrabinnik, P.A.; Pivkin, P.M.; Kozochkin, M.P.; Porvatov, A.N. Electrical Discharge Machining of Oxide Nanocomposite: Nanomodification of Surface and Subsurface Layers. J. Manuf. Mater. Process. 2020, 4, 96. https://doi.org/10.3390/jmmp4030096
Grigoriev SN, Volosova MA, Okunkova AA, Fedorov SV, Hamdy K, Podrabinnik PA, Pivkin PM, Kozochkin MP, Porvatov AN. Electrical Discharge Machining of Oxide Nanocomposite: Nanomodification of Surface and Subsurface Layers. Journal of Manufacturing and Materials Processing. 2020; 4(3):96. https://doi.org/10.3390/jmmp4030096
Chicago/Turabian StyleGrigoriev, Sergey N., Marina A. Volosova, Anna A. Okunkova, Sergey V. Fedorov, Khaled Hamdy, Pavel A. Podrabinnik, Petr M. Pivkin, Mikhail P. Kozochkin, and Artur N. Porvatov. 2020. "Electrical Discharge Machining of Oxide Nanocomposite: Nanomodification of Surface and Subsurface Layers" Journal of Manufacturing and Materials Processing 4, no. 3: 96. https://doi.org/10.3390/jmmp4030096