Laser-Based Additive Manufacturing of Magnesium Alloys for Bone Tissue Engineering Applications: From Chemistry to Clinic
Abstract
:1. Introduction
2. AM of Mg for Bone Tissue Engineering
3. Laser Powder-Bed Fusion (LPBF)
4. The Advantages of LPBF over Other AM Processes
5. Properties of LPBF’ed Mg Implants
5.1. Mechanical Properties
5.2. Corrosion Behavior
5.3. Biocompatibility
6. Conclusions, Challenges, and Future Perspectives
6.1. Bottlenecks
- (1)
- Laser-based additive manufacturing presents a challenge in terms of producing pre-alloyed powder. More research is needed in the area of blending magnesium powders and building consistency.
- (2)
- How the topology of scaffolds affects cell proliferation, new cell growth, and the lattice structure of the fabricated Mg components that are fabricated using the additive manufacturing process are still unexplored. This suggests that further studies should be conducted using in vitro and in vivo methods for the Mg scaffolds manufactured through LPBF.
- (3)
- Mg implants are evaluated in vivo for their biodegradation performance out of both processes. Therefore, the study of in vivo processes should be carried out in great detail in order to succeed in clinical applications. For biomedical implants, LPBF of Mg components has been shown to be an appropriate and promising alternative. An alloy that is suitable for bio-implant application could be developed by evaluating the Mg alloys used currently. An alloying element would be added according to the strength considerations of the implant in question and its biocompatibility. This will be considered in future work if a new Mg-based alloy is created.
6.2. Prospects
- (1)
- As a result of the efficient infiltration and complete melting of Mg alloy, LPBF is a suitable AM technology for the fabrication of Mg implants. This resulted in the removal of voids and the creation of high-density components. The powder properties that were used in the manufacturing process of the Mg scaffolds and implants, as well as the printing parameters used in printing, play a major role in determining their biological and mechanical properties.
- (2)
- LPBF produces the Mg scaffold with a hierarchical porous structure that mimics the structure of the human bone in terms of micro- and macro-pores for personalized medicine.
- (3)
- In comparison with other AM techniques, LPBF provides better dimensional accuracy, because it has a smaller beam spot, finer powder, and a thinner layer. Additionally, LPBF technology offers high-energy density, no sacrificial binder, and near-complete densification of metal parts, which makes it superior to other metal additive manufacturing processes such as binder jetting and WAAM.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Sezer, N.; Evis, Z.; Kayhan, S.M.; Tahmasebifar, A.; Koç, M. Review of magnesium-based biomaterials and their applications. J. Magnes. Alloy. 2018, 6, 23–43. [Google Scholar] [CrossRef]
- Aidin, B.-K.; Yarmand, B.; Mozafari, M. Emerging magnesium-based biomaterials for orthopedic implantation. Emerg. Mater. Res. 2019, 305–319. [Google Scholar] [CrossRef] [Green Version]
- Aidin, B.-K.; Yarmand, B.; Mozafari, M. Functional PEO layers on magnesium alloys: Innovative polymer-free drug-eluting stents. Surf. Innov. 2018, 4–5, 237–243. [Google Scholar] [CrossRef]
- Prasadh, S.; Ratheesh, V.; Manakari, V.; Parande, G.; Gupta, M.; Wong, R. The Potential of Magnesium Based Materials in Mandibular Reconstruction. Metals 2019, 9, 302. [Google Scholar] [CrossRef] [Green Version]
- On, S.-W.; Cho, S.-W.; Byun, S.-H.; Yang, B.-E. Bioabsorbable osteofixation materials for maxillofacial bone surgery: A review on polymers and mag-nesium-based materials. Biomedicines 2020, 9, 300. [Google Scholar] [CrossRef]
- Dutta, S.; Gupta, S.; Roy, M. Recent Developments in Magnesium Metal–Matrix Composites for Biomedical Applications: A Review. ACS Biomater. Sci. Eng. 2020, 6, 4748–4773. [Google Scholar] [CrossRef] [PubMed]
- Tan, J.; Ramakrishna, S. Applications of Magnesium and Its Alloys: A Review. Appl. Sci. 2021, 11, 6861. [Google Scholar] [CrossRef]
- Sepideh, K.; Fleck, C. Biodegradable magnesium alloys as temporary orthopaedic implants: A re-view. Biometals 2019, 32, 185–193. [Google Scholar]
- Ali, M.; Hussein, M.; Al-Aqeeli, N. Magnesium-based composites and alloys for medical applications: A review of mechanical and corrosion properties. J. Alloy. Compd. 2019, 792, 1162–1190. [Google Scholar] [CrossRef]
- Krishnan, R.; Pandiaraj, S.; Muthusamy, S.; Panchal, H.; Alsoufi, M.S.; Ibrahim, A.M.M.; Elsheikh, A. Biodegradable Magnesium Metal Matrix Composites for Biomedical Implants: Synthesis, Mechanical Performance, and Corrosion Behavior—A Review. J. Mater. Res. Technol. 2022, 20, 650–670. [Google Scholar] [CrossRef]
- Shah, F.A.; Thomsen, P.; Palmquist, A. Osseointegration and current interpretations of the bone-implant interface. Acta Biomater. 2018, 84, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Guzzi, E.A.; Tibbitt, M.W. Additive Manufacturing of Precision Biomaterials. Adv. Mater. 2020, 32, e1901994. [Google Scholar] [CrossRef] [PubMed]
- Telang, V.S.; Pemmada, R.; Thomas, V.; Ramakrishna, S.; Tandon, P.; Nanda, H.S. Harnessing additive manufacturing for magnesium-based metallic bioimplants: Recent advances and future perspectives. Curr. Opin. Biomed. Eng. 2021, 17, 100264. [Google Scholar] [CrossRef]
- Allavikutty, R.; Gupta, P.; Santra, T.S.; Rengaswamy, J. Additive manufacturing of Mg alloys for biomedical applications: Current status and challeng-es. Curr. Opin. Biomed. Eng. 2021, 18, 100276. [Google Scholar] [CrossRef]
- Bai, L.; Gong, C.; Chen, X.; Sun, Y.; Zhang, J.; Cai, L.; Zhu, S.; Xie, S.Q. Additive manufacturing of customized metallic orthopedic implants: Materials, structures, and surface modifications. Metals 2019, 9, 1004. [Google Scholar] [CrossRef] [Green Version]
- Velu, R.; Calais, T.; Jayakumar, A.; Raspall, F. A Comprehensive Review on Bio-Nanomaterials for Medical Implants and Feasibility Studies on Fabrication of Such Implants by Additive Manufacturing Technique. Materials 2019, 13, 92. [Google Scholar] [CrossRef] [Green Version]
- Gao, C.; Wang, C.; Jin, H.; Wang, Z.; Li, Z.; Shi, C.; Leng, Y.; Yang, F.; Liu, H.; Wang, J. Additive manufacturing technique-designed metallic porous implants for clinical application in ortho-pedics. RSC Adv. 2018, 44, 25210–25227. [Google Scholar] [CrossRef] [Green Version]
- Dzogbewu, T.C.; du Preez, W.B. Additive manufacturing of titanium-based implants with met-al-based antimicrobial agents. Metals 2021, 11, 453. [Google Scholar] [CrossRef]
- Ghomi, E.R.; Khosravi, F.; Neisiany, R.E.; Singh, S.; Ramakrishna, S. Future of additive manufacturing in healthcare. Curr. Opin. Biomed. Eng. 2021, 17, 100255. [Google Scholar] [CrossRef]
- Safavi, M.S.; Bordbar-Khiabani, A.; Khalil-Allafi, J.; Mozafari, M.; Visai, L. Additive Manufacturing: An Opportunity for the Fabrication of Near-Net-Shape NiTi Implants. J. Manuf. Mater. Process. 2022, 6, 65. [Google Scholar] [CrossRef]
- Aidin, B.-K.; Yarmand, B.; Mozafari, M. Enhanced corrosion resistance and in-vitro biodegradation of plasma electrolytic oxidation coatings prepared on AZ91 Mg alloy using ZnO nanoparticles-incorporated electrolyte. Surf. Coat. Technol. 2019, 360, 153–171. [Google Scholar]
- Khiabani, A.B.; Ghanbari, A.; Yarmand, B.; Zamanian, A.; Mozafari, M. Improving corrosion behavior and in vitro bioactivity of plasma electrolytic oxidized AZ91 magnesium alloy using calcium fluoride containing electrolyte. Mater. Lett. 2018, 212, 98–102. [Google Scholar] [CrossRef]
- Jiao, L.; Chua, Z.Y.; Moon, S.K.; Song, J.; Bi, G.; Zheng, H. Femtosecond Laser Produced Hydrophobic Hierarchical Structures on Additive Manufacturing Parts. Nanomaterials 2018, 8, 601. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodrigues, T.A.; Duarte, V.; Miranda, R.M.; Santos, T.G.; Oliveira, J.P. Current Status and Perspectives on Wire and Arc Additive Manufacturing (WAAM). Materials 2019, 12, 1121. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Farag, M.; Yun, H.-S. Effect of gelatin addition on fabrication of magnesium phosphate-based scaffolds prepared by additive manufacturing system. Mater. Lett. 2014, 132, 111–115. [Google Scholar] [CrossRef]
- Williams, M.B.; Robinson, T.W.; Williamson, C.J.; Kinser, R.P.; Ashmore, N.A.; Allison, P.G.; Jordon, J.B. Elucidating the Effect of Additive Friction Stir Deposition on the Resulting Microstructure and Mechanical Properties of Magnesium Alloy WE43. Metals 2021, 11, 1739. [Google Scholar] [CrossRef]
- Mirzababaei, S.; Pasebani, S. A Review on Binder Jet Additive Manufacturing of 316L Stainless Steel. J. Manuf. Mater. Process. 2019, 3, 82. [Google Scholar] [CrossRef] [Green Version]
- Bär, F.; Berger, L.; Jauer, L.; Kurtuldu, G.; Schäublin, R.; Schleifenbaum, J.H.; Löffler, J.F. Laser additive manufacturing of biodegradable magnesium alloy WE43: A detailed microstructure analysis. Acta Biomater. 2019, 98, 36–49. [Google Scholar] [CrossRef]
- Hyer, H.; Zhou, L.; Benson, G.; McWilliams, B.; Cho, K.; Sohn, Y. Additive manufacturing of dense WE43 Mg alloy by laser powder bed fusion. Addit. Manuf. 2020, 33, 101123. [Google Scholar] [CrossRef]
- Black, C.R.M.; Goriainov, V.; Gibbs, D.; Kanczler, J.; Tare, R.S.; Oreffo, R.O.C. Bone tissue engineering. Curr. Mol. Biol. Rep. 2015, 1, 132–140. [Google Scholar] [CrossRef]
- Amini, A.R.; Cato, T.L.; Nukavarapu, S.P. Bone tissue engineering: Recent advances and challenges. Crit. Rev. Biomed. Eng. 2012, 40, 363–408. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qu, H.; Fu, H.; Han, Z.; Sun, Y. Biomaterials for bone tissue engineering scaffolds: A review. RSC Adv. 2019, 9, 26252–26262. [Google Scholar] [CrossRef] [PubMed]
- Rajeshkumar, S.; Subramanian, A.K.; Prabhakar, R. In vitro Anti-inflammatory activity of Si-lymarin/Hydroxyapatite/Chitosan Nanocomposites and its cytotoxic effect using Brine shrimp lethality assay: Nanocomposite for biomedical applications. J. Popul. Ther. Clin. Pharmacol. 2021, 28, 71–77. [Google Scholar]
- Seyyedi, M.; Molajou, A. Nanohydroxyapatite loaded-acrylated polyurethane nanofibrous scaffolds for con-trolled release of paclitaxel anticancer drug. J. Res. Sci. Eng. Technol. 2021, 9, 50–61. [Google Scholar]
- Wan, Z.; Zhang, P.; Liu, Y.; Lv, L.; Zhou, Y. Four-dimensional bioprinting: Current developments and applications in bone tissue engineering. Acta Biomater. 2020, 101, 26–42. [Google Scholar] [CrossRef]
- Aghili, A.; Kamrani, M.R. Modeling of the thermal degradation of poly (methyl methacrylate) and its nanocomposite with multi-walled carbon nanotubes. Adv. Appl. NanoBio-Technol. 2021, 2, 22–34. [Google Scholar]
- Wang, X.; Xu, S.; Zhou, S.; Xu, W.; Leary, M.; Choong, P.; Qian, M.; Brandt, M.; Xie, Y.M. Topological design and additive manufacturing of porous metals for bone scaffolds and orthopaedic implants: A review. Biomaterials 2016, 83, 127–141. [Google Scholar] [CrossRef]
- Müssig, J.; Graupner, N. Test Methods for Fibre/Matrix Adhesion in Cellulose Fibre-Reinforced Thermoplastic Composite Materials: A Critical Review. Rev. Adhes. Adhes. 2021, 8.2, 68–129. [Google Scholar] [CrossRef]
- Du, X.; Fu, S.; Zhu, Y. 3D printing of ceramic-based scaffolds for bone tissue engineering: An over-view. J. Mater. Chem. B 2018, 6, 4397–4412. [Google Scholar] [CrossRef]
- Ji, K.; Wang, Y.; Wei, Q.; Zhang, K.; Jiang, A.; Rao, Y.; Cai, X. Application of 3D printing technology in bone tissue engineering. Bio-Design Manuf. 2018, 1, 203–210. [Google Scholar] [CrossRef]
- Lewin, S.; Fleps, I.; Åberg, J.; Ferguson, S.J.; Engqvist, H.; Öhman-Mägi, C.; Helgason, B.; Persson, C. Additively manufactured mesh-type titanium structures for cranial implants: E-PBF vs. L-PBF. Mater. Des. 2021, 197, 109207. [Google Scholar] [CrossRef]
- Tilton, M.; Lewis, G.S.; Manogharan, G.P. Additive Manufacturing of Orthopedic Implants. In Orthopedic Biomaterials; Springer: Cham, Switzerland, 2018; pp. 21–55. [Google Scholar] [CrossRef]
- Majumdar, T.; Bazin, T.; Ribeiro, E.M.C.; Frith, J.E.; Birbilis, N. Understanding the effects of PBF process parameter interplay on Ti-6Al-4V surface properties. PLoS ONE 2019, 14, e0221198. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; He, C.; Dianyu, E.; Yang, W.; Qi, F.; Xie, D.; Shen, L.; Peng, S.; Shuai, C. Mg bone implant: Features, developments and perspectives. Mater. Des. 2020, 185, 108259. [Google Scholar] [CrossRef]
- Murr, L. Metallurgy principles applied to powder bed fusion 3D printing/additive manufacturing of personalized and optimized metal and alloy biomedical implants: An overview. J. Mater. Res. Technol. 2019, 9, 1087–1103. [Google Scholar] [CrossRef]
- Wang, C.; Shuai, Y.; Yang, Y.; Zeng, D.; Liang, X.; Peng, S.; Shuai, C. Amorphous magnesium alloy with high corrosion resistance fabricated by laser powder bed fusion. J. Alloy. Compd. 2022, 897, 163247. [Google Scholar] [CrossRef]
- Niu, X.; Shen, H.; Fu, J.; Feng, J. Effective control of microstructure evolution in AZ91D magnesium alloy by SiC nanoparticles in laser powder-bed fusion. Mater. Des. 2021, 206, 109787. [Google Scholar] [CrossRef]
- Deng, Q.; Wang, X.; Lan, Q.; Chang, Z.; Liu, Z.; Su, N.; Wu, Y.; Liu, D.; Peng, L.; Ding, W. Limitations of linear energy density for laser powder bed fusion of Mg-15Gd-1Zn-0.4Zr alloy. Mater. Charact. 2022, 190, 112071. [Google Scholar] [CrossRef]
- Deng, Q.; Wu, Y.; Wu, Q.; Xue, Y.; Zhang, Y.; Peng, L.; Ding, W. Microstructure evolution and mechanical properties of a high-strength Mg-10Gd-3Y–1Zn-0.4 Zr alloy fabricated by laser powder bed fusion. Addit. Manuf. 2022, 49, 102517. [Google Scholar]
- Liu, J.; Yin, B.; Sun, Z.; Wen, P.; Zheng, Y.; Tian, Y. Hot cracking in ZK60 magnesium alloy produced by laser powder bed fusion process. Mater. Lett. 2021, 301, 130283. [Google Scholar] [CrossRef]
- Liang, J.; Lei, Z.; Chen, Y.; Fu, W.; Wu, S.; Chen, X.; Yang, Y. Microstructure evolution of laser powder bed fusion ZK60 Mg alloy after different heat treatment. J. Alloy. Compd. 2022, 898, 163046. [Google Scholar] [CrossRef]
- Julmi, S.; Abel, A.; Gerdes, N.; Hoff, C.; Hermsdorf, J.; Overmeyer, L.; Klose, C.; Maier, H. Development of a Laser Powder Bed Fusion Process Tailored for the Additive Manufacturing of High-Quality Components Made of the Commercial Magnesium Alloy WE43. Materials 2021, 14, 887. [Google Scholar] [CrossRef] [PubMed]
- Xu, R.; Yuan, T.; Zhu, H.; Wang, M.; Li, J.; Zhang, W.; Cao, P. Laser powder bed fusion of Al–Mg–Zr alloy: Microstructure, mechanical properties and dynamic precipitation. Mater. Sci. Eng. A 2022, 859, 144181. [Google Scholar] [CrossRef]
- Attarzadeh, F.; Asadi, E. Analysis of element loss, densification, and defects in laser-based powder-bed fusion of magnesium alloy WE43. J. Magnes. Alloy. 2022, 10, 2118–2136. [Google Scholar] [CrossRef]
- Dobkowska, A.; Żrodowski, L.; Chlewicka, M.; Koralnik, M.; Adamczyk-Cieślak, B.; Ciftci, J.; Morończyk, B.; Kruszewski, M.; Jaroszewicz, J.; Kuc, D.; et al. A comparison of the microstructure-dependent corrosion of dual-structured Mg-Li alloys fabricated by powder consolidation methods: Laser powder bed fusion vs pulse plasma sintering. J. Magnes. Alloy. 2022. (accessed on 5 December 2022). [Google Scholar] [CrossRef]
- Liang, J.; Wu, S.; Lei, Z.; Chen, Y.; Zhang, X.; Li, B.; Jiang, M.; Chen, Y. In-situ aging treatment by preheating to obtain high-strength ZK60 Mg alloy processed by laser powder bed fusion. Mater. Charact. 2022, 194, 112361. [Google Scholar] [CrossRef]
- Deng, Q.; Zhang, Y.; Liu, Z.; Chang, Z.; Su, N.; Wu, Y.; Hao, L.; Peng, L.; Ding, W. Laser powder bed fusion of an age-hardenable Mg-10Gd-0.2 Zr alloy with excellent strength-ductility synergy. J. Alloy. Compd. 2022, 910, 164863. [Google Scholar] [CrossRef]
- Hwang, Y.-J.; Kim, K.-S.; AlMangour, B.; Grzesiak, D.; Lee, K.-A. A New Approach for Manufacturing Stochastic Pure Magnesium Foam by Laser Powder Bed Fusion: Fabrication, Geometrical Characteristics, and Compressive Mechanical Properties. Adv. Eng. Mater. 2021, 23, 2100483. [Google Scholar] [CrossRef]
- Hendea, R.E.; Raducanu, D.; Nocivin, A.; Ivanescu, S.; Stanciu, D.; Trisca-Rusu, C.; Campian, R.S.; Drob, S.I.; Cojocaru, V.D.; Gălbinașu, B.M. Laser Powder Bed Fusion Applied to a New Biodegradable Mg-Zn-Zr-Ca Alloy. Materials 2022, 15, 2561. [Google Scholar] [CrossRef]
- Liang, J.; Lei, Z.; Chen, Y.; Fu, W.; Chen, X.; Ma, S. Elimination of extraordinarily high cracking susceptibility of ZK60 Mg alloy fabricated by laser powder bed fusion. Mater. Lett. 2022, 312, 131731. [Google Scholar] [CrossRef]
- Nilsson, Å.; Hanna Thorsson, L.; Mellin, P.; Lindwall, G.; Persson, C. An Enhanced Understanding of the Powder Bed Fusion–Laser Beam Processing of Mg-Y3. 9wt%-Nd3wt%-Zr0. 5wt%(WE43) Alloy through Thermodynamic Modeling and Experimental Characterization. Materials 2022, 15, 417. [Google Scholar] [CrossRef]
- Liu, J.; Yin, B.; Wen, P.; Tian, Y. Laser powder bed fusion of WE43 magnesium alloy porous scaffolds: Investigation on densification behavior and dimensional accuracy. Adv. Laser Process. Manuf. 2021, 11892, 9–19. [Google Scholar] [CrossRef]
- Abel, A.; Wessarges, Y.; Julmi, S.; Hoff, C.; Hermsdorf, J.; Klose, C.; Maier, H.J.; Kaierle, S.; Overmeyer, L. Laser powder bed fusion of WE43 in hydrogen-argon-gas atmosphere. Procedia CIRP 2020, 94, 21–24. [Google Scholar] [CrossRef]
- Hanna, N.Å.; Mellin, P.; Persson, C. Influence of Hot Isostatic Pressing on the corrosion resistance of Mg-4wt% Y-3wt% Nd processed by Laser-Powder Bed Fusion. In Proceedings of the 31st Annual Conference of the European Society for Biomaterials (ESB 2021), virtually, 5–9 September 2021. [Google Scholar]
- Wei, K.; Wang, Z.; Zeng, X. Influence of element vaporization on formability, composition, microstructure, and mechanical performance of the selective laser melted Mg–Zn–Zr components. Mater. Lett. 2015, 156, 187–190. [Google Scholar] [CrossRef]
- Zhao, Z.; Yang, G.; Zhao, K. 3D Printing of Mg-Based Bulk Metallic Glasses with Proper Laser Power and Scanning Speed. Metals 2022, 12, 1318. [Google Scholar] [CrossRef]
- Zumdick, N.A.; Jauer, L.; Kersting, L.C.; Kutz, T.N.; Schleifenbaum, J.H.; Zander, D. Additive manufactured WE43 magnesium: A comparative study of the microstructure and mechanical properties with those of powder extruded and as-cast WE43. Mater. Charact. 2019, 147, 384–397. [Google Scholar] [CrossRef]
- Lietaert, K.; Zadpoor, A.A.; Sonnaert, M.; Schrooten, J.; Weber, L.; Mortensen, A.; Vleugels, J. Mechanical properties and cytocompatibility of dense and porous Zn produced by laser powder bed fusion for biodegradable implant applications. Acta Biomater. 2020, 110, 289–302. [Google Scholar] [CrossRef]
- Chowdhury, S.; Yadaiah, N.; Prakash, C.; Ramakrishna, S.; Dixit, S.; Gupta, L.R.; Buddhi, D. Laser Powder Bed Fusion: A State-of-the-Art Review of the Technology, Materials, Properties & Defects, and Numerical Modelling. J. Mater. Res. Technol. 2022, 20, 2109–2172. [Google Scholar]
- Zhang, B.; Liao, H.; Coddet, C. Effects of processing parameters on properties of selective laser melting Mg–9%Al powder mixture. Mater. Des. 2012, 34, 753–758. [Google Scholar] [CrossRef]
- Han, Y.; Wang, L.; Liu, K.; Yan, W. Numerical modeling of laser powder bed fusion of metallic glasses: Prediction of crystallization. J. Micromechanics Mol. Phys. 2020, 5, 2050013. [Google Scholar] [CrossRef]
- Mair, P.; Letofsky-Papst, I.; Leichtfried, G. Microstructural features and mechanical properties of a novel Ti- and Zr-modified Al-Mn alloy processed by laser powder bed fusion. J. Alloy. Compd. 2022, 897, 163156. [Google Scholar] [CrossRef]
- Li, Z.; Li, H.; Yin, J.; Li, Y.; Nie, Z.; Li, X.; You, D.; Guan, K.; Duan, W.; Cao, L.; et al. A Review of Spatter in Laser Powder Bed Fusion Additive Manufacturing: In Situ Detection, Generation, Effects, and Countermeasures. Micromachines 2022, 13, 1366. [Google Scholar] [CrossRef]
- Sing, S.L.; Yeong, W.Y. Laser powder bed fusion for metal additive manufacturing: Perspectives on recent developments. Virtual Phys. Prototyp. 2020, 15, 359–370. [Google Scholar] [CrossRef]
- Kotadia, H.; Gibbons, G.; Das, A.; Howes, P. A review of Laser Powder Bed Fusion Additive Manufacturing of aluminium alloys: Microstructure and properties. Addit. Manuf. 2021, 46, 102155. [Google Scholar] [CrossRef]
- Sun, J.; Zhang, B.; Qu, X. High strength Al alloy development for laser powder bed fusion. J. Micromechanics Mol. Phys. 2021, 6, 2141001. [Google Scholar] [CrossRef]
- Hu, D.; Wang, Y.; Zhang, D.; Hao, L.; Jiang, J.; Li, Z.; Chen, Y. Experimental Investigation on Selective Laser Melting of Bulk Net-Shape Pure Magnesium. Mater. Manuf. Process. 2015, 30, 1298–1304. [Google Scholar] [CrossRef]
- Niu, X.; Shen, H.; Fu, J.; Yan, J.; Wang, Y. Corrosion behaviour of laser powder bed fused bulk pure magnesium in hank’s solution. Corros. Sci. 2019, 157, 284–294. [Google Scholar] [CrossRef]
- Esmaily, M.; Zeng, Z.; Mortazavi, A.; Gullino, A.; Choudhary, S.; Derra, T.; Benn, F.; D’Elia, F.; Müther, M.; Thomas, S.; et al. A detailed microstructural and corrosion analysis of magnesium alloy WE43 manufactured by selective laser melting. Addit. Manuf. 2020, 35, 101321. [Google Scholar] [CrossRef]
- Gangireddy, S.; Gwalani, B.; Liu, K.; Faierson, E.J.; Mishra, R.S. Microstructure and mechanical behavior of an additive manufactured (AM) WE43-Mg alloy. Addit. Manuf. 2019, 26, 53–64. [Google Scholar] [CrossRef]
- Deng, Q.; Wu, Y.; Su, N.; Chang, Z.; Chen, J.; Peng, L.; Ding, W. Influence of friction stir processing and aging heat treatment on microstructure and mechanical properties of selective laser melted Mg-Gd-Zr alloy. Addit. Manuf. 2021, 44, 102036. [Google Scholar] [CrossRef]
- Deng, Q.; Wu, Y.; Luo, Y.; Su, N.; Xue, X.; Chang, Z.; Wu, Q.; Xue, Y.; Peng, L. Fabrication of high-strength Mg-Gd-Zn-Zr alloy via selective laser melting. Mater. Charact. 2020, 165, 110377. [Google Scholar] [CrossRef]
- Fu, P.-H.; Wang, N.-Q.; Liao, H.-G.; Xu, W.-Y.; Peng, L.-M.; Chen, J.; Hu, G.-Q.; Ding, W.-J. Microstructure and mechanical properties of high strength Mg-15Gd-1Zn-0.4 Zr alloy additive-manufactured by selective laser melting process. Trans. Nonferrous Met. Soc. China 2021, 31, 1969–1978. [Google Scholar] [CrossRef]
- Shuai, C.; Yang, Y.; Wu, P.; Lin, X.; Liu, Y.; Zhou, Y.; Feng, P.; Liu, X.; Peng, S. Laser rapid solidification improves corrosion behavior of Mg-Zn-Zr alloy. J. Alloy. Compd. 2016, 691, 961–969. [Google Scholar] [CrossRef]
- Ng, C.C.; Savalani, M.M.; Man, H.; Gibson, I. Layer manufacturing of magnesium and its alloy structures for future applications. Virtual Phys. Prototyp. 2010, 5, 13–19. [Google Scholar] [CrossRef]
- Ng, C.; Savalani, M.; Lau, M.; Man, H. Microstructure and mechanical properties of selective laser melted magnesium. Appl. Surf. Sci. 2011, 257, 7447–7454. [Google Scholar] [CrossRef]
- Polmear, I.J.; Stjohn, D.; Nie, J.-F.; Qian, M. Metallurgy of the Light Metals. In Light Alloys, 4th ed.; Elsevier: New York, NY, USA, 2005; pp. 237–297. [Google Scholar]
- Liu, S.; Yang, W.; Shi, X.; Li, B.; Duan, S.; Guo, H.; Guo, J. Influence of laser process parameters on the densification, microstructure, and mechanical properties of a selective laser melted AZ61 magnesium alloy. J. Alloy. Compd. 2019, 808, 151160. [Google Scholar] [CrossRef]
- He, C.; Bin, S.; Wu, P.; Gao, C.; Feng, P.; Yang, Y.; Liu, L.; Zhou, Y.; Zhao, M.; Yang, S.; et al. Microstructure evolution and biodegradation behavior of laser rapid solidified Mg–Al–Zn alloy. Metals 2017, 7, 105. [Google Scholar] [CrossRef]
- Wei, K.; Gao, M.; Wang, Z.; Zeng, X. Effect of energy input on formability, microstructure and mechanical properties of selective laser melted AZ91D magnesium alloy. Mater. Sci. Eng. A 2014, 611, 212–222. [Google Scholar] [CrossRef]
- Zhu, Z.; Zhang, M.; Chen, C. Effect of selective laser melting on microstructure and properties of AZ91D alloy. Mater. Und Werkst. 2019, 50, 1484–1494. [Google Scholar] [CrossRef]
- Jauer, L.; Meiners, W.; Vervoort, S.; Gayer, C.; Zumdick, N.; Zander, D. Selective Laser Melting of Magnesium Alloys. European Congress and Exhibition on Powder Metallurgy. Eu-ropean PM Conference Proceedings. The European Powder Metallurgy Association. 2016. Available online: https://www.epma.com/publications/euro-pm-proceedings/product/ep16-3291874 (accessed on 5 December 2022).
- Proaño, B.; Miyahara, H.; Matsumoto, T.; Hamada, S.; Sakai, H.; Ogawa, K.; Suyalatu; Noguchi, H. Weakest region analysis of non-combustible Mg products fabricated by selective laser melting. Theor. Appl. Fract. Mech. 2019, 103, 102291. [Google Scholar] [CrossRef]
- Wei, K.; Zeng, X.; Wang, Z.; Deng, J.; Liu, M.; Huang, G.; Yuan, X. Selective laser melting of Mg-Zn binary alloys: Effects of Zn content on densification behavior, micro-structure, and mechanical property. Mater. Sci. Eng. A 2019, 756, 226–236. [Google Scholar] [CrossRef]
- Xu, R.; Zhao, M.-C.; Zhao, Y.-C.; Liu, L.; Liu, C.; Gao, C.; Shuai, C.; Atrens, A. Improved biodegradation resistance by grain refinement of novel antibacterial ZK30-Cu alloys produced via selective laser melting. Mater. Lett. 2019, 237, 253–257. [Google Scholar] [CrossRef]
- Gnedenkov, S.; Sinebryukhov, S.; Egorkin, V.; Mashtalyar, D.; Vyaliy, I.; Nadaraia, K.; Imshinetskiy, I.; Nikitin, A.; Subbotin, E.; Gnedenkov, A. Magnesium fabricated using additive technology: Specificity of corrosion and protection. J. Alloy. Compd. 2019, 808, 151629. [Google Scholar] [CrossRef]
- Khiabani, A.B.; Rahimi, S.; Yarmand, B.; Mozafari, M. Electrophoretic deposition of graphene oxide on plasma electrolytic oxidized-magnesium implants for bone tissue engineering applications. Mater. Today Proc. 2018, 5, 15603–15612. [Google Scholar] [CrossRef]
- Ghanbari, A.; Khiabani, A.B.; Zamanian, A.; Yarmand, B.; Mozafari, M. The competitive mechanism of plasma electrolyte oxidation for the formation of magnesium oxide bioceramic coatings. Mater. Today Proc. 2018, 5, 15677–15685. [Google Scholar] [CrossRef]
- Sharifi, S.; Lotfipour, F.; Ghavimi, M.A.; Dizaj, S.M.; Shahi, S.; Yazdani, J.; Mokhtarpour, M.; Khalilov, R. Hydroxyapatite-gelatin and calcium carbonate- gelatin nanocomposite scaffolds: Production, physicochemical characterization and comparison of their bioactivity in simulated body fluid. Eurasian Chem. Commun. 2021, 3, 70–80. [Google Scholar] [CrossRef]
- Shuai, C.; Liu, L.; Zhao, M.; Feng, P.; Yang, Y.; Guo, W.; Gao, C.; Yuan, F. Microstructure, biodegradation, antibacterial and mechanical properties of ZK60-Cu alloys prepared by selective laser melting technique. J. Mater. Sci. Technol. 2018, 34, 1944–1952. [Google Scholar] [CrossRef]
- Ding, Y.; Li, R.W.; Nakai, M.; Majumdar, T.; Zhang, D.; Niinomi, M.; Birbilis, N.; Smith, P.N.; Chen, X. Osteoanabolic Implant Materials for Orthopedic Treatment. Adv. Health Mater. 2016, 5, 1740–1752. [Google Scholar] [CrossRef]
- Bordbar-Khiabani, A.; Yarmand, B.; Mozafari, M. Effect of ZnO pore-sealing layer on anti-corrosion and in-vitro bioactivity behavior of plasma electrolytic oxidized AZ91 magnesium alloy. Mater. Lett. 2019, 258, 126779. [Google Scholar] [CrossRef]
- Bordbar-Khiabani, A.; Yarmand, B.; Sharifi-Asl, S.; Mozafari, M. Improved corrosion performance of biodegradable magnesium in simulated inflammatory condition via drug-loaded plasma electrolytic oxidation coatings. Mater. Chem. Phys. 2020, 239, 122003. [Google Scholar] [CrossRef]
- Li, R.W.; Kirkland, N.T.; Truong, J.; Wang, J.; Smith, P.N.; Birbilis, N.; Nisbet, D.R. The influence of biodegradable magnesium alloys on the osteogenic differentiation of human mesenchymal stem cells. J. Biomed. Mater. Res. Part A 2014, 102, 4346–4357. [Google Scholar] [CrossRef]
- Wani, S.D. A Review: Emerging Trends in Bionanocomposites. Int. J. Pharm. Res. Technol. 2021, 11, 1. [Google Scholar]
- Nemati, E. Cell Membrane Coated Nanoparticles for Biomedical Applications. Adv. Appl. Nano-Bio-Technol. 2022, 3, 49–59. [Google Scholar]
- Ghavimi, M.A.; Negahdari, R.; Shahabadi, A.B.; Sharifi, S.; Kazeminejad, E.; Shahi, S.; Dizaj, S.M. Preparation and study of starch/collagen/polycaprolactone nanofiber scaffolds for bone tissue engineering using electrospinning technique. Eurasian Chem. Commun. 2020, 2, 122–127. [Google Scholar] [CrossRef] [Green Version]
- Song, M.-S.; Zeng, R.-C.; Ding, Y.-F.; Li, R.W.; Easton, M.; Cole, I.; Birbilis, N.; Chen, X.-B. Recent advances in biodegradation controls over Mg alloys for bone fracture management: A review. J. Mater. Sci. Technol. 2019, 35, 535–544. [Google Scholar] [CrossRef]
- Cortizo, M.C.; Mónica, F.L.; De, M.; Cortizo, A.M. Metallic dental material biocompatibility in osteo-blastlike cells. Biol. Trace Elem. Res. 2004, 100, 151–168. [Google Scholar] [CrossRef]
- Kopp, A.; Derra, T.; Müther, M.; Jauer, L.; Schleifenbaum, J.H.; Voshage, M.; Jung, O.; Smeets, R.; Kröger, N. Influence of design and postprocessing parameters on the degradation behavior and mechanical properties of additively manufactured magnesium scaffolds. Acta Biomater. 2019, 98, 23–35. [Google Scholar] [CrossRef]
- Li, M.; Benn, F.; Derra, T.; Kröger, N.; Zinser, M.; Smeets, R.; Molina-Aldareguia, J.M.; Kopp, A.; Llorca, J. Microstructure, mechanical properties, corrosion resistance and cytocompatibility of WE43 Mg alloy scaffolds fabricated by laser powder bed fusion for biomedical applications. Mater. Sci. Eng. C 2021, 119, 111623. [Google Scholar] [CrossRef]
- Benn, F.; Kröger, N.; Zinser, M.; van Gaalen, K.; Vaughan, T.J.; Yan, M.; Smeets, R.; Bibiza, E.; Malinov, S.; Buchanan, F.; et al. Influence of surface condition on the degradation behaviour and biocompatibility of additively manufactured WE43. Mater. Sci. Eng. C 2021, 124, 112016. [Google Scholar] [CrossRef]
- Wang, Y.; Fu, P.; Wang, N.; Ping, L.; Kang, B.; Zeng, H.; Yuan, G.; Ding, W. Challenges and solutions for the additive manufacturing of biodegradable magnesium implants. Engineering 2020, 6, 1267–1275. [Google Scholar] [CrossRef]
- Koopaie, M.; Bordbar-Khiabani, A.; Kolahdooz, S.; Darbandsari, A.K.; Mozafari, M. Advanced surface treatment techniques counteract biofilm-associated infections on dental implants. Mater. Res. Express 2020, 7, 015417. [Google Scholar] [CrossRef]
- Alipour, A. Virus decorated nanobiomaterials as scaffolds for tissue engineering. Adv. Appl. Nano-Bio-Technol. 2021, 79-85, 4. [Google Scholar]
- Desai, H.; Arun, K.; Tanna, A. Structural and magnetic properties of MgFe2O4 ferrite nanoparticle synthesis through auto combustion technique. Eur. Chem. Bull. 2021, 10, 186–190. [Google Scholar]
- Pourshadloo, M.; Rezaei, H.A.; Saeidnia, M.; Alkokab, H.; Bathaei, M.S. Effect of graphene-family incorporation on corrosion performance of PEO coatings formed on titanium alloys: A mini review. Surf. Innov. 2022, 1–10. [Google Scholar] [CrossRef]
- Bahaa, M.; Daily, Z.A.; Alsharbaty, M.H.M.H.; Abullais, S.S.; Arora, S.; Lafta, H.A.; Turki Jalil, A.; Almulla, A.F.; Ramírez-Coronel, A.A.; Aravindhan, S. Effect of PMMA sealing treatment on the corrosion behavior of plasma electrolytic oxidized titanium dental implants in fluoride-containing saliva solution. Mater. Res. Express 2022, 1–15. [Google Scholar] [CrossRef]
- Jamali, R.; Bordbar-Khiabani, A.; Yarmand, B.; Mozafari, M.; Kolahi, A. Effects of co-incorporated ternary elements on biocorrosion stability, antibacterial efficacy, and cytotoxicity of plasma electrolytic oxidized titanium for implant dentistry. Mater. Chem. Phys. 2021, 276, 125436. [Google Scholar] [CrossRef]

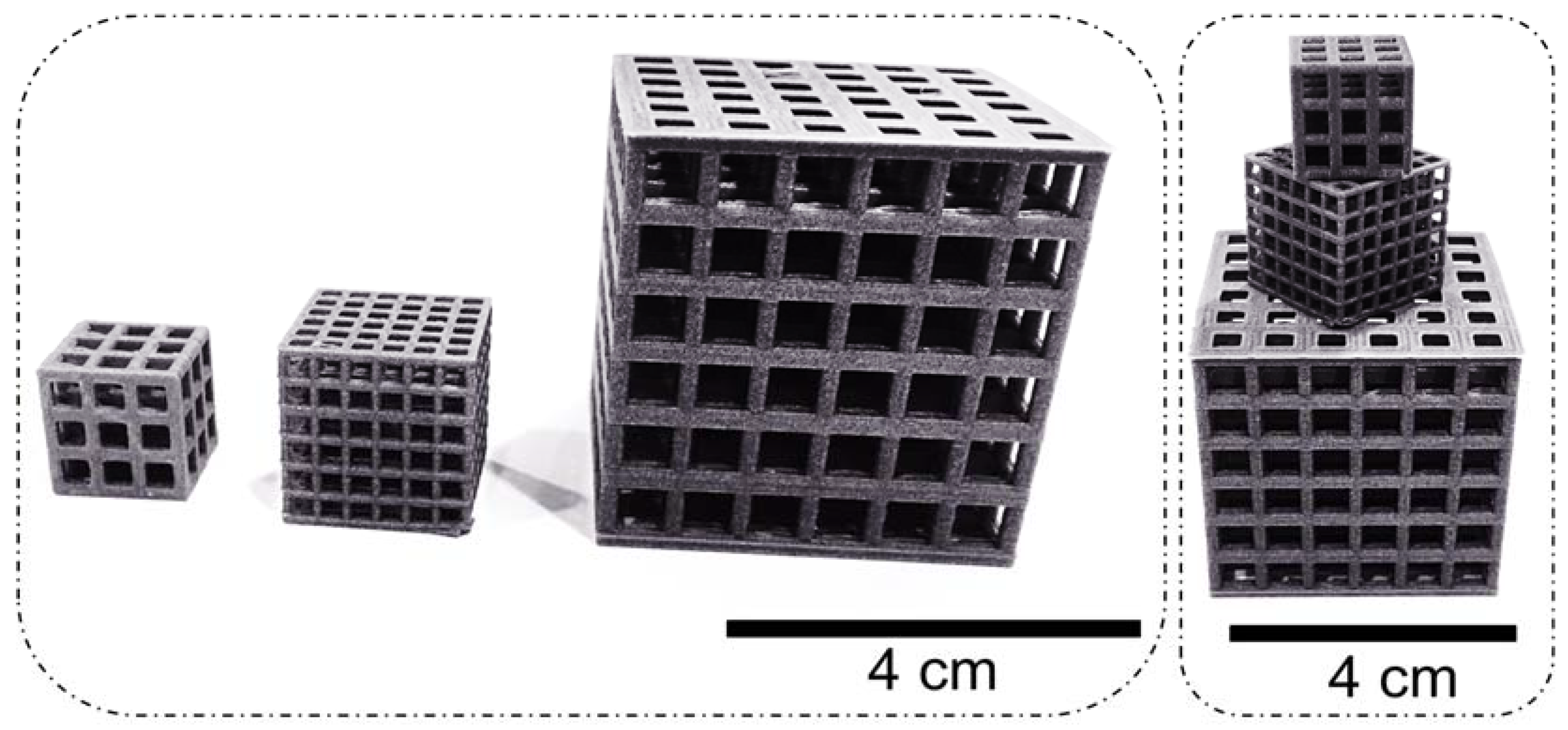




| Tissue/Material | Young’s Modulus (GPa) | Yield Strength (MPa) | Compression Strength (MPa) | Tensile Strength (MPa) |
|---|---|---|---|---|
| Cortical bone | 7–30 | - | 100–230 | 164–240 |
| Cancellous bone | 0.01–3.0 | - | 2–12 | - |
| Ti-6Al-4V (casted) | 114 | 760–880 | - | 895–930 |
| Ti-6Al-4V (wrought) | 114 | 827–1103 | 896–1172 | 860–965 |
| Stainless steel 316 L | 193 | 170–310 | 480–620 | 540–1000 |
| CoCrMo Alloy | 240 | 500–1500 | - | 900–1540 |
| Mg (99.9%, casted) | 41 | 21 | 40 | 87 |
| Mg (99.9%, wrought) | 41 | 100 | 100–140 | 180 |
| Alloys | Powder Size and Shape (μm) | Parameters | Input Energy Density (J/mm3) | Relative Density (%) | Ref. | ||||
|---|---|---|---|---|---|---|---|---|---|
| Power (W) | Spot Size (μm) | Speed (mm/s) | Thickness (μm) | Hatch Spacing (μm) | |||||
| Mg | Pre-alloyed 43, s 1 | 90 | 100 | 100 | 30 | 100 | 300 | 96.1 | [77] |
| 90 | 100 | >300 | Evaporated | ||||||
| Mg | Pre-alloyed 24, s | 70 | 80 | 500 | 30 | 30 | 156 | 97.5 | [78] |
| 1250 | 63 | 88.2 | [79] | ||||||
| WE43 | 25–63, s | 195 | 100 | 800 | 30 | 200 | 41 | 99.7 | [67] |
| 195 | 800 | 250 | 33 | 98.3 | |||||
| 195 | 1200 | 200 | 27 | 96.6 | |||||
| 135 | 1200 | 200 | 19 | 87.6 | |||||
| WE43 | 25–63, s | 200 | 125 | 700 | 30 | 40 | 238 | 99.9 | [28] |
| WE43 | 25–63, s | 200 | 70 | 1100 | 40 | 130 | 35 | 99.7 | [80] |
| WE43 | 25–63, s | 200 | 90 | 700 | 30 | 40 | 238 | 99.8 | [81] |
| WE43 | 30, p 45, 63, s | 120 | 90 | 960 | 30 | 40 | 104 | 98.6 | [82] |
| 150 | 1200 | 104 | 99.0 | ||||||
| 300 | 1200 | 208 | 99.5 | ||||||
| GZ151K | 25–65, s | 200 | – | 700 | 30 | 70 | 136 | 97.9 | [83] |
| GZ112K | 31–44, s | 80 | 100 | 100 | 30 | 100 | 267 | 98.7 | [65] |
| 300 | 89 | 99.9 | |||||||
| 500 | 53 | 99.7 | |||||||
| 700 | 38 | 99.8 | |||||||
| 1000 | 27 | 96.9 | |||||||
| 1500 | 18 | 71.8 | |||||||
| 500 | 50 | 107 | 99.5 | ||||||
| 500 | 150 | 36 | 96.5 | ||||||
| G10K | 63, s | 80 | – | 200 | 30 | 100 | 133 | 99.2 | [84] |
| Mg-1Zn | Blended Mg-5.5 Zn (36, s), Mg (31, s) and Zn (19, s) | 180 | 150 | 700 | 20 | 70 | 183 | 99.4 | [47] |
| Mg-2Zn | 98.2 | ||||||||
| Mg-6Zn | 94.7 | ||||||||
| Mg-12Zn | 98.9 | ||||||||
| ZK60 | 30, s | 50 | 150 | 6.7 | 100 | 100 | 750 | 94.5 | [85] |
| 8.3 | 600 | 97.4 | |||||||
| 10 | 500 | 88.6 | |||||||
| 11.7 | 420 | 72.8 | |||||||
| ZK60 | 30, s | 200 | 150 | 300 | 20 | 80 | 417 | 94 | [86] |
| 500 | 250 | 93 | |||||||
| 700 | 179 | 88 | |||||||
| 900 | 139 | 84 | |||||||
| Alloys | Energy Density (J/mm3) | Grain Size (μm) | Mechanical Properties | Electrochemical Properties | Ref. | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| H 1 (HV) | YS 2 (MPa) | UTS 3 (MPa) | EL 4 (%) | Solution | icorr (μA/cm2) | Mass Loss (mm/year) | |||||
| Mg | 97.5 | 1–5 | – | – | – | – | Hank’s solution | 74 | 3 | [77] | |
| 88.2 | – | 177 | 32 | ||||||||
| Mg | 300 | – | 52.4 | – | – | – | – | – | [70] | ||
| Mg-9Al | 250 | 10–20 | 70 | – | – | – | – | – | [88] | ||
| Mg-9Al | 156 | 1–3 | – | 274 | 1 | – | – | [89] | |||
| AZ61 | 139 | 1.6 | – | 219 | 273 | 3.3 | – | – | [90] | ||
| 156 | 1.8 | 233 | 287 | 3.1 | |||||||
| 179 | 2.1 | 225 | 261 | 2.8 | |||||||
| 208 | 2.5 | 216 | 239 | 2.1 | |||||||
| AZ61 | 120 | 4.5 | 70 | – | – | – | SBF solution | – | 2.7 | [91] | |
| 140 | 8 | 80 | 2.4 | ||||||||
| 160 | 10 | 93 | 12 | ||||||||
| 180 | 13 | 90 | 1.5 | ||||||||
| AZ91 | 167 | 1–2.9 | 85–100 | 274 | 296 | 1.2 | – | – | [92] | ||
| 83 | 237 | 254 | 1.8 | – | – | ||||||
| AZ91 | 68.6 | 1–10 | 115 | – | – | – | – | – | [47] | ||
| AZ91 | 104 | 1–1.5 | – | 265 | 328 | 3.8 | – | – | [93] | ||
| AZ91 | 278 | 3.3 | – | 308 | 345 | 1 | – | – | [79] | ||
| AZ91-SiC | 278 | 1.1 | – | 260 | 300 | 2 | – | – | [67] | ||
| AZ91–2Ca | – | – | – | 235 | 332 | 3.2 | – | – | [29] | ||
| WE43 | 120 | 34 | - | - | - | - | 0.1 M NaCl | 5.1 | 6–7.2 | [28] | |
| 150 | 27 | 5.0 | |||||||||
| 300 | 18 | 4.4 | |||||||||
| WE43 | 238 | 1 | – | 296 | 308 | 12.2 | – | – | [80] | ||
| WE43 | 35 | 1–3 | – | 214 | 251 | 2.6 | – | – | [82] | ||
| WE43 | 238 | 20.4 | – | – | – | – | – | – | [82] | ||
| G10K | 133 | 27 | 80 | 180 | 228 | 2.2 | – | – | [83] | ||
| GZ151K | 136 | 2 | 345 | 368 | 3 | – | – | [94] | |||
| Mg-1Zn | 183 | – | 50 | 145 | 11 | – | – | [95] | |||
| Mg-2Zn | 46 | 70 | 2.5 | ||||||||
| Mg-6Zn | 65 | 50 | 1.5 | ||||||||
| Mg-12Zn | 83 | 75 | 3.2 | ||||||||
| ZK30 | 2000 | – | 80 | – | – | – | SBF solution | 17.8 | 1.23 | [96] | |
| ZK30-Cu | 98 | 47.8 | 2.12 | ||||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fard, M.G.; Sharifianjazi, F.; Kazemi, S.S.; Rostamani, H.; Bathaei, M.S. Laser-Based Additive Manufacturing of Magnesium Alloys for Bone Tissue Engineering Applications: From Chemistry to Clinic. J. Manuf. Mater. Process. 2022, 6, 158. https://doi.org/10.3390/jmmp6060158
Fard MG, Sharifianjazi F, Kazemi SS, Rostamani H, Bathaei MS. Laser-Based Additive Manufacturing of Magnesium Alloys for Bone Tissue Engineering Applications: From Chemistry to Clinic. Journal of Manufacturing and Materials Processing. 2022; 6(6):158. https://doi.org/10.3390/jmmp6060158
Chicago/Turabian StyleFard, Mohammad Ghasemian, Fariborz Sharifianjazi, Sanam Sadat Kazemi, Hosein Rostamani, and Masoud Soroush Bathaei. 2022. "Laser-Based Additive Manufacturing of Magnesium Alloys for Bone Tissue Engineering Applications: From Chemistry to Clinic" Journal of Manufacturing and Materials Processing 6, no. 6: 158. https://doi.org/10.3390/jmmp6060158






