Additive Manufacturing: An Opportunity for the Fabrication of Near-Net-Shape NiTi Implants
Abstract
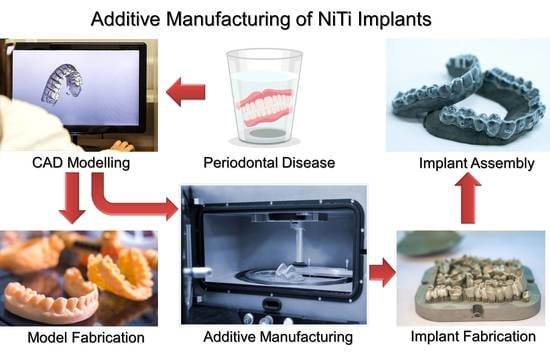
:1. Introduction
2. Application of AM for NiTi Implants
2.1. Background
2.2. Powder-Bed Fusion (PBF)
2.2.1. Laser Powder-Bed Fusion (LPBF)
2.2.2. Electron Powder-Bed Fusion (EPBF)
2.3. Directed Energy Deposition (DED)
3. Surface Modification of AM-fabricated NiTi Implants
4. Concluding Remarks and Future Horizons
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| AM | Additive manufacturing |
| ASCs | Autologous adipose-derived stem cells |
| BJ | Binder jetting |
| CAD | Computer-aided design |
| DED | Directed energy deposition |
| EPBF | Electron-beam powder-bed fusion |
| HAp | Hydroxyapatite |
| LPBF | Laser powder-bed fusion |
| NiTi | Nickel–titanium |
| OCP | Open-circuit potential |
| PBF | Powder-bed fusion |
| PBS | Phosphate-buffered saline |
| PM | Powder metallurgy |
| SBF | Simulated body fluid |
| SEM | Scanning electron microscopy |
References
- Bikas, H.; Stavropoulos, P.; Chryssolouris, G. Additive manufacturing methods and modelling approaches: A critical review. Int. J. Adv. Manuf. Technol. 2016, 83, 389–405. [Google Scholar] [CrossRef] [Green Version]
- Singh, S.; Ramakrishna, S.; Singh, R. Material issues in additive manufacturing: A review. J. Manuf. Process. 2017, 25, 185–200. [Google Scholar] [CrossRef]
- Ngo, T.D.; Kashani, A.; Imbalzano, G.; Nguyen, K.T.Q.; Hui, D. Additive Manufacturing (3D Printing): A Review of Materials, Methods, Applications and Challenges. Compos. Part B Eng. 2018, 143, 172–196. [Google Scholar] [CrossRef]
- Madrid, A.P.M.; Vrech, S.M.; Sanchez, M.A.; Rodriguez, A.P. Advances in additive manufacturing for bone tissue engineering scaffolds. Mater. Sci. Eng. C 2019, 100, 631–644. [Google Scholar] [CrossRef] [PubMed]
- Deckers, J.; Vleugels, J.; Kruth, J.-P. Additive manufacturing of ceramics: A review. J. Ceram. Sci. Technol. 2014, 5, 245–260. [Google Scholar]
- Zocca, A.; Colombo, P.; Gomes, C.M.; Günster, J. Additive Manufacturing of Ceramics: Issues, Potentialities, and Opportunities. J. Am. Ceram. Soc. 2015, 98, 1983–2001. [Google Scholar] [CrossRef]
- Galante, R.; Figueiredo-Pina, C.G.; Serro, A.P. Additive manufacturing of ceramics for dental applications: A review. Dent. Mater. 2019, 35, 825–846. [Google Scholar] [CrossRef]
- Herzog, D.; Seyda, V.; Wycisk, E.; Emmelmann, C. Additive manufacturing of metals. Acta Mater. 2016, 117, 371–392. [Google Scholar] [CrossRef]
- Francois, M.; Sun, A.; King, W.; Henson, N.; Tourret, D.; Bronkhorst, C.; Carlson, N.; Newman, C.; Haut, T.; Bakosi, J.; et al. Modeling of additive manufacturing processes for metals: Challenges and opportunities. Curr. Opin. Solid State Mater. Sci. 2017, 21, 198–206. [Google Scholar] [CrossRef]
- Mirzababaei, S.; Pasebani, S. A Review on Binder Jet Additive Manufacturing of 316L Stainless Steel. J. Manuf. Mater. Process. 2019, 3, 82. [Google Scholar] [CrossRef] [Green Version]
- Taghizadeh, M.; Taghizadeh, A.; Yazdi, M.K.; Zarrintaj, P.; Stadler, F.J.; Ramsey, J.D.; Habibzadeh, S.; Rad, S.H.; Naderi, G.; Saeb, M.R.; et al. Chitosan-based inks for 3D printing and bioprinting. Green Chem. 2022, 24, 62–101. [Google Scholar] [CrossRef]
- Nath, S.D.; Nilufar, S. An Overview of Additive Manufacturing of Polymers and Associated Composites. Polymers 2020, 12, 2719. [Google Scholar] [CrossRef] [PubMed]
- Saberi, A.; Behnamghader, A.; Aghabarari, B.; Yousefi, A.; Majda, D.; Huerta, M.V.M.; Mozafari, M. 3D direct printing of composite bone scaffolds containing polylactic acid and spray dried mesoporous bioactive glass-ceramic microparticles. Int. J. Biol. Macromol. 2022, 207, 9–22. [Google Scholar] [CrossRef] [PubMed]
- Tariverdian, T.; Ghader, A.B.; Milan, P.B.; Bafrooei, H.B.; Mozafari, M. 3D-printed barium strontium titanate-based piezoelectric scaffolds for bone tissue engineering. Ceram. Int. 2019, 45, 14029–14038. [Google Scholar] [CrossRef]
- El Moumen, A.; Tarfaoui, M.; Lafdi, K. Additive manufacturing of polymer composites: Processing and modeling approaches. Compos. Part B Eng. 2019, 171, 166–182. [Google Scholar] [CrossRef]
- Zhang, B.; Goel, A.; Ghalsasi, O.; Anand, S. CAD-based design and pre-processing tools for additive manufacturing. J. Manuf. Syst. 2019, 52, 227–241. [Google Scholar] [CrossRef]
- Hasanov, S.; Alkunte, S.; Rajeshirke, M.; Gupta, A.; Huseynov, O.; Fidan, I.; Alifui-Segbaya, F.; Rennie, A. Review on Additive Manufacturing of Multi-Material Parts: Progress and Challenges. J. Manuf. Mater. Process. 2021, 6, 4. [Google Scholar] [CrossRef]
- Attaran, M. The rise of 3-D printing: The advantages of additive manufacturing over traditional manufacturing. Bus. Horiz. 2017, 60, 677–688. [Google Scholar] [CrossRef]
- Mohajeri, B.; Khajavi, S.H.; Nyberg, T.; Khajavi, S.H. Supply Chain Modifications to Improve Additive Manufacturing. In Proceedings of the 2014 International Solid Freeform Fabrication Symposium, Austin, TX, USA, 4–6 August 2014. [Google Scholar]
- Khajavi, S.H.; Holmström, J.; Partanen, J. Additive manufacturing in the spare parts supply chain: Hub configuration and technology maturity. Rapid Prototyp. J. 2018, 24, 1178–1192. [Google Scholar] [CrossRef] [Green Version]
- Mota, C.; Puppi, D.; Chiellini, F.; Chiellini, E. Additive manufacturing techniques for the production of tissue engineering constructs. J. Tissue Eng. Regen. Med. 2012, 9, 174–190. [Google Scholar] [CrossRef]
- Entezari, M.; Mozafari, M.; Bakhtiyari, M.; Moradi, F.; Bagher, Z.; Soleimani, M. Three-dimensional-printed polycaprolactone/polypyrrole conducting scaffolds for differentiation of human olfactory ecto-mesenchymal stem cells into Schwann cell-like phenotypes and promotion of neurite outgrowth. J. Biomed. Mater. Res. Part A 2022, 110, 1134–1146. [Google Scholar] [CrossRef] [PubMed]
- ISO/ASTM 52900; F42 Committee. ISO/ASTM 52900: 2021 Additive Manufacturing–General Principles–Terminology. ASTM International: West Conshohocken, PA, USA, 2021.
- Fatma, N.; Haleem, A.; Javaid, M.; Khan, S. Comparison of Fused Deposition Modeling and Color Jet 3D Printing Technologies for the Printing of Mathematical Geometries. J. Ind. Integr. Manag. 2021, 06, 93–105. [Google Scholar] [CrossRef]
- Grubb, P.M.; Koushyar, F.M.; Lenz, T.; Asghari, A.; Gan, G.; Xia, W.; Dalir, H.; Subbaraman, H.; Chen, R.T. High Speed Roll-to-Roll Printable Transistor Enabled by a Pulsed Light Curable CNT Ink. J. Manuf. Mater. Process. 2019, 3, 33. [Google Scholar] [CrossRef] [Green Version]
- Wheat, E.; Vlasea, M.; Hinebaugh, J.; Metcalfe, C. Sinter structure analysis of titanium structures fabricated via binder jetting additive manufacturing. Mater. Des. 2018, 156, 167–183. [Google Scholar] [CrossRef]
- Das, M.; Balla, V.; Kumar, T.S.S.; Manna, I. Fabrication of Biomedical Implants using Laser Engineered Net Shaping (LENS™). Trans. Indian Ceram. Soc. 2013, 72, 169–174. [Google Scholar] [CrossRef]
- Tang, Z.-J.; Liu, W.-W.; Wang, Y.-W.; Saleheen, K.M.; Liu, Z.-C.; Peng, S.-T.; Zhang, Z.; Zhang, H.-C. A review on in situ monitoring technology for directed energy deposition of metals. Int. J. Adv. Manuf. Technol. 2020, 108, 3437–3463. [Google Scholar] [CrossRef]
- Borovkov, H.; de la Yedra, A.; Zurutuza, X.; Angulo, X.; Alvarez, P.; Pereira, J.; Cortes, F. In-Line Height Measurement Technique for Directed Energy Deposition Processes. J. Manuf. Mater. Process. 2021, 5, 85. [Google Scholar] [CrossRef]
- Davis, A.; Kennedy, J.; Strong, D.; Kovalchuk, D.; Porter, S.; Prangnell, P. Tailoring equiaxed β-grain structures in Ti-6Al-4V coaxial electron beam wire additive manufacturing. Materialia 2021, 20, 101202. [Google Scholar] [CrossRef]
- Shaik, Y.P.; Schuster, J.; Shaik, A.; Mohammed, M.; Katherapalli, H.R. Effect of Autoclave Pressure and Temperature on Consolidation of Layers and Mechanical Properties of Additively Manufactured (FDM) Products with PLA. J. Manuf. Mater. Process. 2021, 5, 114. [Google Scholar] [CrossRef]
- Vyavahare, S.; Kumar, S.; Panghal, D. Experimental study of surface roughness, dimensional accuracy and time of fabrication of parts produced by fused deposition modelling. Rapid Prototyp. J. 2020, 26, 1535–1554. [Google Scholar] [CrossRef]
- Zühlke, A.; Gasik, M.; Vrana, N.E.; Muller, C.B.; Barthes, J.; Bilotsky, Y.; Courtial, E.; Marquette, C. Biomechanical and functional comparison of moulded and 3D printed medical silicones. J. Mech. Behav. Biomed. Mater. 2021, 122, 104649. [Google Scholar] [CrossRef] [PubMed]
- Spece, H.; Yu, T.; Law, A.; Marcolongo, M.; Kurtz, S. 3D printed porous PEEK created via fused filament fabrication for osteoconductive orthopaedic surfaces. J. Mech. Behav. Biomed. Mater. 2020, 109, 103850. [Google Scholar] [CrossRef] [PubMed]
- Oh, Y.; Bharambe, V.; Mummareddy, B.; Martin, J.; McKnight, J.; Abraham, M.A.; Walker, J.M.; Rogers, K.; Conner, B.; Cortes, P.; et al. Microwave dielectric properties of zirconia fabricated using NanoParticle Jetting™. Addit. Manuf. 2019, 27, 586–594. [Google Scholar] [CrossRef]
- Saunders, R.; Gough, J.E.; Derby, B. Delivery of human fibroblast cells by piezoelectric drop-on-demand inkjet printing. Biomaterials 2008, 29, 193–203. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.W.; Fang, H.Y.; Shie, M.Y.; Shen, Y.F. The mussel-inspired assisted apatite mineralized on PolyJet material for artificial bone scaffold. Int. J. Bioprinting 2019, 5, 197. [Google Scholar] [CrossRef]
- Gao, H.; Yang, Z.; Lin, W.S.; Tan, J.; Chen, L. The Effect of Build Orientation on the Dimensional Accuracy of 3D-Printed Mandibular Complete Dentures Manufactured with a Multijet 3D Printer. J. Prosthodont. 2021, 30, 684–689. [Google Scholar] [CrossRef]
- Williams, J.M.; Adewunmi, A.; Schek, R.M.; Flanagan, C.L.; Krebsbach, P.H.; Feinberg, S.E.; Hollister, S.J.; Das, S. Bone tissue engineering using polycaprolactone scaffolds fabricated via selective laser sintering. Biomaterials 2005, 26, 4817–4827. [Google Scholar] [CrossRef]
- Dowling, L.; Kennedy, J.; O’Shaughnessy, S.; Trimble, D. A review of critical repeatability and reproducibility issues in powder bed fusion. Mater. Des. 2020, 186, 108346. [Google Scholar] [CrossRef]
- Bartolomeu, F.; Fonseca, J.; Peixinho, N.; Alves, N.; Gasik, M.; Silva, F.S.; Miranda, G. Predicting the output dimensions, porosity and elastic modulus of additive manufactured bio-material structures targeting orthopedic implants. J. Mech. Behav. Biomed. Mater. 2019, 99, 104–117. [Google Scholar] [CrossRef]
- Singh, D.D.; Mahender, T.; Reddy, A.R. Powder bed fusion process: A brief review. Mater. Today Proc. 2021, 46, 350–355. [Google Scholar] [CrossRef]
- Kim, M.-S.; Chu, W.-S.; Kim, Y.-M.; Avila, A.P.G.; Ahn, S.-H. Direct metal printing of 3D electrical circuit using rapid prototyping. Int. J. Precis. Eng. Manuf. 2009, 10, 147–150. [Google Scholar] [CrossRef]
- Bertol, L.S.; Júnior, W.K.; Da Silva, F.P.; Aumund-Kopp, C. Medical design: Direct metal laser sintering of Ti–6Al–4V. Mater. Des. 2010, 31, 3982–3988. [Google Scholar] [CrossRef]
- Polley, C.; Radlof, W.; Hauschulz, F.; Benz, C.; Sander, M.; Seitz, H. Morphological and mechanical characterisation of three-dimensional gyroid structures fabricated by electron beam melting for the use as a porous biomaterial. J. Mech. Behav. Biomed. Mater. 2022, 125, 104882. [Google Scholar] [CrossRef] [PubMed]
- Habib, F.N.; Iovenitti, P.; Masood, S.H.; Nikzad, M. Fabrication of polymeric lattice structures for optimum energy absorption using Multi Jet Fusion technology. Mater. Des. 2018, 155, 86–98. [Google Scholar] [CrossRef]
- Dermeik, B.; Travitzky, N. Laminated Object Manufacturing of Ceramic-Based Materials. Adv. Eng. Mater. 2020, 22, 2000256. [Google Scholar] [CrossRef]
- Melchels, F.P.W.; Feijen, J.; Grijpma, D.W. A review on stereolithography and its applications in biomedical engineering. Biomaterials 2010, 31, 6121–6130. [Google Scholar] [CrossRef] [Green Version]
- Unkovskiy, A.; Schmidt, F.; Beuer, F.; Li, P.; Spintzyk, S.; Fernandez, P.K. Stereolithography vs. Direct Light Processing for Rapid Manufacturing of Complete Denture Bases: An In Vitro Accuracy Analysis. J. Clin. Med. 2021, 10, 1070. [Google Scholar] [CrossRef]
- Caudill, C.L.; Perry, J.L.; Tian, S.; Luft, J.C.; DeSimone, J.M. Spatially controlled coating of continuous liquid interface production microneedles for transdermal protein delivery. J. Control. Release 2018, 284, 122–132. [Google Scholar] [CrossRef]
- Hu, C.; Ashok, D.; Nisbet, D.R.; Gautam, V. Bioinspired surface modification of orthopedic implants for bone tissue engineering. Biomaterials 2019, 219, 119366. [Google Scholar] [CrossRef]
- Ghaffari, M.; Moztarzadeh, F.; Sepahvandi, A.; Mozafari, M.; Faghihi, S. How bone marrow-derived human mesenchymal stem cells respond to poorly crystalline apatite coated orthopedic and dental titanium implants. Ceram. Int. 2013, 39, 7793–7802. [Google Scholar] [CrossRef]
- Prasad, K.; Bazaka, O.; Chua, M.; Rochford, M.; Fedrick, L.; Spoor, J.; Symes, R.; Tieppo, M.; Collins, C.; Cao, A.; et al. Metallic Biomaterials: Current Challenges and Opportunities. Materials 2017, 10, 884. [Google Scholar] [CrossRef] [PubMed]
- Krishna, B.V.; Xue, W.; Bose, S.; Bandyopadhyay, A. Engineered porous metals for implants. Jom 2008, 60, 45–48. [Google Scholar] [CrossRef]
- Mozafari, M.; Rabiee, M.; Azami, M.; Maleknia, S. Biomimetic formation of apatite on the surface of porous gelatin/bioactive glass nanocomposite scaffolds. Appl. Surf. Sci. 2010, 257, 1740–1749. [Google Scholar] [CrossRef]
- Hacking, S.; Bobyn, J.; Toh, K.-K.; Tanzer, M.; Krygier, J. Fibrous tissue ingrowth and attachment to porous tantalum. J. Biomed. Mater. Res. 2000, 52, 631–638. [Google Scholar] [CrossRef]
- Mozafari, M.; Moztarzadeh, F.; Tahriri, M. Investigation of the physico-chemical reactivity of a mesoporous bioactive SiO2–CaO–P2O5 glass in simulated body fluid. J. Non-Crystalline Solids 2010, 356, 1470–1478. [Google Scholar] [CrossRef]
- Gasik, M. Understanding biomaterial-tissue interface quality: Combined in vitro evaluation. Sci. Technol. Adv. Mater. 2017, 18, 550–562. [Google Scholar] [CrossRef] [Green Version]
- Arabnejad, S.; Johnston, B.; Tanzer, M.; Pasini, D. Fully porous 3D printed titanium femoral stem to reduce stress-shielding following total hip arthroplasty. J. Orthop. Res. 2017, 35, 1774–1783. [Google Scholar] [CrossRef] [Green Version]
- Wang, P.; Li, X.; Luo, S.; Nai, M.L.S.; Ding, J.; Wei, J. Additively manufactured heterogeneously porous metallic bone with biostructural functions and bone-like mechanical properties. J. Mater. Sci. Technol. 2021, 62, 173–179. [Google Scholar] [CrossRef]
- Gorsse, S.; Hutchinson, C.; Gouné, M.; Banerjee, R. Additive manufacturing of metals: A brief review of the characteristic microstructures and properties of steels, Ti-6Al-4V and high-entropy alloys. Sci. Technol. Adv. Mater. 2017, 18, 584–610. [Google Scholar] [CrossRef] [Green Version]
- Mahmoud, D.; Elbestawi, M.A. Lattice structures and functionally graded materials applications in additive manufacturing of orthopedic implants: A review. J. Manuf. Mater. Processing 2017, 1, 13. [Google Scholar] [CrossRef]
- Frazier, W.E. Metal additive manufacturing: A review. J. Mater. Eng. Perform. 2014, 23, 1917–1928. [Google Scholar] [CrossRef]
- Davoodi, E.; Montazerian, H.; Mirhakimi, A.S.; Zhianmanesh, M.; Ibhadode, O.; Shahabad, S.I.; Esmaeilizadeh, R.; Sarikhani, E.; Toorandaz, S.; Sarabi, S.A.; et al. Additively manufactured metallic biomaterials. Bioact. Mater. 2021, 15, 214–249. [Google Scholar] [CrossRef] [PubMed]
- Fukuda, A.; Takemoto, M.; Saito, T.; Fujibayashi, S.; Neo, M.; Pattanayak, D.K.; Matsushita, T.; Sasaki, K.; Nishida, N.; Kokubo, T.; et al. Osteoinduction of porous Ti implants with a channel structure fabricated by selective laser melting. Acta Biomater. 2011, 7, 2327–2336. [Google Scholar] [CrossRef]
- Mangano, F.; Chambrone, L.; Van Noort, R.; Miller, C.; Hatton, P.; Mangano, C. Direct Metal Laser Sintering Titanium Dental Implants: A Review of the Current Literature. Int. J. Biomater. 2014, 2014, 461534. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shishkovsky, I.V.; Volova, L.T.; Kuznetsov, M.V.; Morozov, Y.G.; Parkin, I.P. Porous biocompatible implants and tissue scaffolds synthesized by selective laser sintering from Ti and NiTi. J. Mater. Chem. 2008, 18, 1309–1317. [Google Scholar] [CrossRef]
- Wanjara, P.; Backman, D.; Sikan, F.; Gholipour, J.; Amos, R.; Patnaik, P.; Brochu, M. Microstructure and Mechanical Properties of Ti-6Al-4V Additively Manufactured by Electron Beam Melting with 3D Part Nesting and Powder Reuse Influences. J. Manuf. Mater. Process. 2022, 6, 21. [Google Scholar] [CrossRef]
- Allafi, J.K.; Ren, X.; Eggeler, G. The mechanism of multistage martensitic transformations in aged Ni-rich NiTi shape memory alloys. Acta Mater. 2002, 50, 793–803. [Google Scholar] [CrossRef]
- Dlouhy, A.; Khalil-Allafi, J.; Eggeler, G. Multiple-step martensitic transformations in Ni-rich NiTi alloys—An in-situ transmission electron microscopy investigation. Philos. Mag. 2003, 83, 339–363. [Google Scholar] [CrossRef]
- Kauffman, G.B.; Mayo, I. The Story of Nitinol: The Serendipitous Discovery of the Memory Metal and Its Applications. Chem. Educ. 1997, 2, 1–21. [Google Scholar] [CrossRef]
- Khalil-Allafi, J.; Eggeler, G.; Dlouhy, A.; Schmahl, W.; Somsen, C. On the influence of heterogeneous precipitation on martensitic transformations in a Ni-rich NiTi shape memory alloy. Mater. Sci. Eng. A 2004, 378, 148–151. [Google Scholar] [CrossRef]
- Khalil-Allafi, J.; Dlouhy, A.; Eggeler, G. Ni4Ti3-precipitation during aging of NiTi shape memory alloys and its influence on martensitic phase transformations. Acta Mater. 2002, 50, 4255–4274. [Google Scholar] [CrossRef]
- Patel, S.K.; Behera, B.; Swain, B.; Roshan, R.; Sahoo, D.; Behera, A. A review on NiTi alloys for biomedical applications and their biocompatibility. Mater. Today Proc. 2020, 33, 5548–5551. [Google Scholar] [CrossRef]
- Xu, J.; Jin, X.; Luo, J.; Zhong, Z. Fabrication and properties of porous NiTi alloys by microwave sintering for biomedical applications. Mater. Lett. 2014, 124, 110–112. [Google Scholar] [CrossRef]
- Daneshvar, H.; Safavi, M.S.; Khalili, V.; Khalil-Allafi, J. Influence of aging temperature on phase transformation and mechanical behavior of NiTi thin films deposited by magnetron sputtering technique. J. Ultrafine Grained Nanostruct. Mater. 2020, 53, 15–22. [Google Scholar] [CrossRef]
- Khalil-Allafi, J.; Daneshvar, H.; Safavi, M.S.; Khalili, V. A survey on crystallization kinetic behavior of direct current magnetron sputter deposited NiTi thin films. Phys. B Condens. Matter 2021, 615, 413086. [Google Scholar] [CrossRef]
- Ma, C.; Andani, M.T.; Qin, H.; Moghaddam, N.S.; Ibrahim, H.; Jahadakbar, A.; Amerinatanzi, A.; Ren, Z.; Zhang, H.; Doll, G.L.; et al. Improving surface finish and wear resistance of additive manufactured nickel-titanium by ultrasonic nano-crystal surface modification. J. Mater. Process. Technol. 2017, 249, 433–440. [Google Scholar] [CrossRef]
- Arndt, M.; Brück, A.; Scully, T.; Jäger, A.; Bourauel, C. Nickel ion release from orthodontic NiTi wires under simulation of realistic in-situ conditions. J. Mater. Sci. 2005, 40, 3659–3667. [Google Scholar] [CrossRef]
- Wever, D.; Veldhuizen, A.; Sanders, M.; Schakenraad, J.; van Horn, J. Cytotoxic, allergic and genotoxic activity of a nickel-titanium alloy. Biomaterials 1997, 18, 1115–1120. [Google Scholar] [CrossRef]
- Sheykholeslami, S.O.R.; Khalil-Allafi, J.; Fathyunes, L. Preparation, characterization, and corrosion behavior of calcium phosphate coating electrodeposited on the modified nanoporous surface of NiTi alloy for biomedical applications. Metall. Mater. Trans. A 2018, 49, 5878–5887. [Google Scholar] [CrossRef]
- Patel, S.K.; Swain, B.; Roshan, R.; Sahu, N.K.; Behera, A. A brief review of shape memory effects and fabrication processes of NiTi shape memory alloys. Mater. Today Proc. 2020, 33, 5552–5556. [Google Scholar] [CrossRef]
- Parvizi, S.; Hashemi, S.M.; Asgarinia, F.; Nematollahi, M.; Elahinia, M. Effective parameters on the final properties of NiTi-based alloys manufactured by powder metallurgy methods: A review. Prog. Mater. Sci. 2021, 117, 100739. [Google Scholar] [CrossRef]
- Foroozmehr, A.; Kermanpur, A.; Ashrafizadeh, F.; Kabiri, Y. Investigating microstructural evolution during homogenization of the equiatomic NiTi shape memory alloy produced by vacuum arc remelting. Mater. Sci. Eng. A 2011, 528, 7952–7955. [Google Scholar] [CrossRef]
- Zhang, Z.; Frenzel, J.; Neuking, K.; Eggeler, G. On the reaction between NiTi melts and crucible graphite during vacuum induction melting of NiTi shape memory alloys. Acta Mater. 2005, 53, 3971–3985. [Google Scholar] [CrossRef]
- Tang, C.; Zhang, L.; Wong, C.; Chan, K.; Yue, T. Fabrication and characteristics of porous NiTi shape memory alloy synthesized by microwave sintering. Mater. Sci. Eng. A 2011, 528, 6006–6011. [Google Scholar] [CrossRef]
- Biffi, C.; Bassani, P.; Sajedi, Z.; Giuliani, P.; Tuissi, A. Laser ignition in Self-propagating High temperature Synthesis of porous NiTinol Shape Memory Alloy. Mater. Lett. 2017, 193, 54–57. [Google Scholar] [CrossRef]
- Wu, S.; Liu, X.; Chu, P.; Chung, C.; Chu, C.; Yeung, K. Phase transformation behavior of porous NiTi alloys fabricated by capsule-free hot isostatic pressing. J. Alloy. Compd. 2008, 449, 139–143. [Google Scholar] [CrossRef]
- Guoxin, H.; Lixiang, Z.; Yunliang, F.; Yanhong, L. Fabrication of high porous NiTi shape memory alloy by metal injection molding. J. Mater. Process. Technol. 2008, 206, 395–399. [Google Scholar] [CrossRef]
- Butler, J.; Tiernan, P.; Gandhi, A.A.; McNamara, K.; Tofail, S.A.M. Production of Nitinol Wire from Elemental Nickel and Titanium Powders Through Spark Plasma Sintering and Extrusion. J. Mater. Eng. Perform. 2011, 20, 757–761. [Google Scholar] [CrossRef]
- Novak, P.; Moravec, H.; Salvetr, P.; Průša, F.; Drahokoupil, J.; Kopeček, J.; Karlík, M.; Kubatík, T.F. Preparation of nitinol by non-conventional powder metallurgy techniques. Mater. Sci. Technol. 2015, 31, 1886–1893. [Google Scholar] [CrossRef]
- Chen, X.; Liu, K.; Guo, W.; Gangil, N.; Siddiquee, A.N.; Konovalov, S. The fabrication of NiTi shape memory alloy by selective laser melting: A review. Rapid Prototyp. J. 2019, 25, 1421–1432. [Google Scholar] [CrossRef]
- Mahmud, A.; Wu, Z.; Zhang, J.; Liu, Y.; Yang, H. Surface oxidation of NiTi and its effects on thermal and mechanical properties. Intermetallics 2018, 103, 52–62. [Google Scholar] [CrossRef]
- Dabbaghi, H.; Safaei, K.; Nematollahi, M.; Bayati, P.; Elahinia, M. Additively manufactured NiTi and NiTiHf alloys: Estimating service life in high-temperature oxidation. Materials 2020, 13, 2104. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Liu, Z.; Huang, W.; Wang, B.; Niu, J. Influence of cutting parameters on surface roughness and strain hardening during milling NiTi shape memory alloy. Int. J. Adv. Manuf. Technol. 2019, 102, 2211–2221. [Google Scholar] [CrossRef]
- Zhao, Y.-Z.; Guo, K.; Sivalingam, V.; Li, J.-F.; Sun, Q.-D.; Zhu, Z.-J.; Sun, J. Surface integrity evolution of machined NiTi shape memory alloys after turning process. Adv. Manuf. 2021, 9, 446–456. [Google Scholar] [CrossRef]
- Jahadakbar, A.; Moghaddam, N.S.; Amerinatanzi, A.; Dean, D.; Karaca, H.E.; Elahinia, M. Finite Element Simulation and Additive Manufacturing of Stiffness-Matched NiTi Fixation Hardware for Mandibular Reconstruction Surgery. Bioengineering 2016, 3, 36. [Google Scholar] [CrossRef] [PubMed]
- Jahadakbar, A.; Nematollahi, M.; Safaei, K.; Bayati, P.; Giri, G.; Dabbaghi, H.; Dean, D.; Elahinia, M. Design, Modeling, Additive Manufacturing, and Polishing of Stiffness-Modulated Porous Nitinol Bone Fixation Plates Followed by Thermomechanical and Composition Analysis. Metals 2020, 10, 151. [Google Scholar] [CrossRef] [Green Version]
- Elahinia, M.; Shayesteh Moghaddam, N.; Taheri Andani, M.; Amerinatanzi, A.; Bimber, B.A.; Hamilton, R.F. Fabrication of NiTi through additive manufacturing: A review. Prog. Mater. Sci. 2016, 83, 630–663. [Google Scholar] [CrossRef] [Green Version]
- Elahinia, M.H.; Hashemi, M.; Tabesh, M.; Bhaduri, S.B. Manufacturing and processing of NiTi implants: A review. Prog. Mater. Sci. 2012, 57, 911–946. [Google Scholar] [CrossRef]
- Safaei, K.; Abedi, H.; Nematollahi, M.; Kordizadeh, F.; Dabbaghi, H.; Bayati, P.; Javanbakht, R.; Jahadakbar, A.; Elahinia, M.; Poorganji, B. Additive Manufacturing of NiTi Shape Memory Alloy for Biomedical Applications: Review of the LPBF Process Ecosystem. Jom 2021, 73, 3771–3786. [Google Scholar] [CrossRef]
- Haberland, C.; Elahinia, M.; Walker, J.M.; Meier, H.; Frenzel, J. On the development of high quality NiTi shape memory and pseudoelastic parts by additive manufacturing. Smart Mater. Struct. 2014, 23, 104002. [Google Scholar] [CrossRef]
- Wang, X.; Xu, S.; Zhou, S.; Xu, W.; Leary, M.; Choong, P.; Qian, M.; Brandt, M.; Xie, Y.M. Topological design and additive manufacturing of porous metals for bone scaffolds and orthopaedic implants: A review. Biomaterials 2016, 83, 127–141. [Google Scholar] [CrossRef] [PubMed]
- Walker, J.M.; Haberland, C.; Andani, M.T.; Karaca, H.E.; Dean, D.; Elahinia, M. Process development and characterization of additively manufactured nickel–titanium shape memory parts. J. Intell. Mater. Syst. Struct. 2016, 27, 2653–2660. [Google Scholar] [CrossRef]
- Zhang, L.; Song, B.; Choi, S.-K.; Shi, Y. A topology strategy to reduce stress shielding of additively manufactured porous metallic biomaterials. Int. J. Mech. Sci. 2021, 197, 106331. [Google Scholar] [CrossRef]
- Wang, L.; Xie, L.; Zhang, L.C.; Chen, L.; Ding, Z.; Lv, Y.; Zhang, W.; Lu, W.; Zhang, D. Microstructure evolution and superelasticity of layer-like NiTiNb porous metal prepared by eutectic reaction. Acta Mater. 2018, 143, 214–226. [Google Scholar] [CrossRef]
- Wang, P.; Li, X.; Jiang, Y.; Nai, M.L.S.; Ding, J.; Wei, J. Electron beam melted heterogeneously porous microlattices for metallic bone applications: Design and investigations of boundary and edge effects. Addit. Manuf. 2020, 36, 101566. [Google Scholar] [CrossRef]
- Ming, H.W.; Wu, K. Fabrication of nitinol materials and components. In Proceedings of the International Conference on Shape Memory and Superelastic Technologies, Kunming, China, 2–6 September 2001; pp. 285–292. [Google Scholar]
- Azarniya, A.; Azarniya, A.; Safavi, M.S.; Farshbaf Ahmadipour, M.; Esmaeeli Seraji, M.; Sovizi, S.; Saqaei, M.; Yamanoglu, R.; Soltaninejad, M.; Madaah Hosseini, H.R.; et al. Physicomechanical properties of porous materials by spark plasma sintering. Crit. Rev. Solid State Mater. Sci. 2020, 45, 22–65. [Google Scholar] [CrossRef]
- Bartolomeu, F.; Buciumeanu, M.; Costa, M.; Alves, N.; Gasik, M.; Silva, F.; Miranda, G. Multi-material Ti6Al4V & PEEK cellular structures produced by Selective Laser Melting and Hot Pressing: A tribocorrosion study targeting orthopedic applications. J. Mech. Behav. Biomed. Mater. 2019, 89, 54–64. [Google Scholar] [CrossRef]
- Jones, A.C.; Arns, C.; Hutmacher, D.W.; Milthorpe, B.K.; Sheppard, A.; Knackstedt, M.A. The correlation of pore morphology, interconnectivity and physical properties of 3D ceramic scaffolds with bone ingrowth. Biomaterials 2009, 30, 1440–1451. [Google Scholar] [CrossRef]
- Abidi, I.H.; Khalid, F.A.; Farooq, M.U.; Hussain, M.A.; Maqbool, A. Tailoring the pore morphology of porous nitinol with suitable mechanical properties for biomedical applications. Mater. Lett. 2015, 154, 17–20. [Google Scholar] [CrossRef]
- Somo, S.I.; Akar, B.; Bayrak, E.S.; Larson, J.C.; Appel, A.A.; Mehdizadeh, H.; Cinar, A.; Brey, E.M. Pore interconnectivity influences growth factor-mediated vascularization in sphere-templated hydrogels. Tissue Eng. Part C Methods 2015, 21, 773–785. [Google Scholar] [CrossRef] [Green Version]
- Zhu, C.; Liu, T.; Qian, F.; Chen, W.; Chandrasekaran, S.; Yao, B.; Song, Y.; Duoss, E.B.; Kuntz, J.; Spadaccini, C.M.; et al. 3D printed functional nanomaterials for electrochemical energy storage. Nano Today 2017, 15, 107–120. [Google Scholar] [CrossRef]
- Khoo, Z.X.; Liu, Y.; Low, Z.H.; An, J.; Chua, C.K.; Leong, K.F. Fabrication of SLM NiTi Shape Memory Alloy via Repetitive Laser Scanning. Shape Mem. Superelasticity 2018, 4, 112–120. [Google Scholar] [CrossRef]
- Wang, X.; Kustov, S.; van Humbeeck, J. A short review on the microstructure, transformation behavior and functional properties of NiTi shape memory alloys fabricated by selective laser melting. Materials 2018, 11, 1683. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andani, M.T.; Haberland, C.; Walker, J.M.; Karamooz-Ravari, M.R.; Turabi, A.S.; Saedi, S.; Rahmanian, R.; Karaca, H.; Dean, D.; Kadkhodaei, M.; et al. Achieving biocompatible stiffness in NiTi through additive manufacturing. J. Intell. Mater. Syst. Struct. 2016, 27, 2661–2671. [Google Scholar] [CrossRef]
- Hamilton, R.F.; Palmer, T.A.; Bimber, B.A. Spatial characterization of the thermal-induced phase transformation throughout as-deposited additive manufactured NiTi bulk builds. Scr. Mater. 2015, 101, 56–59. [Google Scholar] [CrossRef]
- Kok, Y.; Tan, X.P.; Wang, P.; Nai, M.L.; Loh, N.H.; Liu, E.; Tor, S.B. Anisotropy and heterogeneity of microstructure and mechanical properties in metal additive manufacturing: A critical review. Mater. Des. 2018, 139, 565–586. [Google Scholar] [CrossRef]
- Berbenni, S.; Favier, V.; Berveiller, M. Impact of the grain size distribution on the yield stress of heterogeneous materials. Int. J. Plast. 2007, 23, 114–142. [Google Scholar] [CrossRef]
- Zhang, Y.; Attarilar, S.; Wang, L.; Lu, W.; Yang, J.; Fu, Y. A review on design and mechanical properties of additively manufactured NiTi implants for orthopedic applications. Int. J. Bioprinting 2021, 7, 340. [Google Scholar] [CrossRef]
- Lu, B.; Cui, X.; Ma, W.; Dong, M.; Fang, Y.; Wen, X.; Jin, G.; Zeng, D. Promoting the heterogeneous nucleation and the functional properties of directed energy deposited NiTi alloy by addition of La2O3. Addit. Manuf. 2020, 33, 101150. [Google Scholar] [CrossRef]
- Farber, E.; Zhu, J.-N.; Popovich, A.; Popovich, V. A review of NiTi shape memory alloy as a smart material produced by additive manufacturing. Mater. Today Proc. 2020, 30, 761–767. [Google Scholar] [CrossRef]
- Dadbakhsh, S.; Speirs, M.; Van Humbeeck, J.; Kruth, J.-P. Laser additive manufacturing of bulk and porous shape-memory NiTi alloys: From processes to potential biomedical applications. MRS Bull. 2016, 41, 765–774. [Google Scholar] [CrossRef] [Green Version]
- Khoo, Z.X.; Liu, Y.; An, J.; Chua, C.K.; Shen, Y.F.; Kuo, C.N. A Review of Selective Laser Melted NiTi Shape Memory Alloy. Materials 2018, 11, 519. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bormann, T.; Müller, B.; Schinhammer, M.; Kessler, A.; Thalmann, P.; de Wild, M. Microstructure of selective laser melted nickel–titanium. Mater. Charact. 2014, 94, 189–202. [Google Scholar] [CrossRef]
- Safdel, A.; Elbestawi, M. Distortion and printability of stent structures in laser powder bed fusion processing of NiTi alloys. Mater. Lett. 2021, 300, 130163. [Google Scholar] [CrossRef]
- Bormann, T.; Schumacher, R.; Müller, B.; Mertmann, M.; de Wild, M. Tailoring Selective Laser Melting Process Parameters for NiTi Implants. J. Mater. Eng. Perform. 2012, 21, 2519–2524. [Google Scholar] [CrossRef] [Green Version]
- Wen, S.; Liu, Y.; Zhou, Y.; Zhao, A.; Yan, C.; Shi, Y. Effect of Ni content on the transformation behavior and mechanical property of NiTi shape memory alloys fabricated by laser powder bed fusion. Opt. Laser Technol. 2021, 134, 106653. [Google Scholar] [CrossRef]
- Xue, L.; Atli, K.; Picak, S.; Zhang, C.; Zhang, B.; Elwany, A.; Arroyave, R.; Karaman, I. Controlling martensitic transformation characteristics in defect-free NiTi shape memory alloys fabricated using laser powder bed fusion and a process optimization framework. Acta Mater. 2021, 215, 117017. [Google Scholar] [CrossRef]
- Van Humbeeck, J. Additive Manufacturing of Shape Memory Alloys. Shape Mem. Superelasticity 2018, 4, 309–312. [Google Scholar] [CrossRef]
- Khanlari, K.; Shi, Q.; Yan, X.; Hu, K.; Tan, C.; Kelly, P.; Zhang, W.; Cao, P.; Wang, X.; Liu, X. Printing of NiTinol parts with characteristics respecting the general microstructural, compositional and mechanical requirements of bone replacement implants. Mater. Sci. Eng. A 2022, 839, 142839. [Google Scholar] [CrossRef]
- Khanlari, K.; Shi, Q.; Li, K.; Hu, K.; Cao, P.; Liu, X. Effects of printing volumetric energy densities and post-processing treatments on the microstructural properties, phase transformation temperatures and hardness of near-equiatomic NiTinol parts fabricated by a laser powder bed fusion technique. Intermetallics 2021, 131, 107088. [Google Scholar] [CrossRef]
- Chen, G.; Liu, S.; Huang, C.; Ma, Y.; Li, Y.; Zhang, B.; Gao, L.; Zhang, B.; Wang, P.; Qu, X. In-situ phase transformation and corrosion behavior of TiNi via LPBF. Corros. Sci. 2022, 203, 110348. [Google Scholar] [CrossRef]
- Strauß, S.; Dudziak, S.; Hagemann, R.; Barcikowski, S.; Fliess, M.; Israelowitz, M.; Kracht, D.; Kuhbier, J.W.; Radtke, C.; Reimers, K.; et al. Induction of osteogenic differentiation of adipose derived stem cells by microstructured nitinol actua-tor-mediated mechanical stress. PLoS ONE 2012, 7, e51264. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dadbakhsh, S.; Vrancken, B.; Kruth, J.-P.; Luyten, J.; Van Humbeeck, J. Texture and anisotropy in selective laser melting of NiTi alloy. Mater. Sci. Eng. A 2016, 650, 225–232. [Google Scholar] [CrossRef]
- Andani, M.T.; Saedi, S.; Turabi, A.S.; Karamooz, M.R.; Haberland, C.; Karaca, H.E.; Elahinia, M. Mechanical and shape memory properties of porous Ni50. 1Ti49. 9 alloys manufactured by selective laser melting. J. Mech. Behav. Biomed. Mater. 2017, 68, 224–231. [Google Scholar] [CrossRef] [PubMed]
- Pauzon, C.; Forêt, P.; Hryha, E.; Arunprasad, T.; Nyborg, L. Argon-helium mixtures as Laser-Powder Bed Fusion atmospheres: Towards increased build rate of Ti-6Al-4V. J. Mater. Process. Technol. 2020, 279, 116555. [Google Scholar] [CrossRef]
- Shi, Q.; Zhang, Y.; Tan, C.; Mao, X.; Khanlari, K.; Liu, X. Preparation of Ni–Ti composite powder using radio frequency plasma spheroidization and its laser powder bed fusion densification. Intermetallics 2021, 136, 107273. [Google Scholar] [CrossRef]
- Habijan, T.; Haberland, C.; Meier, H.; Frenzel, J.; Wittsiepe, J.; Wuwer, C.; Greulich, C.; Schildhauer, T.; Köller, M. The biocompatibility of dense and porous Nickel–Titanium produced by selective laser melting. Mater. Sci. Eng. C 2013, 33, 419–426. [Google Scholar] [CrossRef]
- Dadbakhsh, S.; Speirs, M.; Kruth, J.-P.; Schrooten, J.; Luyten, J.; Van Humbeeck, J. Effect of SLM Parameters on Transformation Temperatures of Shape Memory Nickel Titanium Parts. Adv. Eng. Mater. 2014, 16, 1140–1146. [Google Scholar] [CrossRef]
- Khademzadeh, S.; Zanini, F.; Bariani, P.F.; Carmignato, S. Precision additive manufacturing of NiTi parts using micro direct metal deposition. Int. J. Adv. Manuf. Technol. 2018, 96, 3729–3736. [Google Scholar] [CrossRef]
- Guo, W.; Feng, B.; Yang, Y.; Ren, Y.; Liu, Y.; Yang, H.; Yang, Q.; Cui, L.; Tong, X.; Hao, S. Effect of laser scanning speed on the microstructure, phase transformation and mechanical property of NiTi alloys fabricated by LPBF. Mater. Des. 2022, 215, 110460. [Google Scholar] [CrossRef]
- Yu, Z.; Xu, Z.; Guo, Y.; Sha, P.; Liu, R.; Xin, R.; Li, L.; Chen, L.; Wang, X.; Zhang, Z.; et al. Analysis of microstructure, mechanical properties, wear characteristics and corrosion behavior of SLM-NiTi under different process parameters. J. Manuf. Processes 2022, 75, 637–650. [Google Scholar] [CrossRef]
- Zhu, J.-N.; Borisov, E.; Liang, X.; Huizenga, R.; Popovich, A.; Bliznuk, V.; Petrov, R.; Hermans, M.; Popovich, V. Controlling microstructure evolution and phase transformation behavior in additive manufacturing of nitinol shape memory alloys by tuning hatch distance. J. Mater. Sci. 2022, 57, 6066–6084. [Google Scholar] [CrossRef]
- Khorasani, A.M.; Gibson, I.; Ghasemi, A.; Ghaderi, A. A comprehensive study on variability of relative density in selective laser melting of Ti-6Al-4V. Virtual Phys. Prototyp. 2019, 14, 349–359. [Google Scholar] [CrossRef]
- Obeidi, M.A.; Monu, M.; Hughes, C.; Bourke, D.; Dogu, M.N.; Francis, J.; Zhang, M.; Ahad, I.U.; Brabazon, D. Laser beam powder bed fusion of nitinol shape memory alloy (SMA). J. Mater. Res. Technol. 2021, 14, 2554–2570. [Google Scholar] [CrossRef]
- Rasooli, A.; Safavi, M.S.; Ahmadiyeh, S.; Jalali, A. Evaluation of TiO2 Nanoparticles Concentration and Applied Current Density Role in Determination of Microstructural, Mechanical, and Corrosion Properties of Ni–Co Alloy Coatings. Prot. Met. Phys. Chem. Surfaces 2020, 56, 320–327. [Google Scholar] [CrossRef]
- Safavi, M.S.; Fathi, M.; Ahadzadeh, I. Feasible strategies for promoting the mechano-corrosion performance of Ni-Co based coatings: Which one is better? Surf. Coat. Technol. 2021, 420, 127337. [Google Scholar] [CrossRef]
- Safavi, M.S.; Walsh, F.C. Electrodeposited Co-P alloy and composite coatings: A review of progress towards replacement of conventional hard chromium deposits. Surf. Coat. Technol. 2021, 422, 127564. [Google Scholar] [CrossRef]
- Afrouzian, A.; Groden, C.J.; Field, D.P.; Bose, S.; Bandyopadhyay, A. Additive manufacturing of Ti-Ni bimetallic structures. Mater. Des. 2022, 215, 110461. [Google Scholar] [CrossRef]
- Brett, P.; Harle, J.; Salih, V.; Mihoc, R.; Olsen, I.; Jones, F.; Tonetti, M. Roughness response genes in osteoblasts. Bone 2004, 35, 124–133. [Google Scholar] [CrossRef]
- Ibrahim, H.; Jahadakbar, A.; Dehghan, A.; Moghaddam, N.S.; Amerinatanzi, A.; Elahinia, M. In vitro corrosion assessment of additively manufactured porous NiTi structures for bone fixation applications. Metals 2018, 8, 164. [Google Scholar] [CrossRef] [Green Version]
- Farjam, N.; Nematollahi, M.; Andani, M.T.; Mahtabi, M.J.; Elahinia, M. Effects of size and geometry on the thermomechanical properties of additively manufactured NiTi shape memory alloy. Int. J. Adv. Manuf. Technol. 2020, 107, 3145–3154. [Google Scholar] [CrossRef]
- Costa, M.; Lima, R.; Alves, N.; Silva, N.; Gasik, M.; Silva, F.; Bartolomeu, F.; Miranda, G. Multi-material cellular structured orthopedic implants design: In vitro and bio-tribological performance. J. Mech. Behav. Biomed. Mater. 2022, 131, 105246. [Google Scholar] [CrossRef] [PubMed]
- Friedrich, K.; Walter, R. Structure and Properties of Additive Manufactured Polymer Components; Woodhead Publishing: Sawston, UK, 2020. [Google Scholar]
- Bernard, A.; Taillandier, G.; Karunakaran, K. Evolutions of rapid product development with rapid manufacturing: Concepts and applications. Int. J. Rapid Manuf. 2009, 1, 3. [Google Scholar] [CrossRef]
- Mazzoli, A.; Germani, M.; Raffaeli, R. Direct fabrication through electron beam melting technology of custom cranial implants designed in a PHANToM-based haptic environment. Mater. Des. 2009, 30, 3186–3192. [Google Scholar] [CrossRef]
- Zhou, Q.; Hayat, M.; Chen, G.; Cai, S.; Qu, X.; Tang, H.; Cao, P. Selective electron beam melting of NiTi: Microstructure, phase transformation and mechanical properties. Mater. Sci. Eng. A 2019, 744, 290–298. [Google Scholar] [CrossRef]
- Hayat, M.D.; Chen, G.; Liu, N.; Khan, S.; Tang, H.P.; Cao, P. Physical and Tensile Properties of NiTi Alloy by Selective Electron Beam Melting. Key Eng. Mater. 2018, 770, 148–154. [Google Scholar] [CrossRef]
- Wang, P.; Sin, W.J.; Nai, M.L.S.; Wei, J. Effects of Processing Parameters on Surface Roughness of Additive Manufactured Ti-6Al-4V via Electron Beam Melting. Materials 2017, 10, 1121. [Google Scholar] [CrossRef] [Green Version]
- Wang, P.; Song, J.; Nai, M.L.S.; Wei, J. Experimental analysis of additively manufactured component and design guidelines for lightweight structures: A case study using electron beam melting. Addit. Manuf. 2020, 33, 101088. [Google Scholar] [CrossRef]
- Gibson, I.; Rosen, D.W.; Stucker, B.; Khorasani, M.; Rosen, D.; Stucker, B.; Khorasani, M. Additive Manufacturing Technologies; Springer: Berlin/Heidelberg, Germany, 2021; Volume 17. [Google Scholar]
- Sharma, N.; Jangra, K.K.; Raj, T. Fabrication of NiTi alloy: A review. Proc. Inst. Mech. Eng. Part L J. Mater. Des. Appl. 2018, 232, 250–269. [Google Scholar] [CrossRef]
- Marattukalam, J.J.; Singh, A.K.; Datta, S.; Das, M.; Balla, V.K.; Bontha, S.; Kalpathy, S.K. Microstructure and corrosion behavior of laser processed NiTi alloy. Mater. Sci. Eng. C 2015, 57, 309–313. [Google Scholar] [CrossRef]
- Krishna, B.V.; Bose, S.; Bandyopadhyay, A. Fabrication of porous NiTi shape memory alloy structures using laser engineered net shaping. J. Biomed. Mater. Res. Part B 2009, 89, 481–490. [Google Scholar] [CrossRef] [PubMed]
- Zheng, D.; Li, R.D.; Yuan, T.C.; Xiong, Y.; Song, B.; Wang, J.X.; Su, Y.D. Microstructure and mechanical property of additively manufactured NiTi alloys: A comparison between selective laser melting and directed energy deposition. J. Cent. South Univ. 2021, 28, 1028–1042. [Google Scholar] [CrossRef]
- Buciumeanu, M.; Bagheri, A.; Silva, F.S.; Henriques, B.; Lasagni, A.F.; Shamsaei, N. Tribocorrosion Behavior of NiTi Biomedical Alloy Processed by an Additive Manufacturing Laser Beam Directed Energy Deposition Technique. Materials 2022, 15, 691. [Google Scholar] [CrossRef] [PubMed]
- Safavi, M.S.; Surmeneva, M.A.; Surmenev, R.A.; Khalil-Allafi, J. RF-magnetron sputter deposited hydroxyapatite-based composite & multilayer coatings: A systematic review from mechanical, corrosion, and biological points of view. Ceram. Int. 2021, 47, 3031–3053. [Google Scholar] [CrossRef]
- Safavi, M.S.; Walsh, F.C.; Surmeneva, M.A.; Surmenev, R.A.; Khalil-Allafi, J. Electrodeposited Hydroxyapatite-Based Biocoatings: Recent Progress and Future Challenges. Coatings 2021, 11, 110. [Google Scholar] [CrossRef]
- Tohidi, P.M.; Safavi, M.S.; Etminanfar, M.; Khalil-Allafi, J. Pulsed electrodeposition of compact, corrosion resistant, and bioactive HAp coatings by application of optimized magnetic field. Mater. Chem. Phys. 2020, 254, 123511. [Google Scholar] [CrossRef]
- Shokri, N.; Safavi, M.S.; Etminanfar, M.; Walsh, F.C.; Khalil-Allafi, J. Enhanced corrosion protection of NiTi orthopedic implants by highly crystalline hydroxyapatite deposited by spin coating: The importance of pretreatment. Mater. Chem. Phys. 2021, 259, 124041. [Google Scholar] [CrossRef]
- Mohandesnezhad, S.; Etminanfar, M.; Mahdavi, S.; Safavi, M.S. Enhanced bioactivity of 316L stainless steel with deposition of polypyrrole/hydroxyapatite layered hybrid coating: Orthopedic applications. Surfaces Interfaces 2022, 28, 101604. [Google Scholar] [CrossRef]
- Safavi, M.S.; Etminanfar, M. A review on the prevalent fabrication methods, microstructural, mechanical properties, and corrosion resistance of nanostructured hydroxyapatite containing bilayer and multilayer coatings used in biomedical applications. J. Ultrafine Grained Nanostruct. Mater. 2019, 52, 1–17. [Google Scholar]
- Safavi, M.S.; Walsh, F.C.; Visai, L.; Khalil-Allafi, J. Progress in Niobium Oxide-Containing Coatings for Biomedical Applications: A Critical Review. ACS Omega 2022, 7, 9088–9107. [Google Scholar] [CrossRef]
- Asghari, R.; Safavi, M.S.; Khalil-Allafi, J. A facile and cost-effective practical approach to develop clinical applications of NiTi: Fenton oxidation process. Trans. IMF 2020, 98, 250–257. [Google Scholar] [CrossRef]
- Nasakina, E.O.; Sudarchikova, M.A.; Sergienko, K.V.; Konushkin, S.V.; Sevost’Yanov, M.A. Ion Release and Surface Characterization of Nanostructured Nitinol during Long-Term Testing. Nanomaterials 2019, 9, 1569. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, Y.; Rong, Y.; Zhao, Y.; Yao, X.; Hang, R. The influence of substrate electropolishing on anodization behavior, corrosion resistance, cytocompatibility and antibacterial ability of NiTi alloy. Mater. Lett. 2020, 268, 127631. [Google Scholar] [CrossRef]
- Mohseni, E.; Zalnezhad, E.; Bushroa, A. Comparative investigation on the adhesion of hydroxyapatite coating on Ti–6Al–4V implant: A review paper. Int. J. Adhes. Adhes. 2014, 48, 238–257. [Google Scholar] [CrossRef]
- Yang, Y.; Kim, K.-H.; Ong, J.L. A review on calcium phosphate coatings produced using a sputtering process—An alternative to plasma spraying. Biomaterials 2005, 26, 327–337. [Google Scholar] [CrossRef]
- Bordbar-Khiabani, A.; Yarmand, B.; Mozafari, M. Enhanced corrosion resistance and in-vitro biodegradation of plasma electrolytic oxidation coatings prepared on AZ91 Mg alloy using ZnO nanoparticles-incorporated electrolyte. Surf. Coatings Technol. 2019, 360, 153–171. [Google Scholar] [CrossRef]
- Ghasali, E.; Bordbar-Khiabani, A.; Alizadeh, M.; Mozafari, M.; Niazmand, M.; Kazemzadeh, H.; Ebadzadeh, T. Corrosion behavior and in-vitro bioactivity of porous Mg/Al2O3 and Mg/Si3N4 metal matrix compo-sites fabricated using microwave sintering process. Mater. Chem. Phys. 2019, 225, 331–339. [Google Scholar] [CrossRef]
- Es-Souni, M.; Wassel, E.; Dietze, M.; Laghrissi, A.; Kloehn, F.; Weyrich, T.; Es-Souni, M. Processing of nanotubes on NiTi-shape memory alloys and their modification with photografted anti-adhesive polymer brushes. Towards smart implant surfaces. Mater. Des. 2019, 182, 108031. [Google Scholar] [CrossRef]
- Deng, B.; Bruzzaniti, A.; Cheng, G.J. Enhancement of osteoblast activity on nanostructured NiTi/hydroxyapatite coatings on additive manufactured NiTi metal implants by nanosecond pulsed laser sintering. Int. J. Nanomed. 2018, ume 13, 8217–8230. [Google Scholar] [CrossRef] [Green Version]






| Generalized Standard Term | Commercialized Term | Short Description |
|---|---|---|
| Binder jetting | A liquid agent is selectively dropped on top of powder media, requiring subsequent heating or infiltration. | |
| Directed energy deposition |
| A build platform or part is selectively melted and fused using the focused application of heat and materials. |
| Material extrusion | The material is dispensed onto the build platform, usually using a heated nozzle. | |
| Material jetting | As each layer is solidified or cured, droplets of media, typically photopolymers, are dispensed from the print head to the build platform. | |
| Powder-bed fusion | The powder media are bonded together by heating and deposited on a build platform. | |
| Sheet lamination |
| Objects are created by fusing or gluing layers of material together. |
| Vat photopolymerization | Layer-by-layer curing is achieved by selectively exposing liquid photopolymer to light. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Safavi, M.S.; Bordbar-Khiabani, A.; Khalil-Allafi, J.; Mozafari, M.; Visai, L. Additive Manufacturing: An Opportunity for the Fabrication of Near-Net-Shape NiTi Implants. J. Manuf. Mater. Process. 2022, 6, 65. https://doi.org/10.3390/jmmp6030065
Safavi MS, Bordbar-Khiabani A, Khalil-Allafi J, Mozafari M, Visai L. Additive Manufacturing: An Opportunity for the Fabrication of Near-Net-Shape NiTi Implants. Journal of Manufacturing and Materials Processing. 2022; 6(3):65. https://doi.org/10.3390/jmmp6030065
Chicago/Turabian StyleSafavi, Mir Saman, Aydin Bordbar-Khiabani, Jafar Khalil-Allafi, Masoud Mozafari, and Livia Visai. 2022. "Additive Manufacturing: An Opportunity for the Fabrication of Near-Net-Shape NiTi Implants" Journal of Manufacturing and Materials Processing 6, no. 3: 65. https://doi.org/10.3390/jmmp6030065