Synthesis and Characterization of Hybrid Particles Obtained in a One-Pot Process through Simultaneous Sol-Gel Reaction of (3-Mercaptopropyl)trimethoxysilane and Emulsion Polymerization of Styrene
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
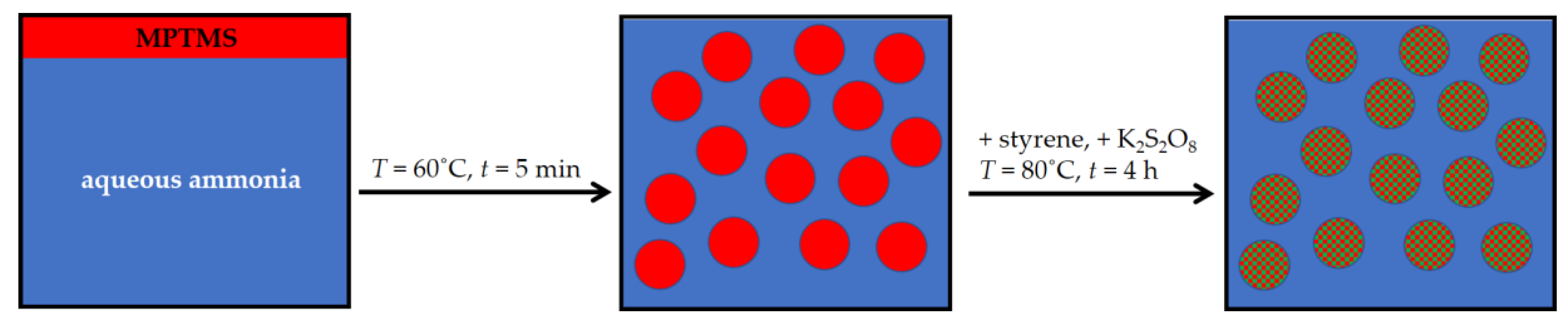
2.2. Synthesis of Hybrid Particles
2.3. Characterization and Measurement Methods
2.3.1. Thermogravimetric Analysis
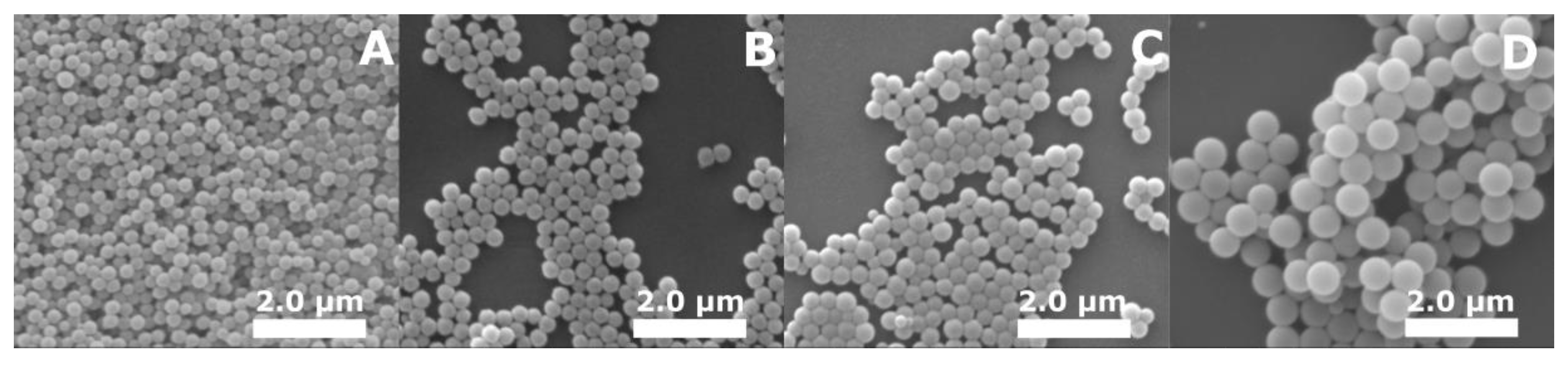
2.3.2. Scanning Electron Microscopy
2.3.3. Transmission Electron Microscopy
3. Results and Discussion
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Van Bommel, K.J.C.; Friggeri, A.; Shinkai, S. Organic templates for the generation of inorganic materials. Angew. Chem. Int. Ed. 2003, 42, 980–999. [Google Scholar] [CrossRef] [PubMed]
- Klapper, M.; Clark, C.J., Jr.; Müllen, K. Application-directed syntheses of surface-functionalized organic and inorganic nanoparticles. Polym. Int. 2008, 57, 181–202. [Google Scholar] [CrossRef]
- Kidsaneepoiboon, P.; Wanichwecharungruang, S.P.; Chooppawa, T.; Deephum, R.; Panyathanmaporn, T. Organic–inorganic hybrid polysilsesquioxane nanospheres as UVA/UVB absorber and fragrance carrier. J. Mater. Chem. 2011, 21, 7922–7930. [Google Scholar] [CrossRef]
- Schottner, G. Hybrid sol-gel-derived polymers: Applications of multifunctional materials. Chem. Mater. 2001, 13, 3422–3435. [Google Scholar] [CrossRef]
- Fielding, L.A.; Mykhaylyk, O.O.; Schmid, A.; Pontoni, D.; Armes, S.P.; Fowler, P.W. Visible Mie scattering from hollow silica particles with particulate shells. Chem. Mater. 2014, 26, 1270–1277. [Google Scholar] [CrossRef]
- Buskens, P.; Burghoorn, M.; Mourad, M.C.D.; Vroon, Z. Antireflective coatings for glass and transparent polymers. Langmuir 2016, 32, 6781–6793. [Google Scholar] [CrossRef] [PubMed]
- Truong, N.P.; Quinn, J.F.; Anastasaki, A.; Rolland, M.; Vu, M.N.; Haddleton, D.M.; Whittaker, M.R.; Davis, T.P. Surfactant-free RAFT emulsion polymerization using a novel biocompatible thermoresponsive polymer. Polym. Chem. 2017, 8, 1353–1363. [Google Scholar] [CrossRef]
- Truong, N.P.; Quinn, J.F.; Anastasaki, A.; Haddleton, D.M.; Whittaker, M.R.; Davis, T.P. Facile access to thermoresponsive filomicelles with tuneable cores. Chem. Commun. 2016, 52, 4497–4500. [Google Scholar] [CrossRef] [PubMed]
- Caruso, F.; Caruso, R.A.; Möhwald, H. Nanoengineering of inorganic and hybrid hollow spheres by colloidal templating. Science 1998, 282, 1111–1114. [Google Scholar] [CrossRef] [PubMed]
- Tissot, I.; Reymond, I.P.; Lefebvre, F.; Bourgeat-Lami, E. SiOH-functionalized polystyrene latexes. A step toward the synthesis of hollow silica nanoparticles. Chem. Mater. 2002, 14, 1325–1331. [Google Scholar] [CrossRef]
- Pyun, J.; Matyjaszewski, K. Synthesis of nanocomposite organic/inorganic hybrid materials using controlled/“living” radical polymerization. Chem. Mater. 2001, 13, 3436–3448. [Google Scholar] [CrossRef]
- Pietrasik, J.; Hui, C.M.; Chaladaj, W.; Dong, H.; Choi, J.; Jurczak, J.; Bockstaller, M.R.; Matyjaszewski, K. Silica-polymethacrylate hybrid particles synthesized using high-pressure atom transfer radical polymerization. Macromol. Rapid Commun. 2011, 32, 295–301. [Google Scholar] [CrossRef] [PubMed]
- Moraes, J.; Ohno, K.; Maschmeyer, T.; Perrier, S. Synthesis of silica–polymer core–shell nanoparticles by reversible addition–fragmentation chain transfer polymerization. Chem. Commun. 2013, 49, 9077–9088. [Google Scholar] [CrossRef] [PubMed]
- Hartono, S.B.; Phuoc, N.T.; Yu, M.; Jia, Z.; Monteiro, M.J.; Qiao, S.; Yu, C. Functionalized large pore mesoporous silica nanoparticles for gene delivery featuring controlled release and co-delivery. J. Mater. Chem. B 2014, 2, 718–726. [Google Scholar] [CrossRef] [Green Version]
- Segers, M.; van Zandvoort, R.; Sliepen, M.; Arfsten, N.; Verheijen, M.; Keul, H.; Buskens, P.; Möller, M. Facile and versatile platform approach for the synthesis of submicrometer-sized hybrid particles with programmable size, composition, and architecture comprising organosiloxanes and/or organosilsesquioxanes. Chem. Mater. 2014, 26, 5718–5724. [Google Scholar] [CrossRef]
- Segers, M.; Arfsten, N.; Buskens, P.; Möller, M. A facile route for the synthesis of sub-micron sized hollow and multiporous organosilica spheres. RSC Adv. 2014, 4, 20673–20676. [Google Scholar] [CrossRef]
- Segers, M.; Sliepen, M.; Kraft, D.J.; Möller, M.; Buskens, P. Synthesis of sub-micron sized hollow, and nanoporous phenylsiloxane spheres through use of phenyltrimethoxysilane as surfmer: Insights into the surfactant and factors influencing the particle architecture. Colloids Surf. Physicochem. Eng. Asp. 2016, 497, 378–384. [Google Scholar] [CrossRef]
- Mann, D.; Voogt, S.; van Zandvoort, R.; Keul, H.; Möller, M.; Verheijen, M.; Nascimento-Duplat, D.; Xu, M.; Urbach, H.P.; Adam, A.J.L.; et al. Protecting patches in colloidal synthesis of Au semishells. Chem. Commun. 2017, 53, 3898–3901. [Google Scholar] [CrossRef] [PubMed]
- Mann, D.; Voogt, S.; Keul, H.; Möller, M.; Verheijen, M.; Buskens, P. Synthesis of polystyrene-polyphenylsiloxane Janus particles through colloidal assembly with unexpected high selectivity: Mechanistic insights and their application in the design of polystyrene particles with multiple polyphenylsiloxane patches. Polymers 2017, 9, 475. [Google Scholar] [CrossRef]
- Mann, D.; Chattopadhyay, S.; Pargen, S.; Verheijen, M.; Keul, H.; Buskens, P.; Möller, M. Glucose-functionalized polystyrene particles designed for selective deposition of silver on the surface. RSC Adv. 2014, 4, 62878–62881. [Google Scholar] [CrossRef]
- Mann, D.; Nascimento-Duplat, D.; Keul, H.; Möller, M.; Verheijen, M.; Xu, M.; Urbach, H.P.; Adam, A.J.L.; Buskens, P. The influence of particle size distribution and shell imperfections on the plasmon resonance of Au and Ag nanoshells. Plasmonics 2017, 12, 929–945. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.; Liu, L.; Ouyang, S.; Xu, H.; Wang, D.; Zhao, N.; Ye, J. Nanometals for solar-to-chemical energy conversion: From semiconductor-based photocatalysis to plasmon-mediated photocatalysis and photo-thermocatalysis. Adv. Mater. 2016, 28, 6781–6803. [Google Scholar] [CrossRef] [PubMed]
- Baffou, G.; Quidant, R. Nanoplasmonics for chemistry. Chem. Soc. Rev. 2014, 43, 3898–3907. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.-J.; Cabello, G.; Wu, D.-Y.; Tian, Z.-Q. Surface-enhanced Raman spectroscopy toward application in plasmonic photocatalysis on metal nanostructures. J. Photochem. Photobiol. C Photochem. Rev. 2014, 21, 54–80. [Google Scholar] [CrossRef]
- Cialla, D.; März, A.; Böhme, R.; Theil, F.; Weber, K.; Schmitt, M.; Popp, J. Surface-enhanced Raman spectroscopy (SERS): Progress and trends. Anal. Bioanal. Chem. 2012, 403, 27–54. [Google Scholar] [CrossRef] [PubMed]
- Boisselier, E.; Astruc, D. Gold nanoparticles in nanomedicine: Preparations, imaging, diagnostics, therapies and toxicity. Chem. Soc. Rev. 2009, 38, 1759–1782. [Google Scholar] [CrossRef] [PubMed]
- Hirsch, L.R.; Stafford, R.J.; Bankson, J.A.; Sershen, S.R.; Rivera, B.; Price, R.E.; Hazle, J.D.; Halas, N.J.; West, J.L. Nanoshell-mediated near-infrared thermal therapy of tumors under magnetic resonance guidance. Proc. Natl. Acad. Sci. USA 2003, 100, 13549–13554. [Google Scholar] [CrossRef] [PubMed]
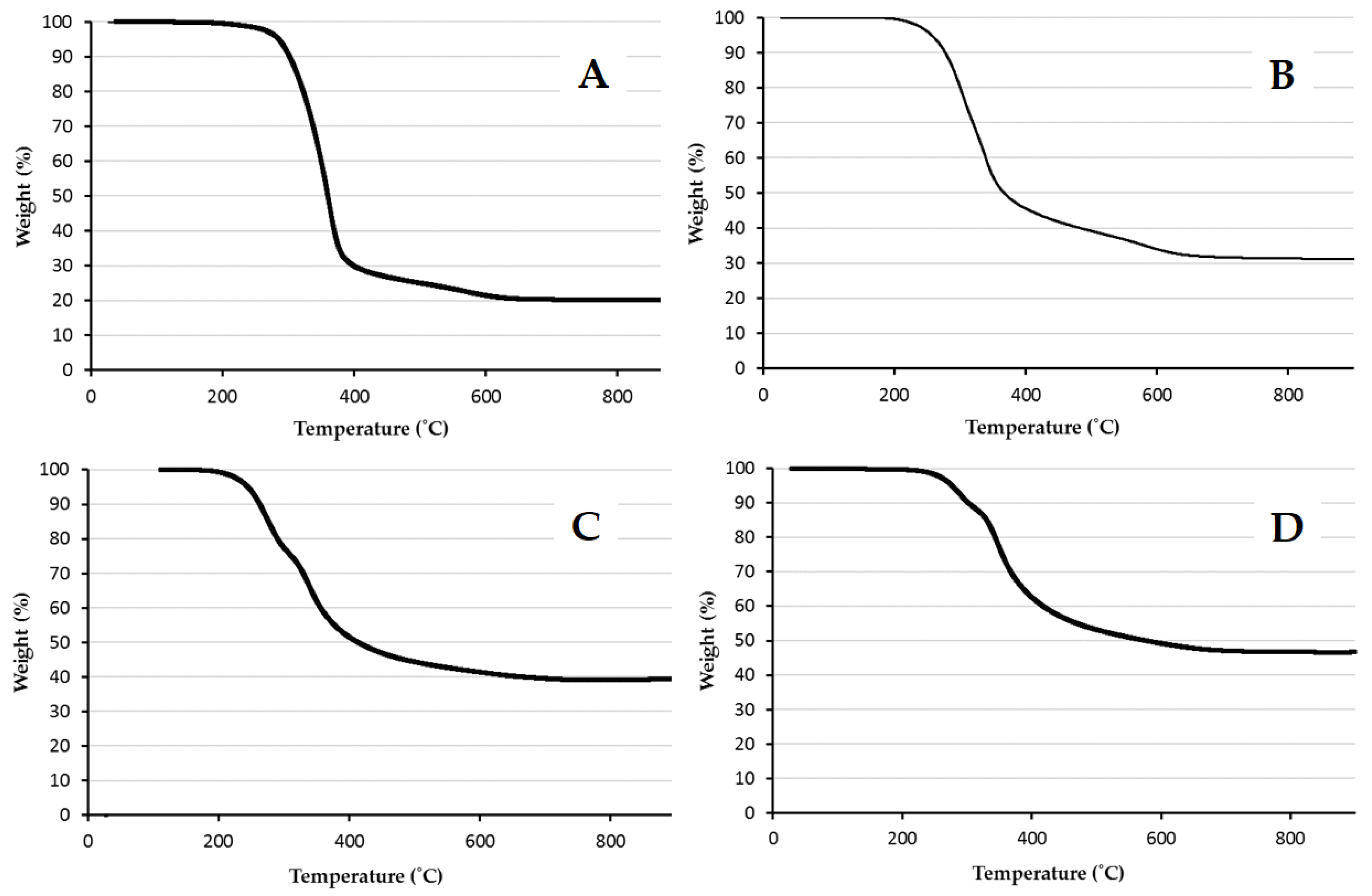

| Ratio MPTMS:Styrene | Theoretical wt % Silica | Experimental wt % Silica |
|---|---|---|
| 20:80 | 7.49 | 19.7 |
| 50:50 | 20.3 | 32.7 |
| 80:20 | 35.5 | 39.0 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Segers, M.; Vermeer, I.; Möller, M.; Verheijen, M.; Buskens, P. Synthesis and Characterization of Hybrid Particles Obtained in a One-Pot Process through Simultaneous Sol-Gel Reaction of (3-Mercaptopropyl)trimethoxysilane and Emulsion Polymerization of Styrene. Colloids Interfaces 2017, 1, 7. https://doi.org/10.3390/colloids1010007
Segers M, Vermeer I, Möller M, Verheijen M, Buskens P. Synthesis and Characterization of Hybrid Particles Obtained in a One-Pot Process through Simultaneous Sol-Gel Reaction of (3-Mercaptopropyl)trimethoxysilane and Emulsion Polymerization of Styrene. Colloids and Interfaces. 2017; 1(1):7. https://doi.org/10.3390/colloids1010007
Chicago/Turabian StyleSegers, Margot, Isabel Vermeer, Martin Möller, Marcel Verheijen, and Pascal Buskens. 2017. "Synthesis and Characterization of Hybrid Particles Obtained in a One-Pot Process through Simultaneous Sol-Gel Reaction of (3-Mercaptopropyl)trimethoxysilane and Emulsion Polymerization of Styrene" Colloids and Interfaces 1, no. 1: 7. https://doi.org/10.3390/colloids1010007




