Cold Atmospheric Plasma Medicine: Applications, Challenges, and Opportunities for Predictive Control
Abstract
:1. Background
1.1. History and Current State of Plasma Medicine
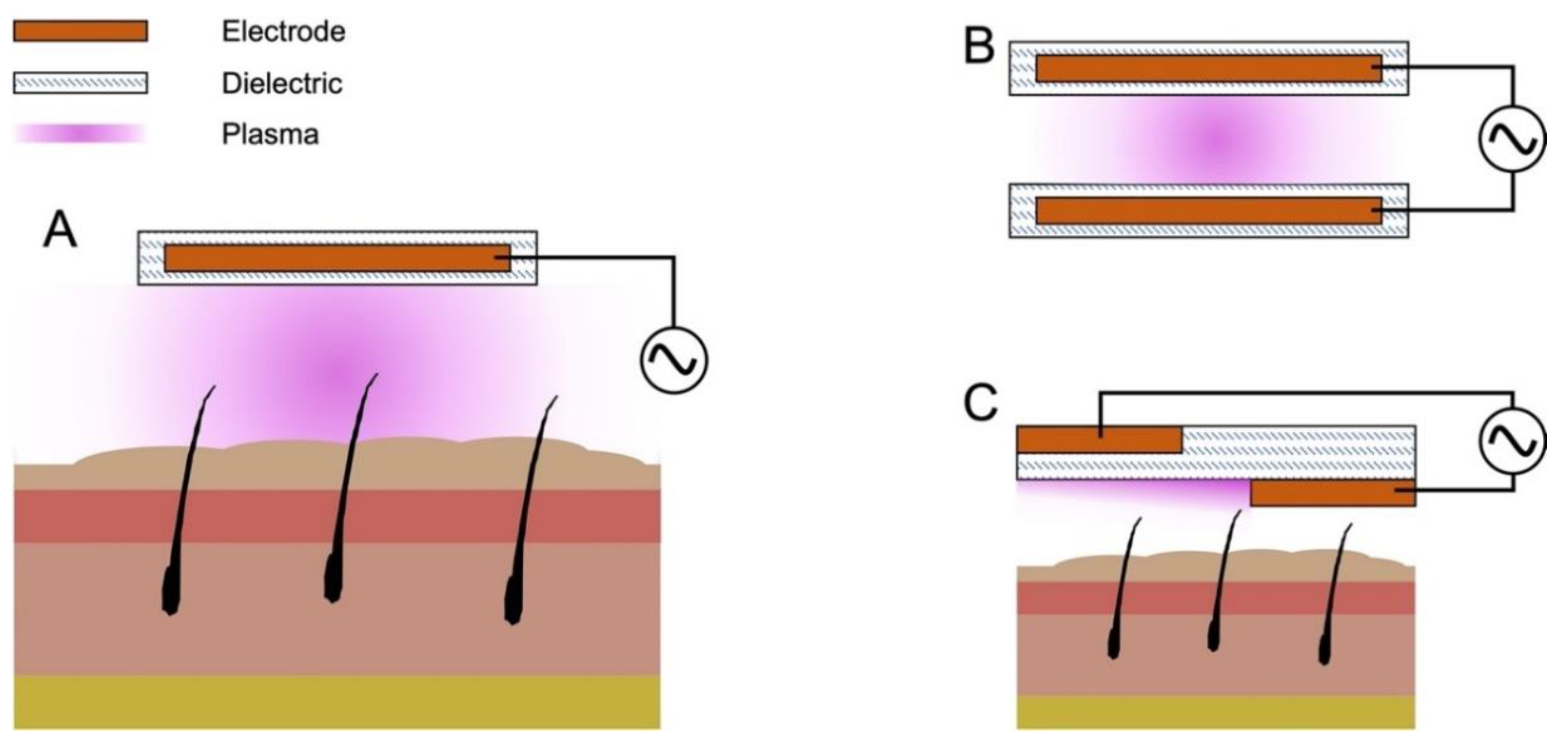
1.2. Common Discharge Systems for Cold Atmospheric Pressure Plasma
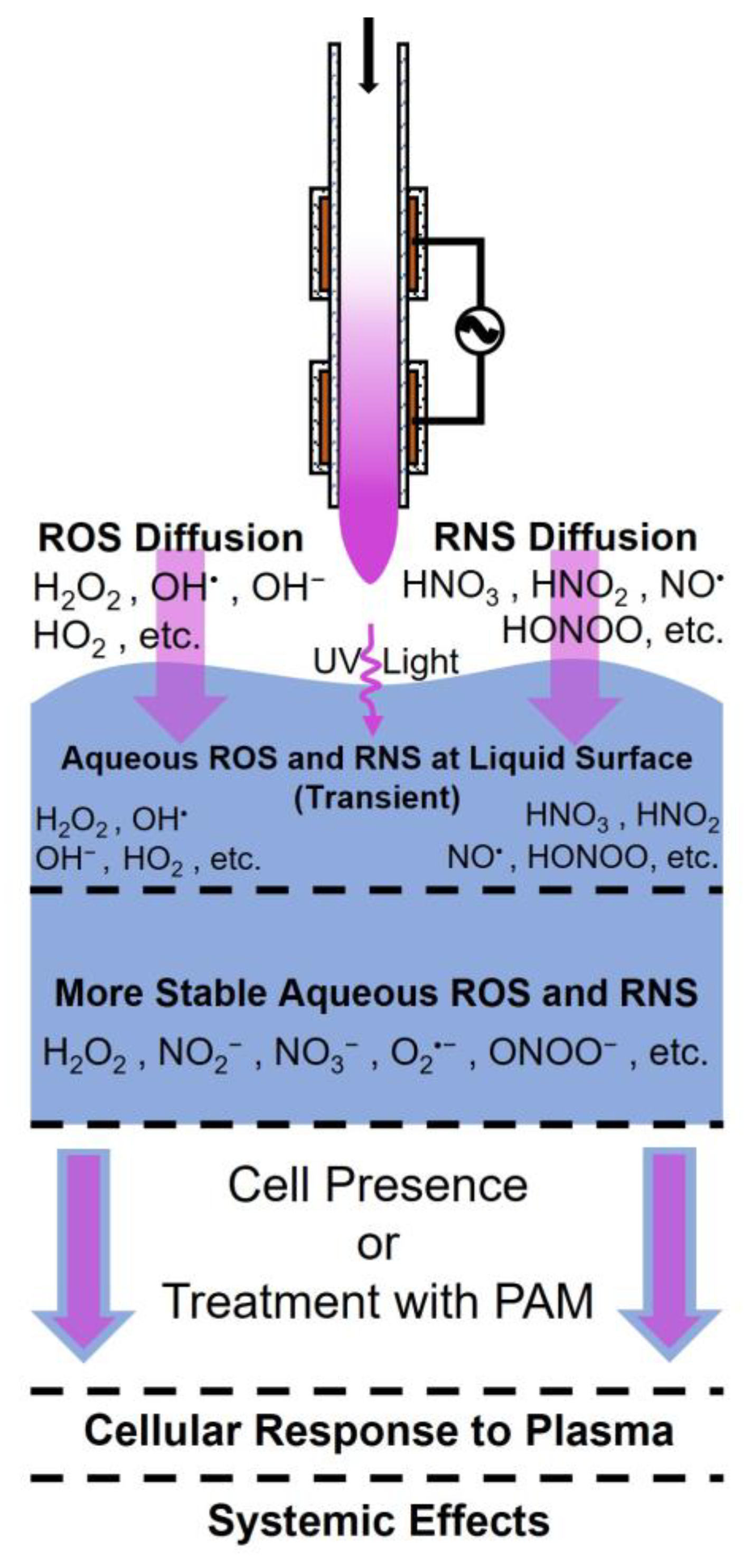
1.3. The Main Effectors of CAP Treatment
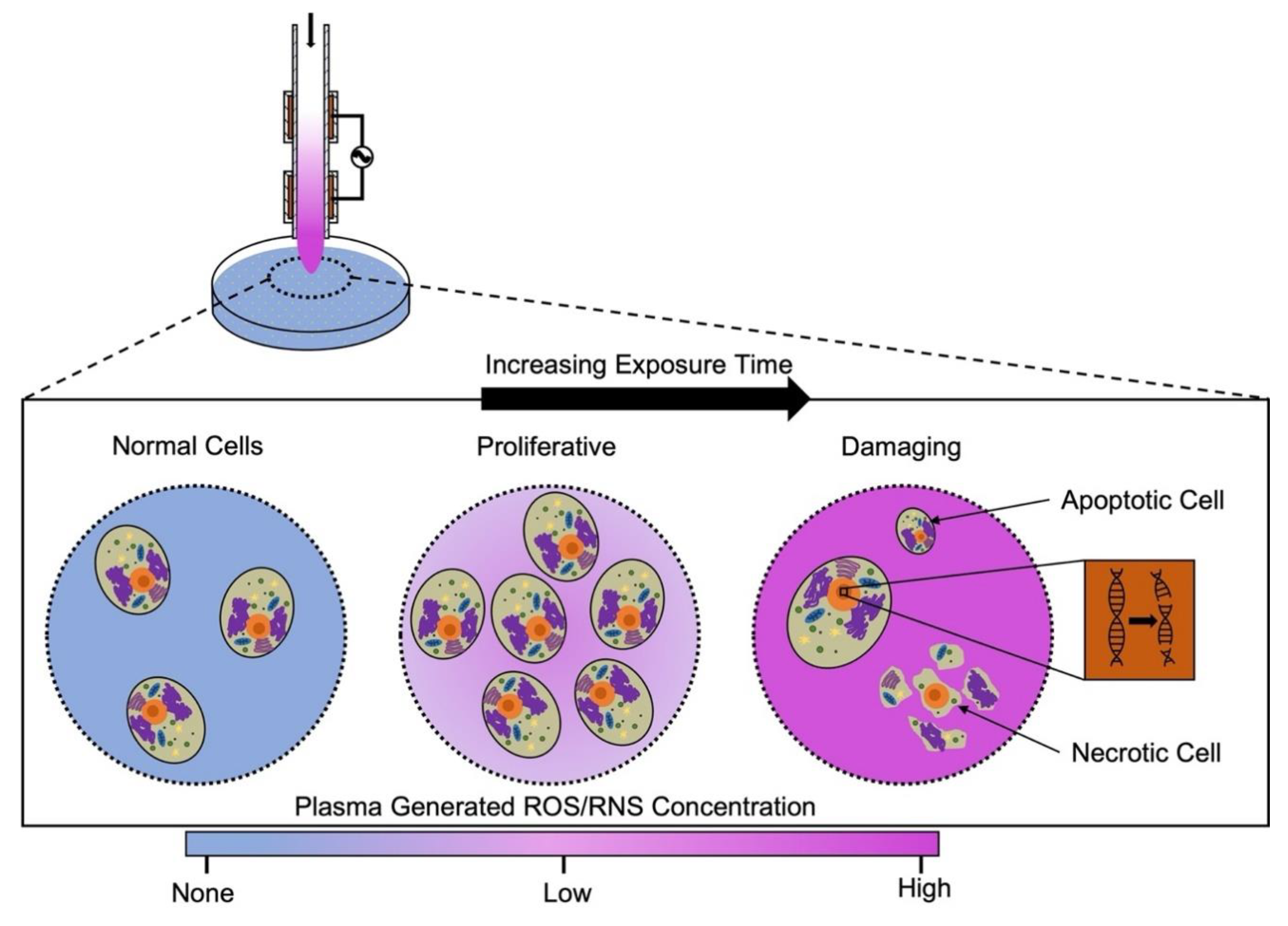
1.4. A Unified Definition of Plasma Dose Is Essential
2. CAP and Dermatology
2.1. Wound Healing
2.2. Sterilization
3. CAP and Oncology
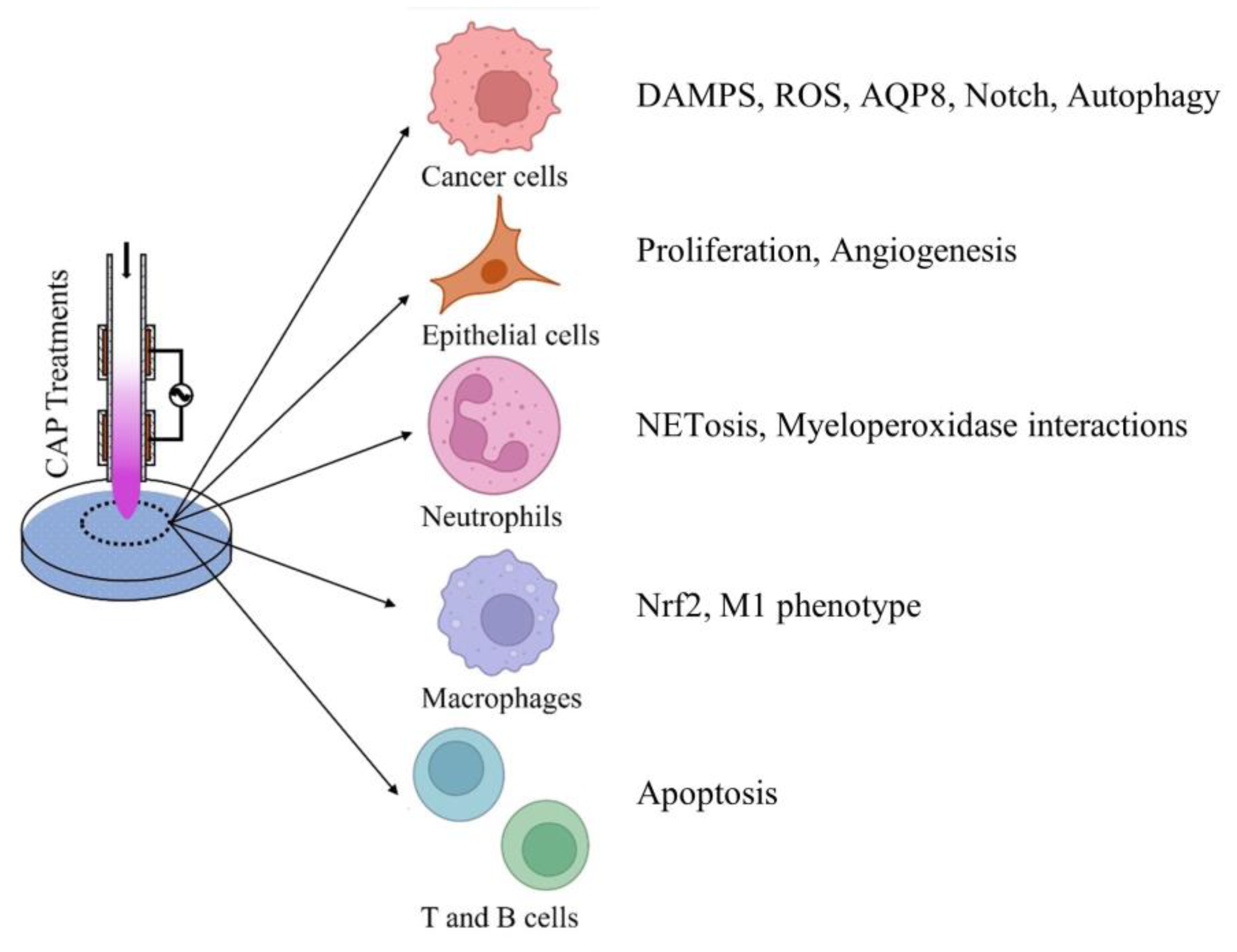
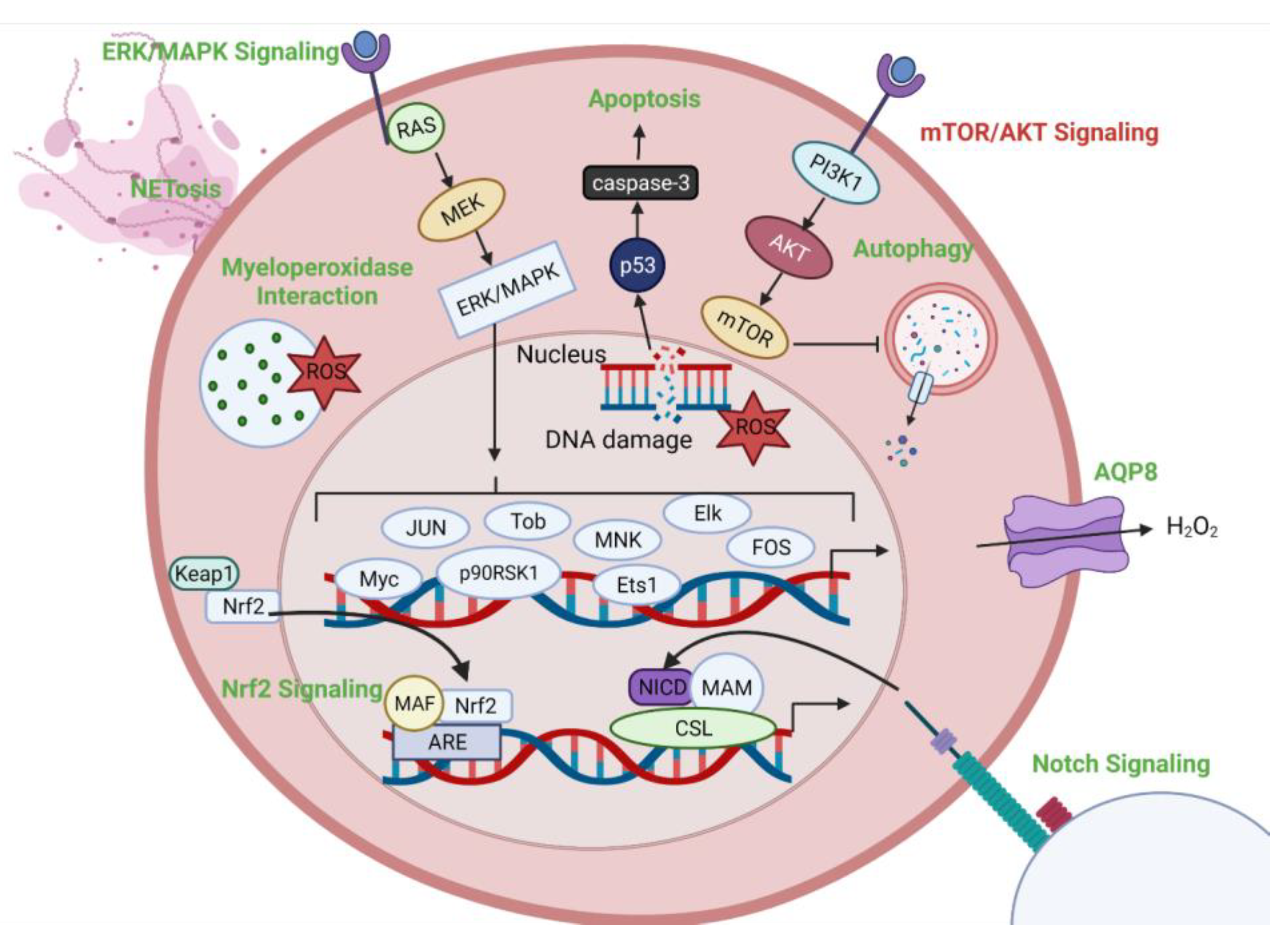
4. Intracellular Targets of CAP
5. Conclusions and Future Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Liley, B.S.; Kelley, M.C.; Potter, S. Plasma|Physics, State of Matter, & Facts|Britannica. Britannica. Available online: https://www.britannica.com/science/plasma-state-of-matter (accessed on 28 December 2023).
- Plasma—Universe Today. Available online: https://www.universetoday.com/84361/plasma/ (accessed on 3 December 2023).
- Kanarik, K.J. Inside the mysterious world of plasma: A process engineer’s perspective. J. Vac. Sci. Technol. A Vac. Surf. Film. 2020, 38, 31004. [Google Scholar] [CrossRef]
- Zhu, X.; Li, G.; Mo, J.; Ding, S. Electrical discharge machining of semiconductor materials: A review. J. Mater. Res. Technol. 2023, 25, 4354–4379. [Google Scholar] [CrossRef]
- Bigot, B. Progress toward ITER’s First Plasma. Nucl. Fusion. 2019, 59, 112001. [Google Scholar] [CrossRef]
- Kikuchi, M. A Review of Fusion and Tokamak Research Towards Steady-State Operation: A JAEA Contribution. Energies 2010, 3, 1741–1789. [Google Scholar] [CrossRef]
- Samukawa, S.; Hori, M.; Rauf, S.; Tachibana, K.; Bruggeman, P.; Kroesen, G.; Whitehead, J.C.; Murphy, A.B.; Gut-sol, A.F.; Starikovskaia, S.; et al. The 2012 Plasma Roadma. J. Phys. D Appl. Phys. 2012, 45, 253001. [Google Scholar] [CrossRef]
- Andreussi, T.; Ferrato, E.; Giannetti, V. A review of air-breathing electric propulsion: From mission studies to technology verification. J. Electr. Propuls. 2022, 1, 1–57. [Google Scholar] [CrossRef]
- Nicol, M.K.J.; Brubaker, T.R.; Honish, B.J.; Simmons, A.N.; Kazemi, A.; Geissel, M.A.; Whalen, C.T.; Siedlecki, C.A.; Bilén, S.G.; Knecht, S.D.; et al. Antibacterial effects of low-temperature plasma generated by atmospheric-pressure plasma jet are mediated by reactive oxygen species. Sci. Rep. 2020, 10, 1–11. [Google Scholar] [CrossRef]
- Von Woedtke, T.; Schmidt, A.; Bekeschus, S.; Wende, K.; Weltmann, K.D. Plasma Medicine: A Field of Applied Redox Biology. In Vivo 2019, 33, 1011–1026. [Google Scholar] [CrossRef]
- Dharini, M.; Jaspin, S.; Mahendran, R. Cold plasma reactive species: Generation, properties, and interaction with food biomolecules. Food Chem. 2023, 405, 134746. [Google Scholar] [CrossRef]
- Yan, D.; Cui, H.; Zhu, W.; Talbot, A.; Zhang, L.G.; Sherman, J.H.; Keidar, M. The Strong Cell-based Hydrogen Peroxide Generation Triggered by Cold Atmospheric Plasma. Sci. Rep. 2017, 7, 1–9. [Google Scholar] [CrossRef]
- Lata, S.; Chakravorty, S.; Mitra, T.; Pradhan, P.K.; Mohanty, S.; Patel, P.; Jha, E.; Panda, P.K.; Verma, S.K.; Suar, M. Aurora Borealis in dentistry: The applications of cold plasma in biomedicine. Mater. Today Bio. 2022, 13, 100200. [Google Scholar] [CrossRef]
- Busco, G.; Robert, E.; Chettouh-Hammas, N.; Pouvesle, J.M.; Grillon, C. The emerging potential of cold atmospheric plasma in skin biology. Free Radic. Biol. Med. 2020, 161, 290–304. [Google Scholar] [CrossRef]
- Tan, F.; Wang, Y.; Zhang, S.; Shui, R.; Chen, J. Plasma Dermatology: Skin Therapy Using Cold Atmospheric Plasma. Front. Oncol. 2022, 12, 918484. [Google Scholar] [CrossRef]
- Tanaka, H.; Mizuno, M.; Ishikawa, K.; Toyokuni, S.; Kajiyama, H.; Kikkawa, F.; Hori, M. Cancer Treatments Using Low-Temperature Plasma. Curr. Med. Chem. 2021, 28, 8549–8558. [Google Scholar] [CrossRef]
- Fu, J.; Xu, Y.; Arts, E.J.; Bai, Z.; Chen, Z.; Zheng, Y. Viral disinfection using nonthermal plasma: A critical review and perspectives on the plasma-catalysis system. Chemosphere 2022, 309, 136655. [Google Scholar] [CrossRef]
- Fallon, M.; Kennedy, S.; Daniels, S.; Humphreys, H. Technologies to decontaminate bacterial biofilm on hospital surfaces: A potential new role for cold plasma? J. Med. Microbiol. 2022, 71, 001582. [Google Scholar] [CrossRef] [PubMed]
- Katsigiannis, A.S.; Bayliss, D.L.; Walsh, J.L. Cold plasma for the disinfection of industrial food-contact surfaces: An overview of current status and opportunities. Compr. Rev. Food Sci. Food Saf. 2022, 21, 1086–1124. [Google Scholar] [CrossRef]
- Huang, H.; Li, S.; Zhong, J.; Wang, B.; Peng, L.; Deng, Y.; Wang, M.; Yuan, J. Evaluation of the Safety and Efficacy of a Low-Temperature Plasma Surgical System for Pterygium. Cornea 2020, 39, 1581. [Google Scholar] [CrossRef]
- Ranjan, R.; Krishnamraju, P.V.; Shankar, T.; Gowd, S. Nonthermal Plasma in Dentistry: An Update. J. Int. Soc. Prev. Community Dent. 2017, 7, 71. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.; Ouyang, J.; Zhang, C.; Shi, Z.; Wang, B.; Ostrikov, K. Plasma medicine for neuroscience—An introduction. Chin. Neurosurg. J. 2019, 5, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Bisag, A.; Isabelli, P.; Laghi, G.; Laurita, R.; Dirani, G.; Taddei, F.; Bucci, C.; Capelli, F.; Gherardi, M.; Paglianti, A.; et al. Cold atmospheric plasma decontamination of SARS-CoV-2 bioaerosols. Plasma Process. Polym. 2022, 19, e2100133. [Google Scholar] [CrossRef]
- Nayak, G.; Andrews, A.J.; Marabella, I.; Aboubakr, H.A.; Goyal, S.M.; Olson, B.A.; Torremorell, M.; Bruggeman, P.J. Rapid inactivation of airborne porcine reproductive and respiratory syndrome virus using an atmospheric pressure air plasma. Plasma Process. Polym. 2020, 17, 1900269. [Google Scholar] [CrossRef]
- Yan, D.; Sherman, J.H.; Keidar, M. Cold atmospheric plasma, a novel promising anti-cancer treatment modality. Oncotarget 2017, 8, 15977. [Google Scholar] [CrossRef] [PubMed]
- Fridman, G.; Shereshevsky, A.; Jost, M.M.; Brooks, A.D.; Fridman, A.; Gutsol, A.; Vasilets, V.; Friedman, G. Floating electrode dielectric barrier discharge plasma in air promoting apoptotic behavior in Melanoma skin cancer cell lines. Plasma Process. Polym. 2007, 27, 163–176. [Google Scholar] [CrossRef]
- Lee, K.H.; Kim, S.; Jo, H.; Son, B.K.; Shin, M.S.; Cho, G. Plasma skincare device based on floating electrode dielectric barrier discharge. Plasma Sci. Technol. 2019, 21, 125403. [Google Scholar] [CrossRef]
- Armenise, V.; Veronico, V.; Cosmai, S.; Benedetti, D.; Gristina, R.; Favia, P.; Fracassi, F.; Sardella, E. The effect of different cold atmospheric plasma sources and treatment modalities on the generation of reactive oxygen and nitrogen species in water. Plasma Process. Polym. 2023, 20, 2200182. [Google Scholar] [CrossRef]
- Stratmann, B.; Costea, T.C.; Nolte, C.; Hiller, J.; Schmidt, J.; Reindel, J.; Masur, K.; Motz, W.; Timm, J.; Kerner, W.; et al. Effect of Cold Atmospheric Plasma Therapy vs Standard Therapy Placebo on Wound Healing in Patients With Diabetic Foot Ulcers: A Randomized Clinical Trial. JAMA Netw. Open 2020, 3, e2010411. [Google Scholar] [CrossRef]
- Brehmer, F.; Haenssle, H.A.; Daeschlein, G.; Ahmed, R.; Pfeiffer, S.; Görlitz, A.; Simon, D.; Schön, M.P.; Wandke, D.; Emmert, S. Alleviation of chronic venous leg ulcers with a hand-held dielectric barrier discharge plasma generator (PlasmaDerm(®) VU-2010): Results of a monocentric, two-armed, open, prospective, randomized and controlled trial (NCT01415622). J. Eur. Acad. Dermatol. Venereol. 2015, 29, 148–155. [Google Scholar] [CrossRef] [PubMed]
- Laroussi, M.; Bekeschus, S.; Keidar, M.; Bogaerts, A.; Fridman, A.; Lu, X.; Ostrikov, K.; Hori, M.; Stapelmann, K.; Miller, V.; et al. Low-Temperature Plasma for Biology, Hygiene, and Medicine: Perspective and Roadma. IEEE Trans. Radiat. Plasma Med. Sci. 2022, 6, 127–157. [Google Scholar] [CrossRef]
- Laroussi, M. Special Issue on Low Temperature Plasma Jets. Plasma 2019, 2, 339–340. [Google Scholar] [CrossRef]
- Canady, J.; Murthy, S.R.K.; Zhuang, T.; Gitelis, S.; Nissan, A.; Ly, L.; Jones, O.Z.; Cheng, X.; Adileh, M.; Blank, A.T.; et al. The First Cold Atmospheric Plasma Phase I Clinical Trial for the Treatment of Advanced Solid Tumors: A Novel Treatment Arm for Cancer. Cancers 2023, 15, 3688. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Kim, C.H. Applications of Plasma-Activated Liquid in the Medical Field. Biomedicines 2021, 9, 1700. [Google Scholar] [CrossRef]
- Kaushik, N.K.; Ghimire, B.; Li, Y.; Adhikari, M.; Veerana, M.; Kaushik, N.; Jha, N.; Adhikari, B.; Lee, S.J.; Masur, K.; et al. Biological and medical applications of plasma-activated media, water and solutions. Biol. Chem. 2018, 400, 39–62. [Google Scholar] [CrossRef] [PubMed]
- Joslin, J.M.; McCall, J.R.; Bzdek, J.P.; Johnson, D.C.; Hybertson, B.M. Aqueous Plasma Pharmacy: Preparation Methods, Chemistry, and Therapeutic Applications. Plasma Med. 2016, 6, 135–177. [Google Scholar] [CrossRef]
- Fang, T.; Cao, X.; Shen, B.; Chen, Z.; Chen, G. Injectable cold atmospheric plasma-activated immunotherapeutic hydrogel for enhanced cancer treatment. Biomaterials 2023, 300, 122189. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Chen, G.; Obenchain, R.; Zhang, R.; Bai, F.; Fang, T.; Wang, H.; Lu, Y.; Wirz, R.E.; Gu, Z. Cold atmospheric plasma delivery for biomedical applications. Mater. Today 2022, 54, 153–188. [Google Scholar] [CrossRef]
- Mai-Prochnow, A.; Zhou, R.; Zhang, T.; Ostrikov, K.; Mugunthan, S.; Rice, S.A.; Cullen, P.J. Interactions of plasma-activated water with biofilms: Inactivation, dispersal effects and mechanisms of action. npj Biofilms Microbiomes 2021, 7, 1–12. [Google Scholar] [CrossRef]
- Tsoukou, E.; Bourke, P.; Boehm, D. Temperature Stability and Effectiveness of Plasma-Activated Liquids over an 18 Months Period. Water 2020, 12, 3021. [Google Scholar] [CrossRef]
- Gao, Y.; Francis, K.; Zhang, X. Review on formation of cold plasma activated water (PAW) and the applications in food and agriculture. Food Res. Int. 2022, 157, 111246. [Google Scholar] [CrossRef]
- Chauvin, J.; Judée, F.; Yousfi, M.; Vicendo, P.; Merbahi, N. Analysis of reactive oxygen and nitrogen species generated in three liquid media by low temperature helium plasma jet. Sci. Rep. 2017, 7, 1–15. [Google Scholar] [CrossRef]
- Kondeti, V.S.S.K.; Phan, C.Q.; Wende, K.; Jablonowski, H.; Gangal, U.; Granick, J.L.; Hunter, R.C.; Bruggeman, P.J. Long-lived and short-lived reactive species produced by a cold atmospheric pressure plasma jet for the inactivation of Pseudomonas aeruginosa and Staphylococcus aureus. Free Radic. Biol. Med. 2018, 124, 275–287. [Google Scholar] [CrossRef]
- Wenzel, T.; Carvajal Berrio, D.A.; Daum, R.; Reisenauer, C.; Weltmann, K.D.; Wallwiener, D.; Brucker, S.Y.; Schenke-Layland, K.; Brauchle, E.M.; Weiss, M. Molecular Effects and Tissue Penetration Depth of Physical Plasma in Human Mucosa Analyzed by Contact- And Marker-Independent Raman Microspectroscopy. ACS Appl. Mater. Interfaces 2019, 11, 42885–42895. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Shi, F.; Wang, Q.; Zhang, Z.; Guo, J.; Zhuang, J. Penetration effect of the kINPen plasma jet investigated with a 3D agar-entrapped bacteria model. Microchem. J. 2022, 183, 107973. [Google Scholar] [CrossRef]
- Sies, H.; Jones, D.P. Reactive oxygen species (ROS) as pleiotropic physiological signalling agents. Nat. Rev. Mol. Cell Biol. 2020, 21, 363–383. [Google Scholar] [CrossRef] [PubMed]
- Sakakita, H.; Shimizu, T.; Kiyama, S. Electrical characteristics of a low-temperature, atmospheric-pressure helium plasma jet. AIP Adv. 2021, 11, 15323. [Google Scholar] [CrossRef]
- Misra, V.C.; Pai B, G.; Tiwari, N.; Patro, B.S.; Ghorui, S. Excitation Frequency Effect on Breast Cancer Cell Death by Atmospheric Pressure Cold Plasma. Plasma Chem. Plasma Process. 2023, 43, 467–490. [Google Scholar] [CrossRef]
- Cheng, X.; Sherman, J.; Murphy, W.; Ratovitski, E.; Canady, J.; Keidar, M. The Effect of Tuning Cold Plasma Composition on Glioblastoma Cell Viability. PLoS ONE 2014, 9, e98652. [Google Scholar] [CrossRef]
- Ermakov, A.; Ermakova, O.; Skavulyak, A.; Kreshchenko, N.; Gudkov, S.; Maevsky, E. The Effects of the Low Temperature Argon Plasma on Stem Cells Proliferation and Regeneration in Planarians. Plasma Process. Polym. 2016, 13, 788–801. [Google Scholar] [CrossRef]
- Privat-Maldonado, A.; Schmidt, A.; Lin, A.; Weltmann, K.D.; Wende, K.; Bogaerts, A.; Bekeschus, S. ROS from Physical Plasmas: Redox Chemistry for Biomedical Therapy. Oxid. Med. Cell Longev. 2019, 2019, 9062098. [Google Scholar] [CrossRef]
- Lu, X.P.; Bruggeman, P.J.; Reuter, S.; Naidis, G.; Bogaerts, A.; Laroussi, M.; Keidar, M.; Robert, E.; Pouvesle, J.M.; Liu, D.W.; et al. Grand challenges in low temperature plasmas. Front. Phys. 2022, 10, 1040658. [Google Scholar] [CrossRef]
- Guo, J.; Huang, Y.; Xu, B.; Yang, J. Efficacy of Cold Atmospheric Plasma Therapy on Chronic Wounds: An Updated Systematic Review and Meta-Analysis of RCTs. Comput. Math. Methods Med. 2022, 2022, 5798857. [Google Scholar] [CrossRef]
- Plattfaut, I.; Besser, M.; Severing, A.L.; Stürmer, E.K.; Opländer, C. Plasma medicine and wound management: Evaluation of the antibacterial efficacy of a medically certified cold atmospheric argon plasma jet. Int. J. Antimicrob. Agents 2021, 57, 106319. [Google Scholar] [CrossRef]
- Kim, Y.J.; Lim, D.J.; Lee, M.Y.; Lee, W.J.; Chang, S.E.; Won, C.H. Prospective, comparative clinical pilot study of cold atmospheric plasma device in the treatment of atopic dermatitis. Sci. Rep. 2021, 11, 14461. [Google Scholar] [CrossRef]
- Kongpanichakul, L.; Chuangsuwanich, A.; Kongkunnavat, N.; Tonaree, W. Efficacy of Low-temperature Plasma for Treatment of Facial Rejuvenation in Asian Population. Plast. Reconstr. Surg. Glob. Open 2021, 9, E3812. [Google Scholar] [CrossRef]
- Wang, X.-F.; Fang, Q.-Q.; Jia, B.; Hu, Y.-Y.; Wang, Z.-C.; Yan, K.-P.; Yin, S.-Y.; Liu, Z.; Tan, W.-Q. Potential effect of non-thermal plasma for the inhibition of scar formation: A preliminary report. Sci. Rep. 2020, 10, 1–10. [Google Scholar] [CrossRef]
- Hubbard, J.A.; Binder, D.K. Astrocytes and Epilepsy; Elsevier BV: Amsterdam, The Netherlands, 2016. [Google Scholar] [CrossRef]
- Bernhardt, T.; Semmler, M.L.; Schäfer, M.; Bekeschus, S.; Emmert, S.; Boeckmann, L. Plasma Medicine: Applications of Cold Atmospheric Pressure Plasma in Dermatology. Oxid. Med. Cell Longev. 2019, 2019, 3873928. [Google Scholar] [CrossRef]
- Liu, J.R.; Xu, G.M.; Shi, X.M.; Zhang, G.J. Low temperature plasma promoting fibroblast proliferation by activating the NF-κB pathway and increasing cyclinD1 expression. Sci. Rep. 2017, 7, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Lertpatipanpong, P.; Sillapachaiyaporn, C.; Oh, G.; Kang, Y.H.; Hwang, C.Y.; Baek, S.J. Effect of cold atmospheric microwave plasma (CAMP) on wound healing in canine keratinocytes. Front. Cell Dev. Biol. 2023, 11, 1105692. [Google Scholar] [CrossRef] [PubMed]
- Sedik, A.A.; Salama, M.; Fathy, K.; Salama, A. Cold plasma approach fortifies the topical application of thymoquinone intended for wound healing via up-regulating the levels of TGF-ß, VEGF, and α-SMA in rats. Int. Immunopharmacol. 2023, 122, 110634. [Google Scholar] [CrossRef] [PubMed]
- Dehghanpisheh, P.; Jahandideh, A.; Asghari, A.; Mortazavi, P.; Ghorannevis, M. Effects of Cold Atmospheric Plasma on Infectious Diabetic Wound Healing in Rat Models. Crescent J. Med. Biol. Sci. 2023, 10, 93–97. [Google Scholar] [CrossRef]
- Jensen, J.O.; Schulz, L.; Schleusser, S.; Matzkeit, N.; Stang, F.H.; Mailaender, P.; Kraemer, R.; Kleemann, M.; Deichmann, H.; Kisch, T. The repetitive application of cold atmospheric plasma (CAP) improves microcirculation parameters in chronic wounds. Microvasc. Res. 2021, 138, 104220. [Google Scholar] [CrossRef]
- Hong, Q.; Dong, X.; Jones, J.E.; Hong, L.; Yu, Q.; Sun, H.; Chen, M. A novel approach to expedite wound healing with plasma brush of cold flame. Rev. Sci. Instrum. 2023, 94, 84102. [Google Scholar] [CrossRef]
- Bekeschus, S.; Winterbourn, C.C.; Kolata, J.; Masur, K.; Hasse, S.; Bröker, B.M.; Parker, H.A. Neutrophil extracellular trap formation is elicited in response to cold physical plasma. J. Leukoc. Biol. 2016, 100, 791–799. [Google Scholar] [CrossRef] [PubMed]
- Papayannopoulos, V. Neutrophil extracellular traps in immunity and disease. Nat. Rev. Immunol. 2017, 18, 134–147. [Google Scholar] [CrossRef] [PubMed]
- Bekeschus, S.; Seebauer, C.; Wende, K.; Schmidt, A. Physical plasma and leukocytes—immune or reactive? Biol. Chem. 2018, 400, 63–75. [Google Scholar] [CrossRef] [PubMed]
- Marches, A.; Clement, E.; Albérola, G.; Rols, M.P.; Cousty, S.; Simon, M.; Merbahi, N. Cold Atmospheric Plasma Jet Treatment Improves Human Keratinocyte Migration and Wound Closure Capacity without Causing Cellular Oxidative Stress. Int. J. Mol. Sci. 2022, 23, 10650. [Google Scholar] [CrossRef]
- Rached, N.A.; Kley, S.; Storck, M.; Meyer, T.; Stücker, M. Cold Plasma Therapy in Chronic Wounds—A Multicenter, Randomized Controlled Clinical Trial (Plasma on Chronic Wounds for Epidermal Regeneration Study): Preliminary Results. J. Clin. Med. 2023, 12, 5121. [Google Scholar] [CrossRef]
- Hiller, J.; Stratmann, B.; Timm, J.; Costea, T.C.; Tschoepe, D. Enhanced growth factor expression in chronic diabetic wounds treated by cold atmospheric plasma. Diabet. Med. 2022, 39, e14787. [Google Scholar] [CrossRef]
- Jasim, M.G.; Humud, H.R. Treatment of Warts by DBD Plasma. J. Surv. Fish. Sci. 2023, 10, 2294–2299. [Google Scholar]
- Kaushik, N.K.; Shin, Y.; Ki, S.; Han, I.; Kaushik, N.; Choi, E.H. Nonthermal Plasma-Based Virus Inactivation and Sterilization. Top. Appl. Phys. 2023, 148, 77–91. [Google Scholar] [CrossRef]
- Matthes, R.; Lührman, A.; Holtfreter, S.; Kolata, J.; Radke, D.; Hübner, N.O.; Assadian, O.; Kramer, A. Antibacterial Activity of Cold Atmospheric Pressure Argon Plasma against 78 Genetically Different (mecA, luk-P, agr or Capsular Polysaccharide Type) Staphylococcus aureus Strains. Skin. Pharmacol. Physiol. 2016, 29, 83–91. [Google Scholar] [CrossRef]
- Molina-Hernandez, J.B.; Capelli, F.; Laurita, R.; Tappi, S.; Laika, J.; Gioia, L.; Valbonetti, L.; Chaves-López, C. A comparative study on the antifungal efficacy of cold atmospheric plasma at low and high surface density on Aspergillus chevalieri and mechanisms of action. Innov. Food Sci. Emerg. Technol. 2022, 82, 103194. [Google Scholar] [CrossRef]
- Filipić, A.; Gutierrez-Aguirre, I.; Primc, G.; Mozetič, M.; Dobnik, D. Cold Plasma, a New Hope in the Field of Virus Inactivation. Trends Biotechnol. 2020, 38, 1278–1291. [Google Scholar] [CrossRef]
- Laroussi, M. Sterilization of contaminated matter with an atmospheric pressure plasma. IEEE Trans. Plasma Sci. 1996, 24, 1188–1191. [Google Scholar] [CrossRef]
- Hydrogen Peroxide Gas Plasma|Disinfection & Sterilization Guidelines|Guidelines Library|Infection Control. Available online: https://www.cdc.gov/infectioncontrol/guidelines/disinfection/sterilization/hydrogen-peroxide-gas.html (accessed on 27 November 2023).
- Shintani, H.; Sakudo, A.; Burke, P.; McDonnell, G. Gas plasma sterilization of microorganisms and mechanisms of action. Exp. Ther. Med. 2010, 1, 731. [Google Scholar] [CrossRef]
- Liao, X.; Liu, D.; Xiang, Q.; Ahn, J.; Chen, S.; Ye, X.; Ding, T. Inactivation mechanisms of non-thermal plasma on microbes: A review. Food Control 2017, 75, 83–91. [Google Scholar] [CrossRef]
- Liao, X.; Muhammad, A.I.; Chen, S.; Hu, Y.; Ye, X.; Liu, D.; Ding, T. Bacterial spore inactivation induced by cold plasma. Crit. Rev. Food Sci. Nutr. 2019, 59, 2562–2572. [Google Scholar] [CrossRef] [PubMed]
- Mai-Prochnow, A.; Clauson, M.; Hong, J.; Murphy, A.B. Gram positive and Gram negative bacteria differ in their sensitivity to cold plasma. Sci. Rep. 2016, 6, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Daeschlein, G.; Scholz, S.; Arnold, A.; Von Podewils, S.; Haase, H.; Emmert, S.; Von Woedtke, T.; Weltmann, K.D.; Jünger, M. In Vitro Susceptibility of Important Skin and Wound Pathogens Against Low Temperature Atmospheric Pressure Plasma Jet (APPJ) and Dielectric Barrier Discharge Plasma (DBD). Plasma Process. Polym. 2012, 9, 380–389. [Google Scholar] [CrossRef]
- Olatunde, O.O.; Benjakul, S.; Vongkamjan, K. Dielectric barrier discharge cold atmospheric plasma: Bacterial inactivation mechanism. J. Food Saf. 2019, 39, e12705. [Google Scholar] [CrossRef]
- Xu, H.; Zhu, Y.; Du, M.; Wang, Y.; Ju, S.; Ma, R.; Jiao, Z. Subcellular mechanism of microbial inactivation during water disinfection by cold atmospheric-pressure plasma. Water Res. 2021, 188, 116513. [Google Scholar] [CrossRef]
- Sakudo, A.; Misawa, T. Antibiotic-Resistant and Non-Resistant Bacteria Display Similar Susceptibility to Dielectric Barrier Discharge Plasma. Int. J. Mol. Sci. 2020, 21, 6326. [Google Scholar] [CrossRef]
- Das, S.; Prakash, G.V.; Mohapatra, S.; Kar, S.; Bhatt, S.; Gautam, H.; Singh, G.; Kapil, A.; Das, B.K.; Sood, S.; et al. Antimicrobial Efficacy of Argon Cold Atmospheric Pressure Plasma Jet on Clinical Isolates of Multidrug-Resistant ESKAPE Bacteria. IEEE Trans. Radiat. Plasma Med. Sci. 2023, 7, 421–428. [Google Scholar] [CrossRef]
- Lunder, M.; Dahle, S.; Fink, R. Cold atmospheric plasma for surface disinfection: A promising weapon against deleterious meticillin-resistant Staphylococcus aureus biofilms. J. Hosp. Infect. 2024, 143, 64–75. [Google Scholar] [CrossRef] [PubMed]
- Qin, H.; Qiu, H.; He, S.T.; Hong, B.; Liu, K.; Lou, F.; Li, M.; Hu, P.; Kong, X.; Song, Y.; et al. Efficient disinfection of SARS-CoV-2-like coronavirus, pseudotyped SARS-CoV-2 and other coronaviruses using cold plasma induces spike protein damage. J. Hazard. Mater. 2022, 430, 128414. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Bai, F.; Jonas, S.J.; Wirz, R.E. Cold atmospheric plasma for addressing the COVID-19 pandemic. Plasma Process. Polym. 2022, 19, 2200012. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Tang, T.; Lee, H.; Song, K. Cold atmospheric pressure plasma-activated medium induces selective cell death in human hepatocellular carcinoma cells independently of singlet oxygen, hydrogen peroxide, nitric oxide and nitrite/nitrate. Int. J. Mol. Sci. 2021, 22, 5548. [Google Scholar] [CrossRef] [PubMed]
- Gangemi, S.; Petrarca, C.; Tonacci, A.; Di Gioacchino, M.; Musolino, C.; Allegra, A. Cold Atmospheric Plasma Targeting Hematological Malignancies: Potentials and Problems of Clinical Translation. Antioxidants 2022, 11, 1592. [Google Scholar] [CrossRef]
- Yu, H.; Song, X.; Yang, F.; Wang, J.; Sun, M.; Liu, G.; Ahmad, N.; Zhou, Y.; Zhang, Y.; Shi, G.; et al. Combined effects of vitamin C and cold atmospheric plasma-conditioned media against glioblastoma via hydrogen peroxide. Free Radic. Biol. Med. 2023, 194, 1–11. [Google Scholar] [CrossRef]
- Wang, Y.; Mang, X.; Li, X.; Cai, Z.; Tan, F. Cold atmospheric plasma induces apoptosis in human colon and lung cancer cells through modulating mitochondrial pathway. Front. Cell Dev. Biol. 2022, 10, 915785. [Google Scholar] [CrossRef]
- Jezeh, M.A.; Tayebi, T.; Khani, M.R.; Niknejad, H.; Shokri, B. Direct cold atmospheric plasma and plasma-activated medium effects on breast and cervix cancer cells. Plasma Process. Polym. 2020, 17, 1900241. [Google Scholar] [CrossRef]
- Jo, A.; Bae, J.H.; Yoon, Y.J.; Chung, T.H.; Lee, E.W.; Kim, Y.H.; Joh, H.M.; Chung, J.W. Plasma-activated medium induces ferroptosis by depleting FSP1 in human lung cancer cells. Cell Death Dis. 2022, 13, 212. [Google Scholar] [CrossRef]
- Kenari, A.J.; Siadati, S.N.; Abedian, Z.; Sohbatzadeh, F.; Amiri, M.; Gorji, K.E.; Babapour, H.; Zabihi, E.; Ghoreishi, S.M.; Mehraeen, R.; et al. Therapeutic effect of cold atmospheric plasma and its combination with radiation as a novel approach on inhibiting cervical cancer cell growth (HeLa cells). Bioorg. Chem. 2021, 111, 104892. [Google Scholar] [CrossRef]
- Azzariti, A.; Iacobazzi, R.M.; Di Fonte, R.; Porcelli, L.; Gristina, R.; Favia, P.; Fracassi, F.; Trizio, I.; Silvestris, N.; Guida, G.; et al. Plasma-activated medium triggers cell death and the presentation of immune activating danger signals in melanoma and pancreatic cancer cells. Sci. Rep. 2019, 9, 4099. [Google Scholar] [CrossRef]
- Szili, E.J.; Harding, F.J.; Hong, S.H.; Herrmann, F.; Voelcker, N.H.; Short, R.D. The hormesis effect of plasma-elevated intracellular ROS on HaCaT cells. J. Phys. D Appl. Phys. 2015, 48, 495401. [Google Scholar] [CrossRef]
- Packer, J.R.; Hirst, A.M.; Droop, A.P.; Adamson, R.; Simms, M.S.; Mann, V.M.; Frame, F.M.; O’Connell, D.; Mait-land, N.J. Notch signalling is a potential resistance mechanism of progenitor cells within patient-derived prostate cultures following ROS-inducing treatments. FEBS Lett. 2020, 594, 209. [Google Scholar] [CrossRef]
- Brullé, L.; Vandamme, M.; Riès, D.; Martel, E.; Robert, E.; Lerondel, S.; Trichet, V.; Richard, S.; Pouvesle, J.M.; Le Pape, A. Effects of a non thermal plasma treatment alone or in combination with gemcitabine in a MIA PaCa2-luc orthotopic pancreatic carcinoma model. PLoS ONE 2012, 7, e52653. [Google Scholar] [CrossRef] [PubMed]
- Vandamme, M.; Robert, E.; Lerondel, S.; Sarron, V.; Ries, D.; Dozias, S.; Sobilo, J.; Gosset, D.; Kieda, C.; Legrain, B.; et al. ROS implication in a new antitumor strategy based on non-thermal plasma. Int. J. Cancer 2012, 130, 2185–2194. [Google Scholar] [CrossRef] [PubMed]
- Vandamme, M.; Robert, E.; Dozias, S.; Sobilo, J.; Lerondel, S.; Le Pape, A.; Pouvesle, J.M. Response of Human Glioma U87 Xenografted on Mice to Non Thermal Plasma Treatment. Plasma Med. 2011, 1, 27–43. [Google Scholar] [CrossRef]
- Solé-Martí, X.; Espona-Noguera, A.; Ginebra, M.P.; Canal, C. Plasma-Conditioned Liquids as Anticancer Therapies In Vivo: Current State and Future Directions. Cancers 2021, 13, 452. [Google Scholar] [CrossRef]
- Saadati, F.; Mahdikia, H.; Abbaszadeh, H.A.; Abdollahifar, M.A.; Khoramgah, M.S.; Shokri, B. Comparison of Direct and Indirect cold atmospheric-pressure plasma methods in the B16F10 melanoma cancer cells treatment. Sci. Rep. 2018, 8, 7689. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Peng, S.; Li, B.; Wang, S.; Zhang, H.; Li, Q.; Liu, Z.; Guo, B.; Liu, D.; Xu, D. Systematic Safety Evaluation of Cold Plasma-Activated Liquid in Rabbits. Front. Phys. 2021, 9, 659227. [Google Scholar] [CrossRef]
- Li, S.; Li, C.; Wang, W. Molecular aspects of aquaporins. Vitam. Horm. 2020, 113, 129–181. [Google Scholar] [CrossRef] [PubMed]
- Roh, J.S.; Sohn, D.H. Damage-Associated Molecular Patterns in Inflammatory Diseases. Immune Netw. 2018, 18, e27. [Google Scholar] [CrossRef]
- Zhao, W.; Jing, X.; Wang, T.; Zhang, F. Glutamine Deprivation Synergizes the Anticancer Effects of Cold Atmospheric Plasma on Esophageal Cancer Cells. Molecules 2023, 28, 1461. [Google Scholar] [CrossRef]
- To, H.D.; Huy, G.; Nguyen, H.; Thi, T.; Le, A.; Moradi, E.; Naserzadeh, P.; Millan, P.B.; Berner, J.; Bekeschus, S.; et al. Combined toxicity of indirubins with cold physical plasma in skin cancer cells in vitro. Jpn. J. Appl. Phys. 2022, 62, SA1006. [Google Scholar] [CrossRef]
- Jung, J.M.; Yoon, H.K.; Kim, S.Y.; Yun, M.R.; Kim, G.H.; Lee, W.J.; Lee, M.W.; Chang, S.E.; Won, C.H. Anticancer Effect of Cold Atmospheric Plasma in Syngeneic Mouse Models of Melanoma and Colon Cancer. Molecules 2023, 28, 4171. [Google Scholar] [CrossRef]
- Li, W.; Yu, K.N.; Bao, L.; Shen, J.; Cheng, C.; Han, W. Non-thermal plasma inhibits human cervical cancer HeLa cells invasiveness by suppressing the MAPK pathway and decreasing matrix metalloproteinase-9 expression. Sci. Rep. 2016, 6, 19720. [Google Scholar] [CrossRef]
- Kaushik, N.K.; Kaushik, N.; Adhikari, M.; Ghimire, B.; Linh, N.N.; Mishra, Y.K.; Lee, S.J.; Choi, E.H. Preventing the Solid Cancer Progression via Release of Anticancer-Cytokines in Co-Culture with Cold Plasma-Stimulated Macrophages. Cancers 2019, 11, 842. [Google Scholar] [CrossRef]
- Florczyk, U.; Jazwa, A.; Maleszewska, M.; Mendel, M.; Szade, K.; Kozakowska, M.; Grochot-Przeczek, A.; Viscardi, M.; Czauderna, S.; Bukowska-Strakova, K.; et al. Nrf2 Regulates Angiogenesis: Effect on Endothelial Cells, Bone Marrow-Derived Proangiogenic Cells and Hind Limb Ischemia. Antioxid. Redox Signal 2014, 20, 1693. [Google Scholar] [CrossRef]
- Kopan, R. Notch Signaling. Cold Spring Harb. Perspect. Biol. 2012, 4, a011213. [Google Scholar] [CrossRef] [PubMed]
- Yahyanejad, S.; Theys, J.; Vooijs, M. Targeting Notch to overcome radiation resistance. Oncotarget 2016, 7, 7610. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Rajendran, V.; Sethumadhavan, R.; Purohit, R. AKT Kinase Pathway: A Leading Target in Cancer Research. Sci. World J. 2013, 2013, 756134. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.Y.; Kim, H.J.; Kang, S.U.; Kim, Y.E.; Park, J.K.; Shin, Y.S.; Kim, Y.S.; Lee, K.; Kim, C.H. Non-thermal plasma induces AKT degradation through turn-on the MUL1 E3 ligase in head and neck cancer. Oncotarget 2015, 6, 33382–33396. [Google Scholar] [CrossRef] [PubMed]
- Glick, D.; Barth, S.; Macleod, K.F. Autophagy: Cellular and molecular mechanisms. J. Pathol. 2010, 221, 3. [Google Scholar] [CrossRef] [PubMed]
- Yoshikawa, N.; Liu, W.; Nakamura, K.; Yoshida, K.; Ikeda, Y.; Tanaka, H.; Mizuno, M.; Toyokuni, S.; Hori, M.; Kikkawa, F.; et al. Plasma-activated medium promotes autophagic cell death along with alteration of the mTOR pathway. Sci. Rep. 2020, 10, 1614. [Google Scholar] [CrossRef]
- Wei, Z.; Liu, H.T. MAPK signal pathways in the regulation of cell proliferation in mammalian cells. Cell Res. 2002, 12, 9–18. [Google Scholar] [CrossRef]
- Turrini, E.; Laurita, R.; Stancampiano, A.; Catanzaro, E.; Calcabrini, C.; Maffei, F.; Gherardi, M.; Colombo, V.; Fi-mognari, C. Cold Atmospheric Plasma Induces Apoptosis and Oxidative Stress Pathway Regulation in T-Lymphoblastoid Leukemia Cells. Oxid. Med. Cell Longev. 2017, 2017, 4271065. [Google Scholar] [CrossRef]
- Dai, X.; Thompson, E.W.; Ostrikov, K. Receptor-Mediated Redox Imbalance: An Emerging Clinical Avenue against Aggressive Cancers. Biomolecules 2022, 12, 1880. [Google Scholar] [CrossRef]
- Guo, B.; Pomicter, A.D.; Li, F.; Bhatt, S.; Chen, C.; Li, W.; Qi, M.; Huang, C.; Deininger, M.W.; Kong, M.G.; et al. Trident cold atmospheric plasma blocks three cancer survival pathways to overcome therapy resistance. Proc. Natl. Acad. Sci. USA 2021, 118, e2107220118. [Google Scholar] [CrossRef]
- Klinkhammer, C.; Verlackt, C.; Śmiłowicz, D.; Kogelheide, F.; Bogaerts, A.; Metzler-Nolte, N.; Stapelmann, K.; Havenith, M.; Lackmann, J.W. Elucidation of Plasma-induced Chemical Modifications on Glutathione and Glutathione Disulphide. Sci. Rep. 2017, 7, 13828. [Google Scholar] [CrossRef]
- Ranieri, P.; Mohamed, H.; Myers, B.; Dobossy, L.; Beyries, K.; Trosan, D.; Krebs, F.C.; Miller, V.; Stapelmann, K. GSH Modification as a Marker for Plasma Source and Biological Response Comparison to Plasma Treatment. Appl. Sci. 2020, 10, 2025. [Google Scholar] [CrossRef]
- Cheng, H.; Luo, J.; Song, K.; Zhao, F.; Liu, D.; Nie, L.; Lu, X. On the dose of plasma medicine: Plasma-activated medium (PAM) and its effect on cell viability. Phys. Plasmas 2022, 29, 063506. [Google Scholar] [CrossRef]
- Cuesta, A. ATR-SEIRAS for time-resolved studies of electrode–electrolyte interfaces. Curr. Opin. Electrochem. 2022, 35, 101041. [Google Scholar] [CrossRef]
- Tshitoyan, V.; Dagdelen, J.; Weston, L.; Dunn, A.; Rong, Z.; Kononova, O.; Persson, K.A.; Ceder, G.; Jain, A. Unsupervised word embeddings capture latent knowledge from materials science literature. Nature 2019, 571, 95–98. [Google Scholar] [CrossRef]
- Pyzer-Knapp, E.O.; Pitera, J.W.; Staar, P.W.J.; Takeda, S.; Laino, T.; Sanders, D.P.; Sexton, J.; Smith, J.R.; Curioni, A. Accelerating materials discovery using artificial intelligence, high performance computing and robotics. npj Comput. Mater. 2022, 8, 84. [Google Scholar] [CrossRef]
- Kuenneth, C.; Lalonde, J.; Marrone, B.L.; Iverson, C.N.; Ramprasad, R.; Pilania, G. Bioplastic design using multitask deep neural networks. Commun. Mater. 2022, 3, 96. [Google Scholar] [CrossRef]
- Degrave, J.; Felici, F.; Buchli, J.; Neunert, M.; Tracey, B.; Carpanese, F.; Ewalds, T.; Hafner, R.; Abdolmaleki, A.; de las Casas, D.; et al. Magnetic control of tokamak plasmas through deep reinforcement learning. Nature 2022, 602, 414–419. [Google Scholar] [CrossRef]
- Abbate, J.; Conlin, R.; Kolemen, E. Data-driven profile prediction for DIII-D. Nuclear Fusion. 2021, 61, 046027. [Google Scholar] [CrossRef]
- Pavone, A.; Merlo, A.; Kwak, S.; Svensson, J. Machine learning and Bayesian inference in nuclear fusion research: An overview. Plasma Phys. Control Fusion. 2023, 65, 053001. [Google Scholar] [CrossRef]
- Franke, S.; Paulet, L.; Schäfer, J.; O’Connell, D.; Becker, M.M. Plasma-MDS, a metadata schema for plasma science with examples from plasma technology. Sci. Data 2020, 7, 439. [Google Scholar] [CrossRef]
- Paz, Y. Transient IR spectroscopy as a tool for studying photocatalytic materials. J. Phys. Condens. Matter 2019, 31, 503004. [Google Scholar] [CrossRef]
- Rimshaw, A.; Grieco, C.; Asbury, J.B. High Sensitivity Nanosecond Mid-Infrared Transient Absorption Spectrometer Enabling Low Excitation Density Measurements of Electronic Materials. Appl. Spectrosc. 2016, 70, 1726–1732. [Google Scholar] [CrossRef]
- Bogaerts, A.; Yusupov, M.; Van Der Paal, J.; Verlackt, C.C.W.; Neyts, E.C. Reactive Molecular Dynamics Simulations for a Better Insight in Plasma Medicine. Plasma Process. Polym. 2014, 11, 1156–1168. [Google Scholar] [CrossRef]
- Neyts, E.C.; Yusupov, M.; Verlackt, C.C.; Bogaerts, A. Computer simulations of plasma–biomolecule and plasma–tissue interactions for a better insight in plasma medicine. J. Phys. D Appl. Phys. 2014, 47, 293001. [Google Scholar] [CrossRef]
- Lu, X.; Naidis, G.V.; Laroussi, M.; Reuter, S.; Graves, D.B.; Ostrikov, K. Reactive species in non-equilibrium atmospheric-pressure plasmas: Generation, transport, and biological effects. Phys. Rep. 2016, 630, 1–84. [Google Scholar] [CrossRef]

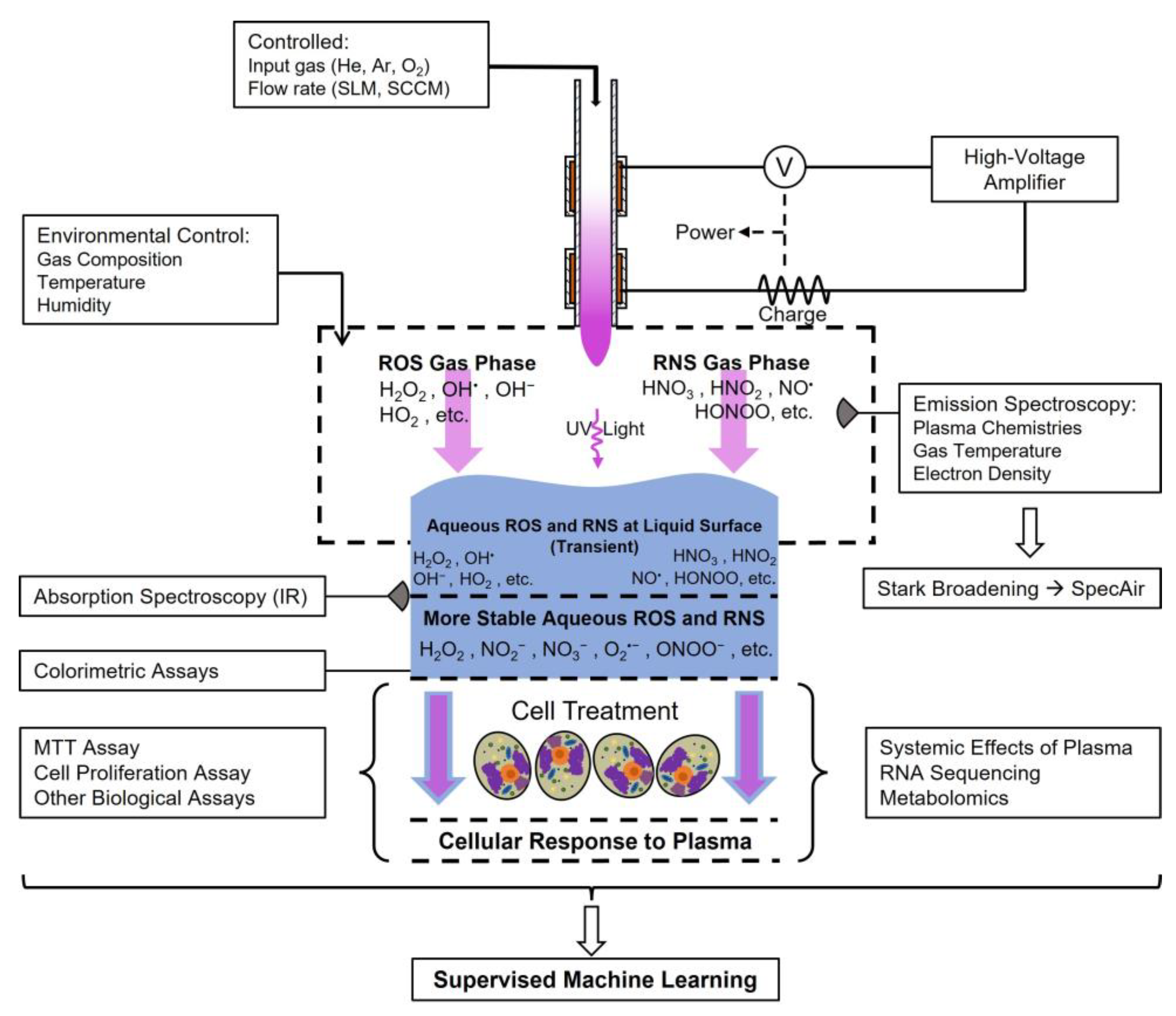
| Plasma Source | Target | Recorded Data | Methods/Techniques |
|---|---|---|---|
| Cold atmospheric microwave plasma (CAMP) [61] | Open wound (canine) Immortalized human keratinocytes (HaCaT) Canine Progenitor epidermal keratinocytes (CPEK) | Input parameters: intensity of electric fields, frequency, ambient environment, treatment distance, etc. | Bio Stimulation Microwave Plasma V1.0, He plasma |
| Direct vs. indirect treatment | CAMP vs. activated media | ||
| Cell viability | CellTiter 96 Aqueous One Solution Assay Kit | ||
| Cell migration parameters | Scratch assay, Transwell migration assay | ||
| Wound area | Imaging | ||
| Gene/protein expression library | RNA-Seq, qRT-PCR, Blotting | ||
| DBD [62] | Thymoquinone treated with CAP was used to treat mouse models of wounds (in vivo) | Input parameters: intensity of electric fields, frequency, ambient environment, treatment distance, etc. | Suzhou Opus Plasma Technology Co. |
| Gene/protein expression | ELISA assays, Flow cytometry | ||
| Wound area | Imaging | ||
| Wound structure | Histology, Transmission electron microscopy (TEM) | ||
| Jet-DBD and planar DBD reactor [28] | Water treated with plasma under different conditions: gas composition, power | Input parameters: intensity of electric fields, frequency, ambient environment, treatment distance, etc. | In-house/modified plasma sources, He + O2, N2, or Air |
| Chemistry: NO2−, H2O2 | Griess assay, Spectrophotometric methods | ||
| RF-plasma jet [63] | S. aureus-infected wounds on Wistar rats with induced diabetes | Input parameters: intensity of electric fields, frequency, ambient environment, treatment distance, etc. | In-house/modified plasma sources, He plasma |
| Wound structure | Histology | ||
| Blood glucose level | Blood test | ||
| Epithelialization, inflammation, collagenization, vascularization | Histology | ||
| DBD- PlasmDerm FLEX9060 [64] | Prospective controlled cohort clinical trial-chronic leg ulcers | Input parameters: intensity of electric fields, frequency, ambient environment, treatment distance, etc. | In-house/modified plasma sources, He + O2, N2, or Air |
| Environmental parameters | Temperature, humidity | ||
| Patient data/medical history | Following patient confidentiality requirements | ||
| Wound area | Imaging | ||
| Capillary blood flow, tissue oxygen saturation, postcapillary venous filling, and microcirculation | Combined laser Doppler and photospectrometry |
| Plasma Source | Target | Recorded Data | Methods/Techniques |
|---|---|---|---|
| DBD [109] | Cultures of esophageal cancer cell lines EC9706 and ECa109 in DMEM medium with 10% FBS | Input parameters: intensity of electric fields, frequency, ambient environment, treatment distance, etc. | In-house plasma sources, Air plasma |
| Cell viability | MTT assay, Apoptosis assay | ||
| Chemistry in medium: NO2−, NO3−, H2O2 | Spectrophotometric techniques and assays | ||
| Levels of glutathione, intracellular ROS | Hydrogen peroxide assay, flow cytometry | ||
| APPJ [110] | Human squamous cell carcinoma cell line A431 and skin malignant melanoma cell line A375 in RPMI 1640 medium with 10% FBS, 1% glutamine, and 1% penicillin + streptomycin | Input parameters: intensity of electric fields, frequency, ambient environment, treatment distance, etc. | kINPen argon plasma, dose of indirubin added |
| Cell viability and metabolic activity | Alamar blue assay, sytox blue assay + flow cytometry | ||
| Cell migration | Scratch assay | ||
| APPJ [37] | DI water used to prepare Pluronic hydrogels intended for intratumoral injection post-plasma treatment | Input parameters: intensity of electric fields, frequency, ambient environment, treatment distance, etc. | In-house plasma sources, air plasma |
| Gas phase chemistry | Optical emission spectroscopy | ||
| Intracellular ROS and RNS | Stained with fluorescent dyes + flowcytometry | ||
| Cell viability, tumor size, and immunological analysis | IVIS imaging and various antibodies + flowcytometry | ||
| APPJ [111] | Various human cancer cell lines treated directly or via PAM (in vitro) Topical treatment of melanoma tumors on mice (in vivo) | Input parameters: intensity of electric fields, frequency, ambient environment, treatment distance, etc. | MediPL plasma torch system, Argon plasma |
| Gene/protein expression | qRT-PCR, Western blot | ||
| In vivo apoptosis/protein expression | Immunohistochemical studies |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kazemi, A.; Nicol, M.J.; Bilén, S.G.; Kirimanjeswara, G.S.; Knecht, S.D. Cold Atmospheric Plasma Medicine: Applications, Challenges, and Opportunities for Predictive Control. Plasma 2024, 7, 233-257. https://doi.org/10.3390/plasma7010014
Kazemi A, Nicol MJ, Bilén SG, Kirimanjeswara GS, Knecht SD. Cold Atmospheric Plasma Medicine: Applications, Challenges, and Opportunities for Predictive Control. Plasma. 2024; 7(1):233-257. https://doi.org/10.3390/plasma7010014
Chicago/Turabian StyleKazemi, Ali, McKayla J. Nicol, Sven G. Bilén, Girish S. Kirimanjeswara, and Sean D. Knecht. 2024. "Cold Atmospheric Plasma Medicine: Applications, Challenges, and Opportunities for Predictive Control" Plasma 7, no. 1: 233-257. https://doi.org/10.3390/plasma7010014






