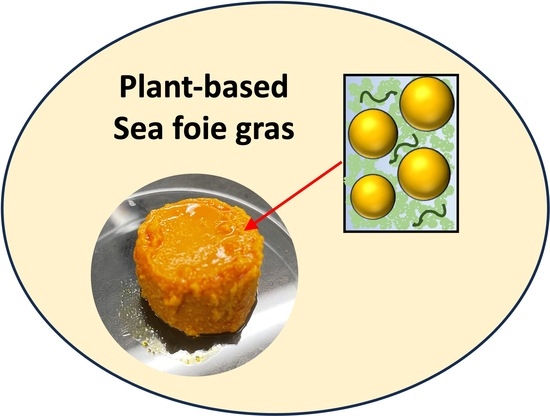
Creation of Next-Generation Plant-Based Seafood Using Emulsion Gel Technology: Omega-3-Enriched Sea Foie Gras Analogs
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Emulsion Preparation
2.2.2. Emulsion Gel Preparation
- Method 1 (fine emulsion + free oil): An oil-in-water emulsion containing 10% RuBisCO protein and 20% flaxseed oil fine emulsion was prepared, and then, colored flaxseed oil was added just before heating to achieve a final flaxseed oil content of 40% w/w.
- Method 2 (coarse emulsion): An oil-in-water emulsion containing 40% w/w colored flaxseed oil and 10% RuBisCO solution was prepared by blending for 2 min using a high-shear mixer.
2.2.3. Emulsion Droplet Characterization
2.2.4. Confocal Laser Scanning Microscopy
2.2.5. Dynamic Shear Rheology Analysis
2.2.6. Texture Profile Analysis
2.2.7. Color Analysis
2.2.8. Scanning Electron Microscopy
2.2.9. Statistical Analysis
3. Results and Discussion
3.1. Preparation and Characterization of Emulsions
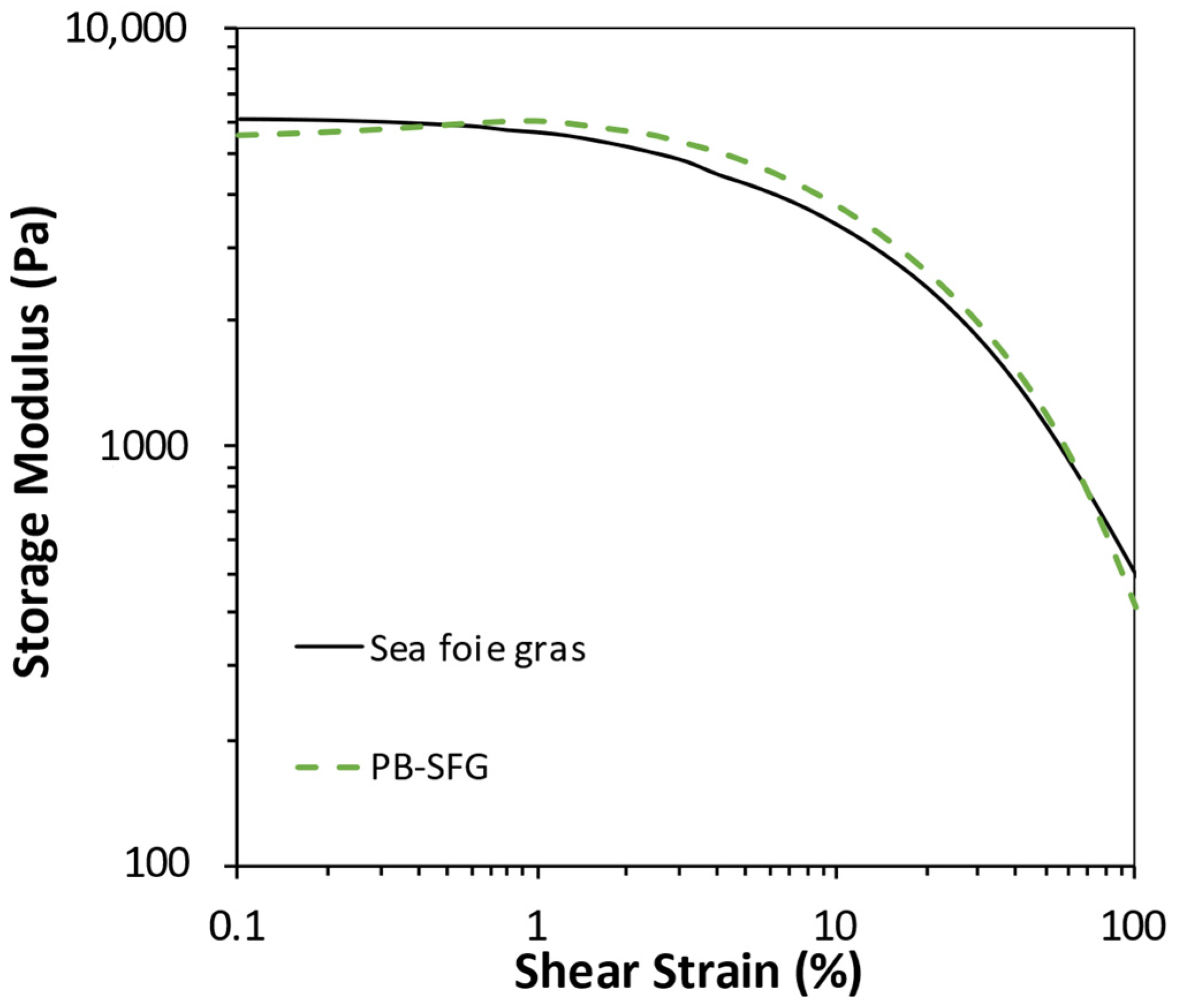
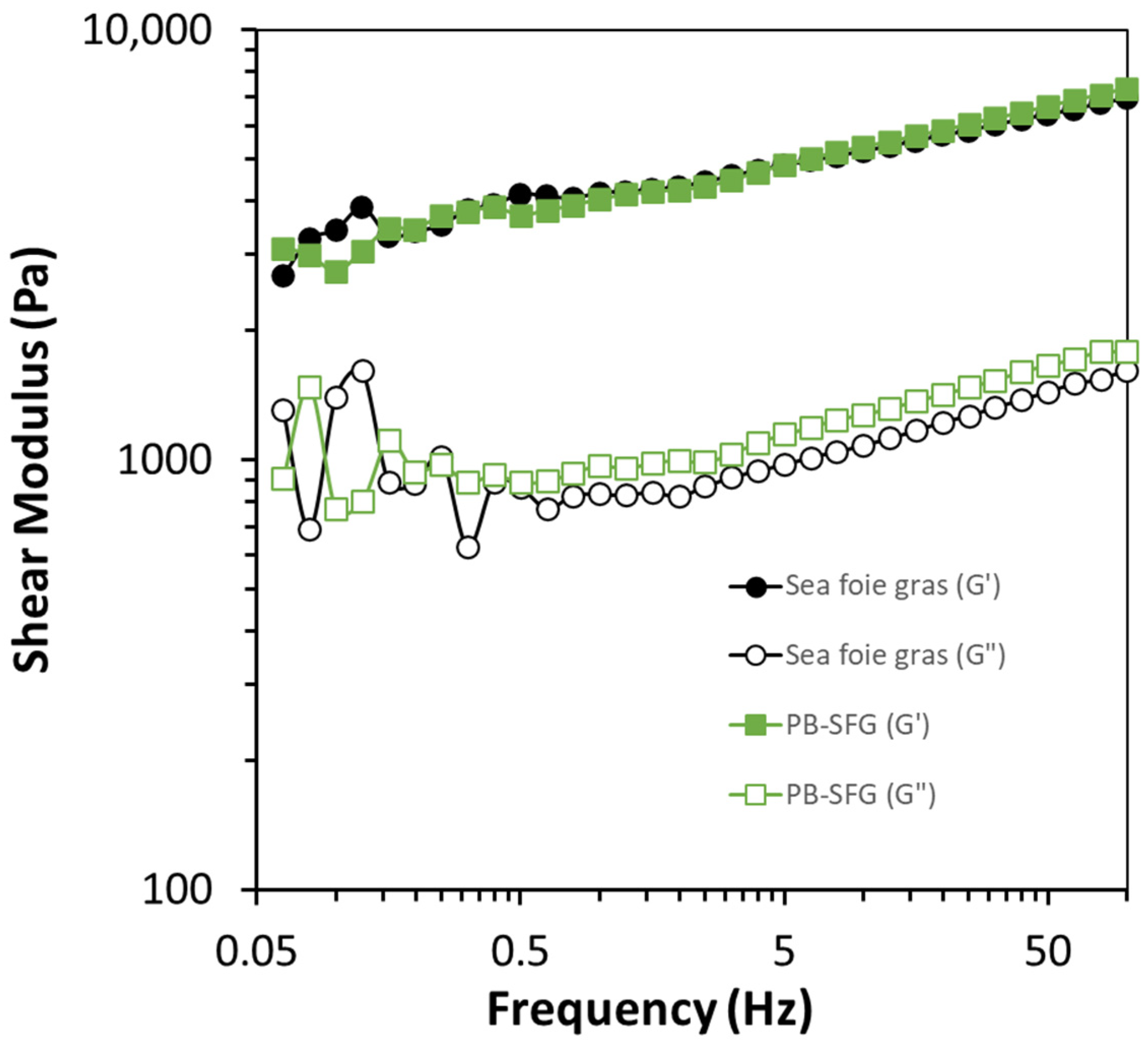
3.2. Rheological Analysis
3.2.1. Thermal Gelation Properties
3.2.2. Strain and Frequency Sweep Analysis
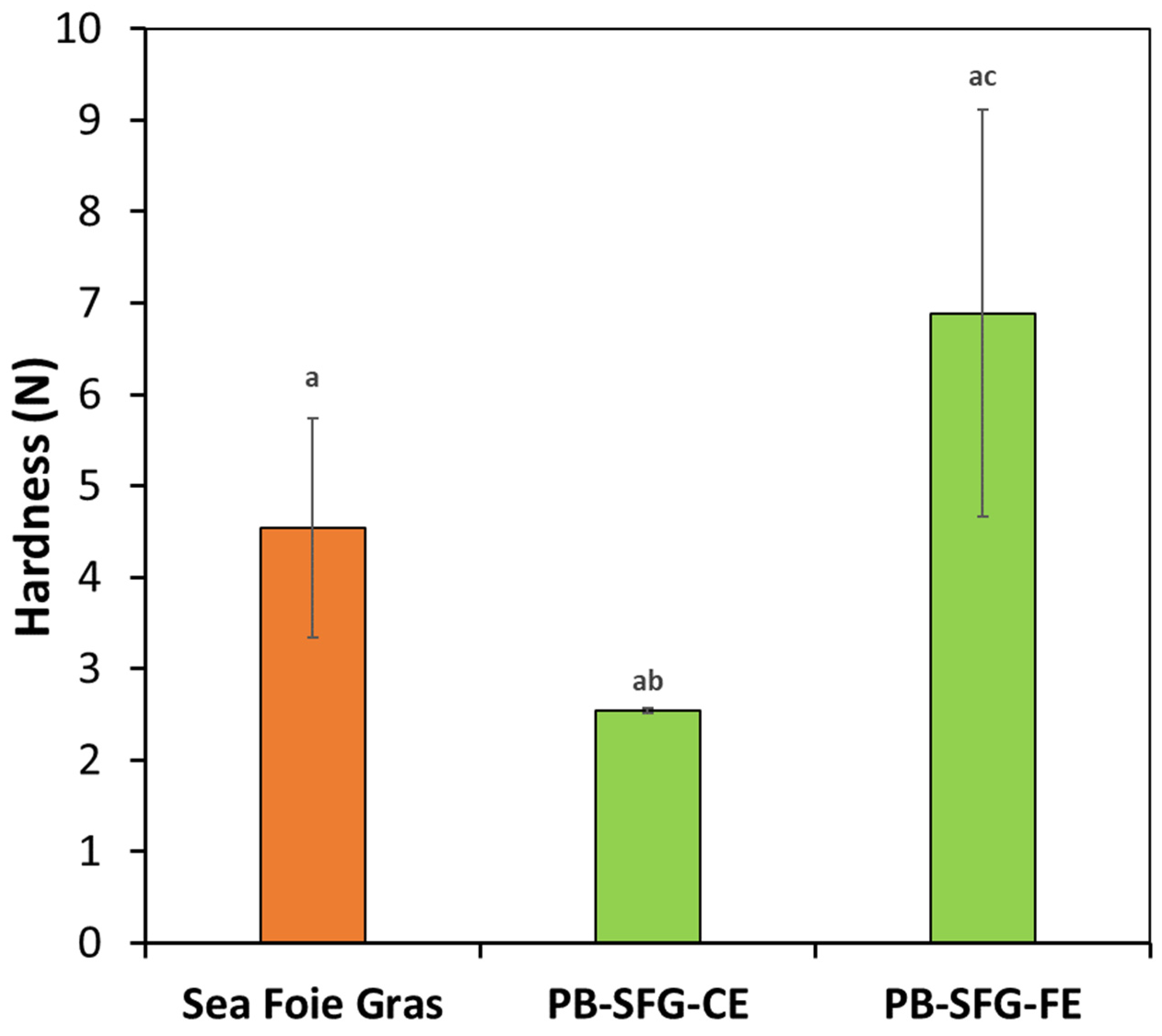
3.3. Textural Characteristics of Real and Plant-Based Sea Foie Gras
3.3.1. Impact of Heating Conditions
3.3.2. Impact of Droplet Size
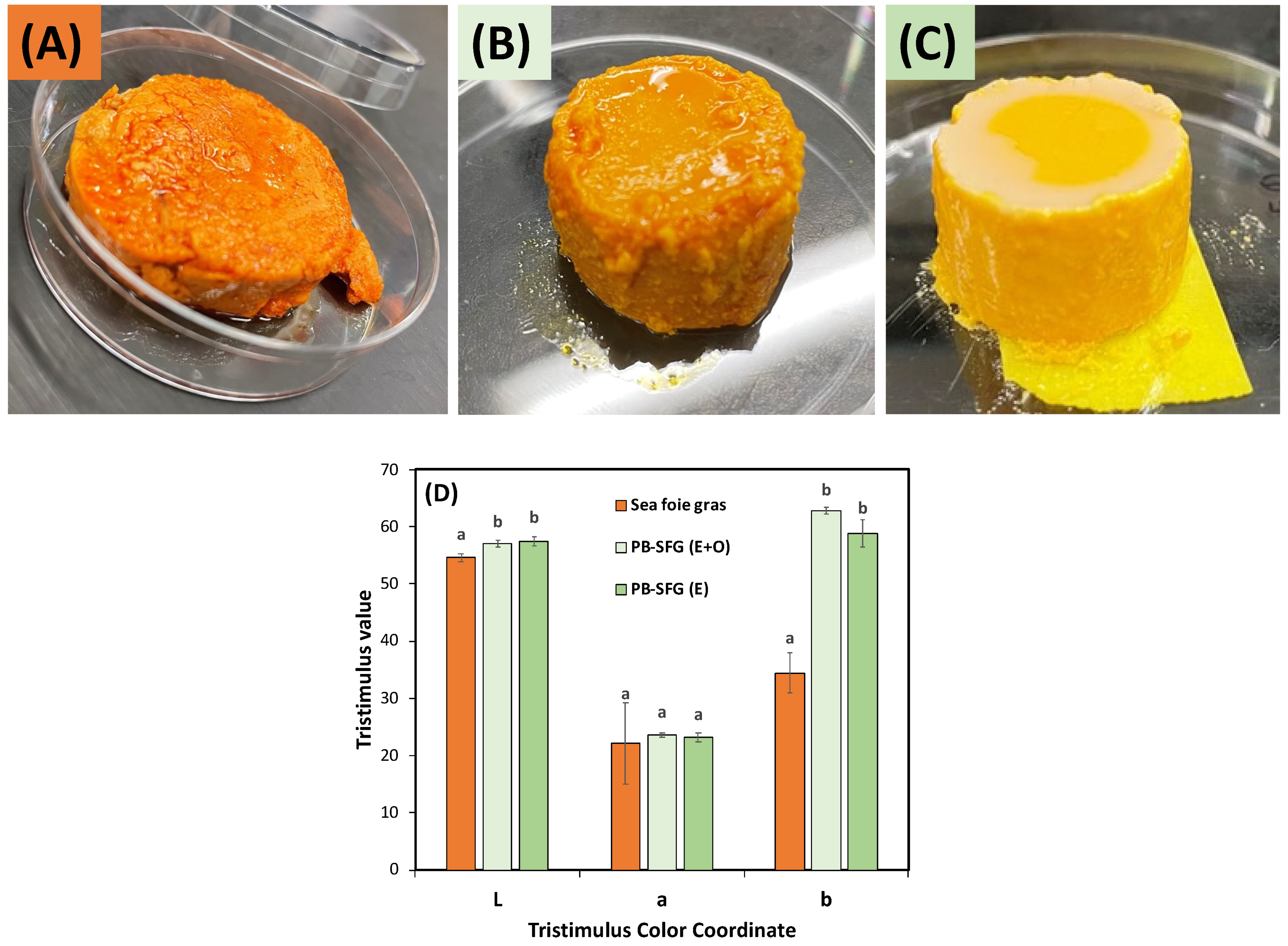
3.4. Matching the Appearance of Sea Foie Gras
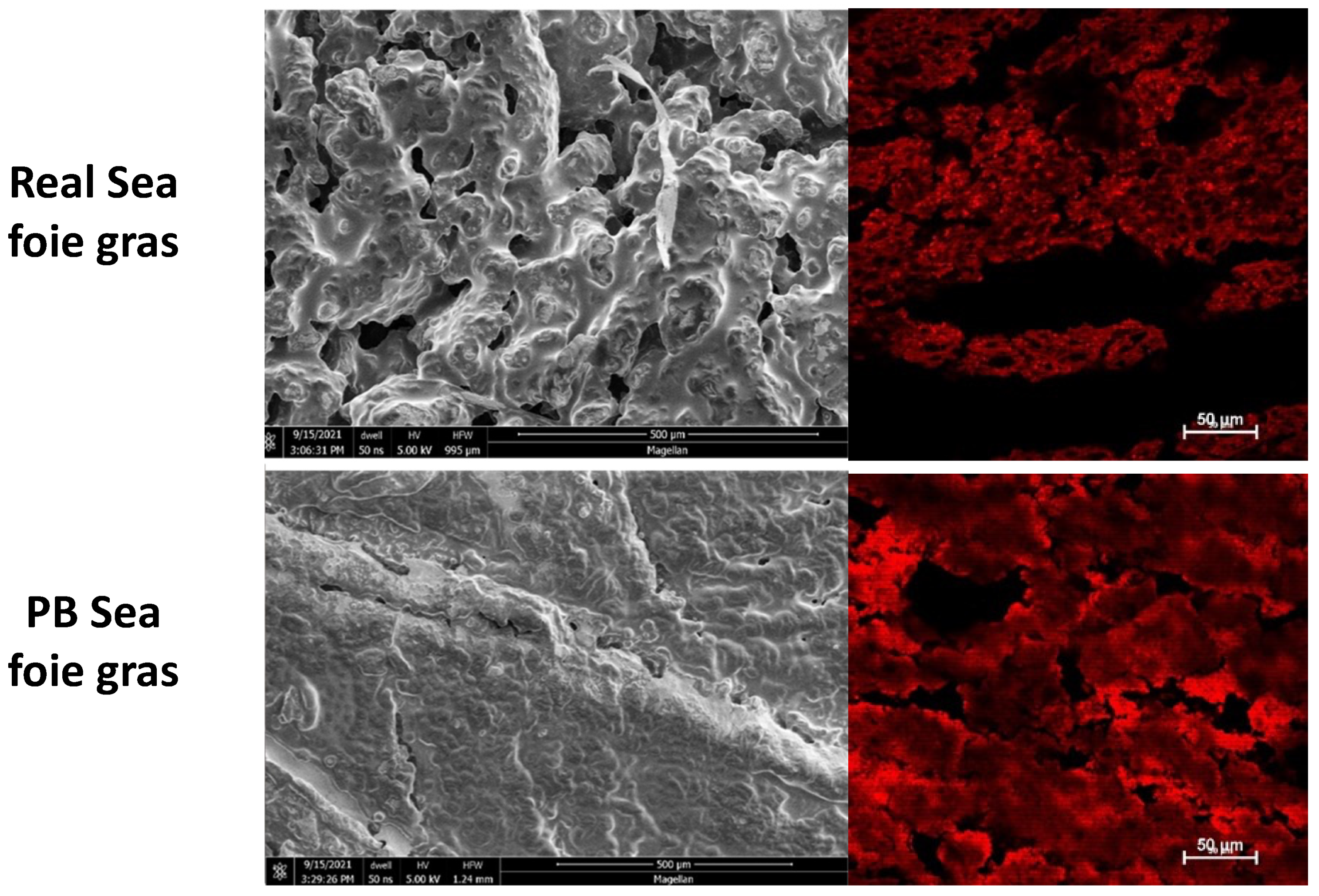
3.5. Microstructure Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Poore, J.; Nemecek, T. Reducing food’s environmental impacts through producers and consumers. Science 2018, 360, 987–992. [Google Scholar] [CrossRef]
- Willett, W.; Rockstrom, J.; Loken, B.; Springmann, M.; Lang, T.; Vermeulen, S.; Garnett, T.; Tilman, D.; DeClerck, F.; Wood, A.; et al. Food in the Anthropocene: The EAT-Lancet Commission on healthy diets from sustainable food systems. Lancet 2019, 393, 447–492. [Google Scholar] [CrossRef]
- Costello, C.; Cao, L.; Gelcich, S.; Cisneros-Mata, M.A.; Free, C.M.; Froehlich, H.E.; Golden, C.D.; Ishimura, G.; Maier, J.; Macadam-Somer, I.; et al. The future of food from the sea. Nature 2020, 588, 95–100. [Google Scholar] [CrossRef]
- FAO. Impacts of Climate Change on Fisheries and Aquaculture: Synthesis of Current Knowledge, Adaptation and Mitigation Options; Food and Agriculture Organization of the United Nations: Rome, Italy, 2018. [Google Scholar]
- Sumaila, U.R.; Tai, T.C. End Overfishing and Increase the Resilience of the Ocean to Climate Change. Front. Mar. Sci. 2020, 7, 523. [Google Scholar] [CrossRef]
- FAO. The State of World Fisheries and Aquaculture 2020. Sustainability in Action; Food and Agriculture Organization of the United Nations: Rome, Italy, 2020. [Google Scholar]
- Martin, A.H.; Ferrer, E.M.; Hunt, C.A.; Bleeker, K.; Villasante, S. Exploring Changes in Fishery Emissions and Organic Carbon Impacts Associated With a Recovering Stock. Front. Mar. Sci. 2022, 9, 788339. [Google Scholar] [CrossRef]
- Chiocchetti, G.; Jadan-Piedra, C.; Velez, D.; Devesa, V. Metal(loid) contamination in seafood products. Crit. Rev. Food Sci. Nutr. 2017, 57, 3715–3728. [Google Scholar] [CrossRef] [PubMed]
- Jinadasa, B.; Jayasinghe, G.; Pohl, P.; Fowler, S.W. Mitigating the impact of mercury contaminants in fish and other seafood-A review. Mar. Pollut. Bull. 2021, 171, 112710. [Google Scholar] [CrossRef]
- Fu, L.L.; Wang, C.; Zhu, Y.; Wang, Y.B. Seafood allergy: Occurrence, mechanisms and measures. Trends Food Sci. Technol. 2019, 88, 80–92. [Google Scholar] [CrossRef]
- Ruethers, T.; Taki, A.C.; Johnston, E.B.; Nugraha, R.; Le, T.T.K.; Kalic, T.; McLean, T.R.; Kamath, S.D.; Lopata, A.L. Seafood allergy: A comprehensive review of fish and shellfish allergens. Mol. Immunol. 2018, 100, 28–57. [Google Scholar] [CrossRef]
- Kazir, M.; Livney, Y.D. Plant-Based Seafood Analogs. Molecules 2021, 26, 1559. [Google Scholar] [CrossRef] [PubMed]
- Dekkers, B.L.; Boom, R.M.; van der Goot, A.J. Structuring processes for meat analogues. Trends Food Sci. Technol. 2018, 81, 25–36. [Google Scholar] [CrossRef]
- McClements, D.J.; Grossmann, L. A brief review of the science behind the design of healthy and sustainable plant-based foods. Npj Sci. Food 2021, 5, 17. [Google Scholar] [CrossRef] [PubMed]
- Kyriakopoulou, K.; Keppler, J.K.; van der Goot, A.J. Functionality of Ingredients and Additives in Plant-Based Meat Analogues. Foods 2021, 10, 600. [Google Scholar] [CrossRef] [PubMed]
- Mattice, K.D.; Marangoni, A.G. Evaluating the use of zein in structuring plant-based products. Curr. Res. Food Sci. 2020, 3, 59–66. [Google Scholar] [CrossRef] [PubMed]
- Aguilera-Oviedo, J.; Yara-Varon, E.; Torres, M.; Canela-Garayoa, R.; Balcells, M. Sustainable Synthesis of Omega-3 Fatty Acid Ethyl Esters from Monkfish Liver Oil. Catalysts 2021, 11, 100. [Google Scholar] [CrossRef]
- Xu, J.J.; Li, Y.Y.; Regenstein, J.; Su, X.R. In vitro and in vivo anti-oxidation and anti-fatigue effect of monkfish liver hydrolysate. Food Biosci. 2017, 18, 9–14. [Google Scholar] [CrossRef]
- Zhu, Y.-C.; Lou, Y.-J.; Xiong, G.-T.; Liu, J.; Liu, T.; Lou, Y. Composition analysis and evaluation of Goosefish liver nutrition. Sci. Technol. Food Ind. 2017, 38, 356–360. [Google Scholar] [CrossRef]
- Dickinson, E. Emulsion gels: The structuring of soft solids with protein-stabilized oil droplets. Food Hydrocoll. 2012, 28, 224–241. [Google Scholar] [CrossRef]
- Farjami, T.; Madadlou, A. An overview on preparation of emulsion-filled gels and emulsion particulate gels. Trends Food Sci. Technol. 2019, 86, 85–94. [Google Scholar] [CrossRef]
- Martin, A.H.; Nieuwland, M.; de Jong, G.A.H. Characterization of Heat-Set Gels from RuBisCO in Comparison to Those from Other Proteins. J. Agric. Food Chem. 2014, 62, 10783–10791. [Google Scholar] [CrossRef]
- Di Stefano, E.; Agyei, D.; Njoku, E.N.; Udenigwe, C.C. Plant RuBisCo: An Underutilized Protein for Food Applications. J. Am. Oil Chem. Soc. 2018, 95, 1063–1074. [Google Scholar] [CrossRef]
- Tan, Y.B.; Lee, P.W.; Martens, T.D.; McClements, D.J. Comparison of Emulsifying Properties of Plant and Animal Proteins in Oil-in-Water Emulsions: Whey, Soy, and RuBisCo Proteins. Food Biophys. 2022, 17, 409–421. [Google Scholar] [CrossRef]
- Kouame, K.; Bora, A.F.M.; Li, X.D.; Sun, Y.; Liu, L. Novel trends and opportunities for microencapsulation of flaxseed oil in foods: A review. J. Funct. Foods 2021, 87, 104812. [Google Scholar] [CrossRef]
- Punia, S.; Sandhu, K.S.; Siroha, A.K.; Dhull, S.B. Omega 3-metabolism, absorption, bioavailability and health benefits-A review. Pharmanutrition 2019, 10, 100162. [Google Scholar] [CrossRef]
- Wang, X.T.; Yu, K.; Cheng, C.; Peng, D.F.; Yu, X.; Chen, H.J.; Chen, Y.S.; McClements, D.J.; Deng, Q.C. Effect of sesamol on the physical and chemical stability of plant-based flaxseed oil-in-water emulsions stabilized by proteins or phospholipids. Food Funct. 2021, 12, 2090–2101. [Google Scholar] [CrossRef] [PubMed]
- Gill, G.W.; Frost, J.K.; Miller, K.A. A new formula for a half-oxidized hematoxylin solution that neither overstains nor requires differentiation. Acta Cytol. 1974, 18, 300–301. [Google Scholar]
- Bohlin, L. A theory of flow as a cooperative phenomenon. J. Colloid Interface Sci. 1980, 74, 423–434. [Google Scholar] [CrossRef]
- McClements, D.J. Food Emulsions: Principles, Practice, and Techniques; CRC Press: Boca Raton, FL, USA, 2015. [Google Scholar]
- Hakansson, A. Emulsion Formation by Homogenization: Current Understanding and Future Perspectives. Annu. Rev. Food Sci. Technol. 2019, 10, 239–258. [Google Scholar] [CrossRef]
- Jafari, S.M.; Assadpoor, E.; He, Y.H.; Bhandari, B. Re-coalescence of emulsion droplets during high-energy emulsification. Food Hydrocoll. 2008, 22, 1191–1202. [Google Scholar] [CrossRef]
- Dickinson, E.; Chen, J.S. Heat-set whey protein emulsion gels: Role of active and inactive filler particles. J. Dispers. Sci. Technol. 1999, 20, 197–213. [Google Scholar] [CrossRef]
- McClements, D.J.; Monahan, F.J.; Kinsella, J.E. Effect of emulsion droplets on the rheology of whey-protein isolate gels. J. Texture Stud. 1993, 24, 411–422. [Google Scholar] [CrossRef]
- Rao, M.A. Rheology of Fluid, Semisolid, and Solid Foods: Principles and Applications, 3rd ed.; Springer Science: New York, NY, USA, 2013. [Google Scholar]







| 10% w/w (Fine) | 20% w/w (Fine) | 40% w/w (Fine) | 40% w/w (Fine-Heat) | 40% w/w (Course) | |
|---|---|---|---|---|---|
| Mean Diameter (nm) | 255.5 ± 2.5 | 282.8 ± 0.1 | 1860 ± 60 | 1810 ± 18 | 8650 |
| Polydispersity Index | 0.230 ± 0.036 | 0.251 ± 0.017 | 3.00 ± 0.11 | 0.291 ± 0.043 | - |
| ζ-Potential (mV) | −47.1 ± 4.6 | −55.6 ± 0.9 | −50.1 ± 6.8 | −42.9 ± 1.6 | −53.8 ± 3.4 |
| Sample | 1/z | z | Log(A) | A [kPa] | R2 |
|---|---|---|---|---|---|
| Sea Foie Gras | 0.118 | 8.46 | 3.62 | 4.16 | 0.939 |
| PB Sea Foie Gras | 0.127 | 7.87 | 4.30 | 19.9 | 0.955 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kobata, K.; Zhang, Z.; McClements, D.J. Creation of Next-Generation Plant-Based Seafood Using Emulsion Gel Technology: Omega-3-Enriched Sea Foie Gras Analogs. Colloids Interfaces 2023, 7, 65. https://doi.org/10.3390/colloids7040065
Kobata K, Zhang Z, McClements DJ. Creation of Next-Generation Plant-Based Seafood Using Emulsion Gel Technology: Omega-3-Enriched Sea Foie Gras Analogs. Colloids and Interfaces. 2023; 7(4):65. https://doi.org/10.3390/colloids7040065
Chicago/Turabian StyleKobata, Kanon, Zhiyun Zhang, and David Julian McClements. 2023. "Creation of Next-Generation Plant-Based Seafood Using Emulsion Gel Technology: Omega-3-Enriched Sea Foie Gras Analogs" Colloids and Interfaces 7, no. 4: 65. https://doi.org/10.3390/colloids7040065