Soil Processes, Pedofeatures and Microscale Metal Distributions: Relevant Study of Contaminant-Dynamics Calls for Pedology-Based Soil-Depth Sampling Strategies
Abstract
:1. Introduction
2. Materials and Methods
2.1. Site and Soil Description
2.2. Methods
3. Results
3.1. Site 1: Zinc-Smelter Waste Contamination
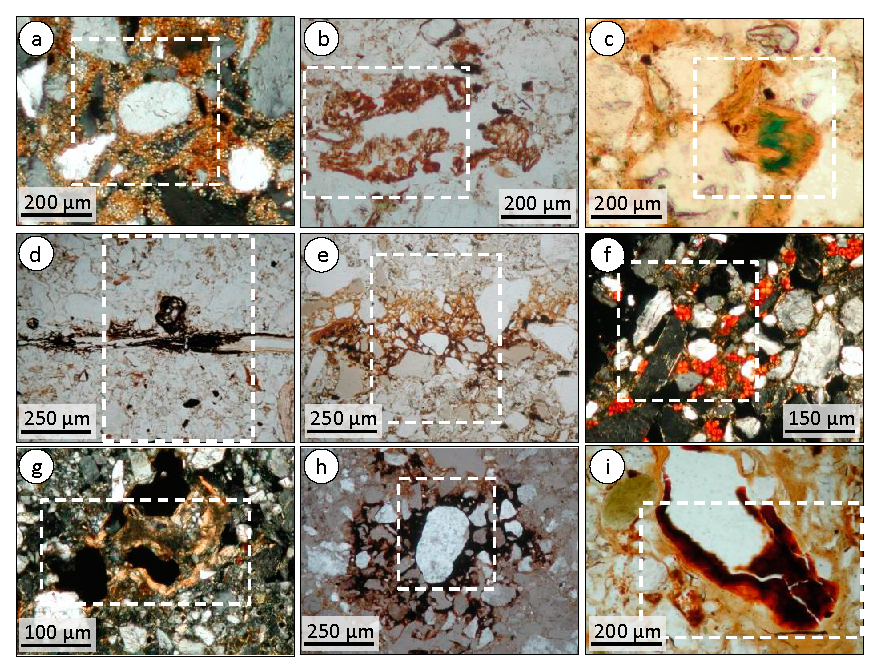
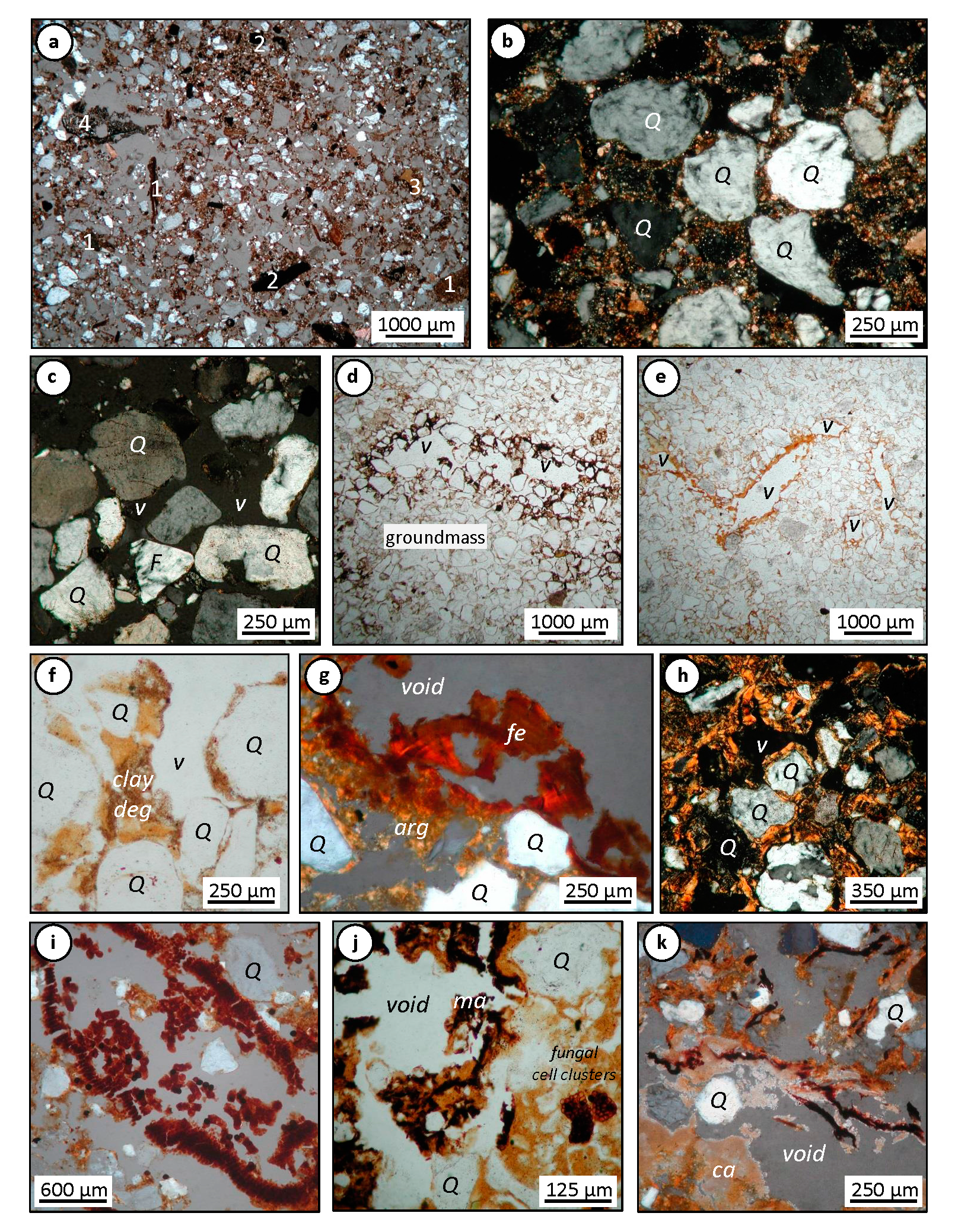
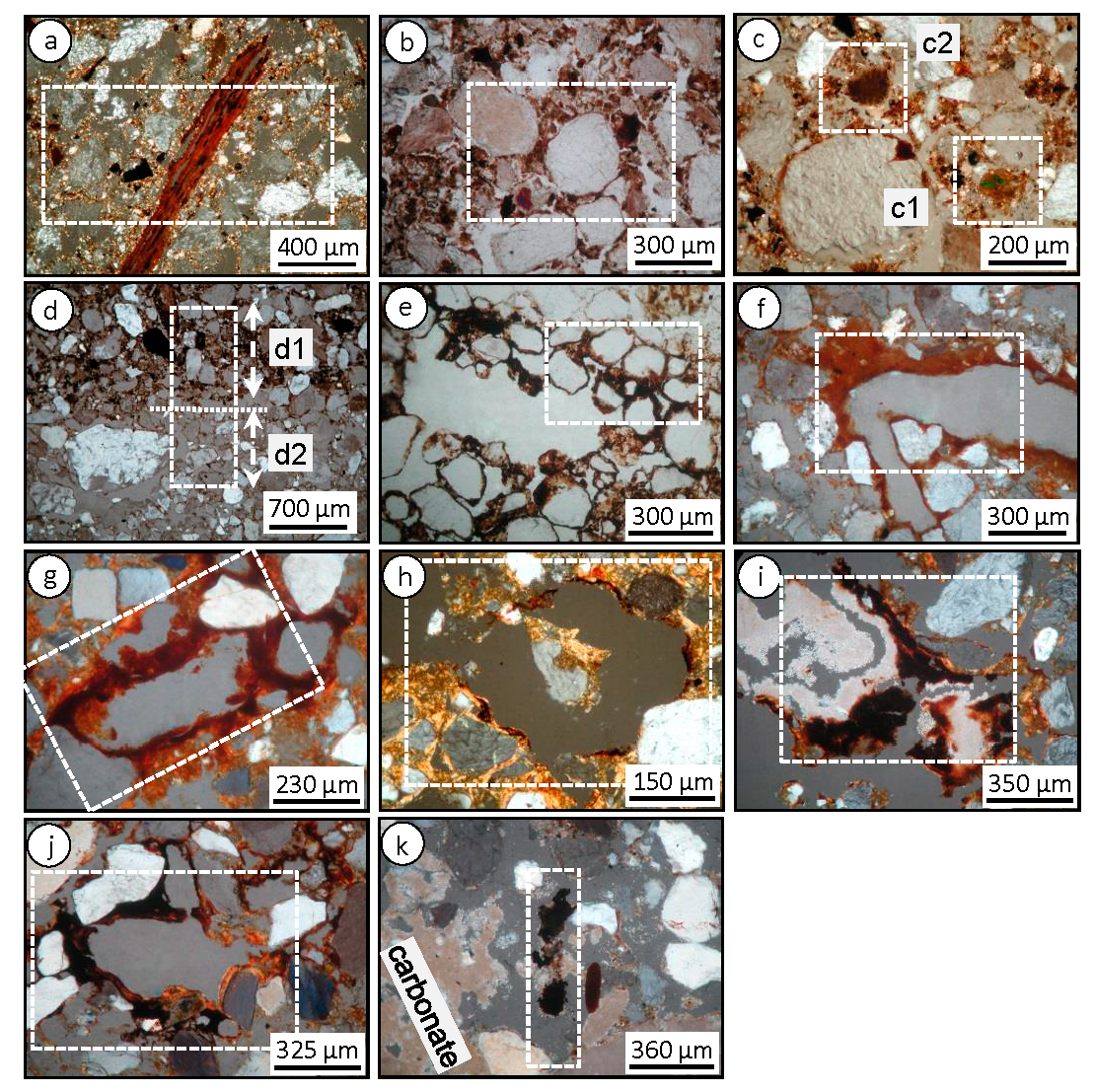
3.1.1. Micromorphology in A, B and C Horizons
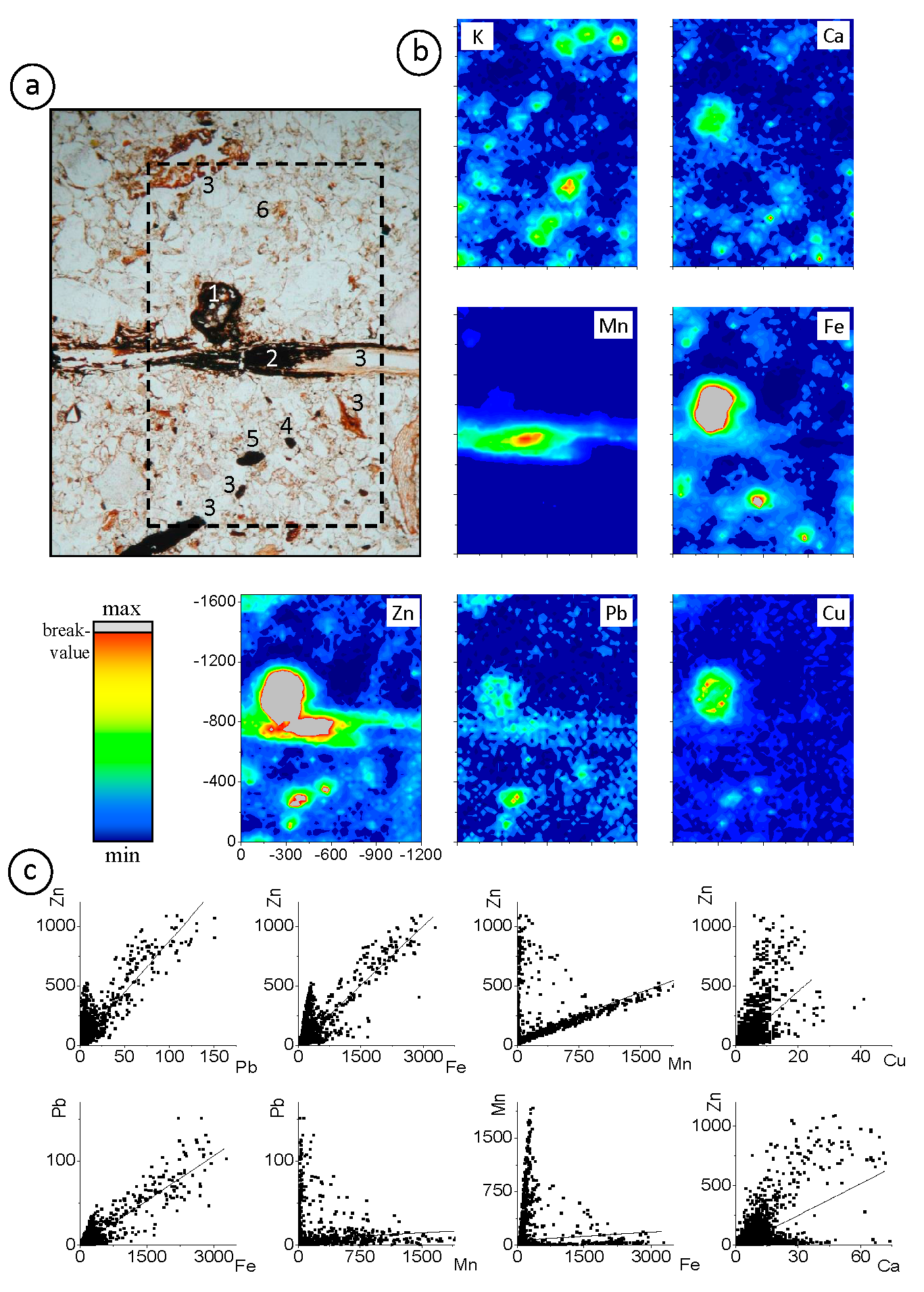
3.1.2. Contrasting Hecto-Micrometer Scale Metal Distributions in the Surface Horizon
3.1.3. Elemental Correlation Analyses on Selected Features of A, B, and C Horizons
3.2. Site 2: Urban Wastewater Contamination
3.2.1. Micromorphology in A, B and C Horizons
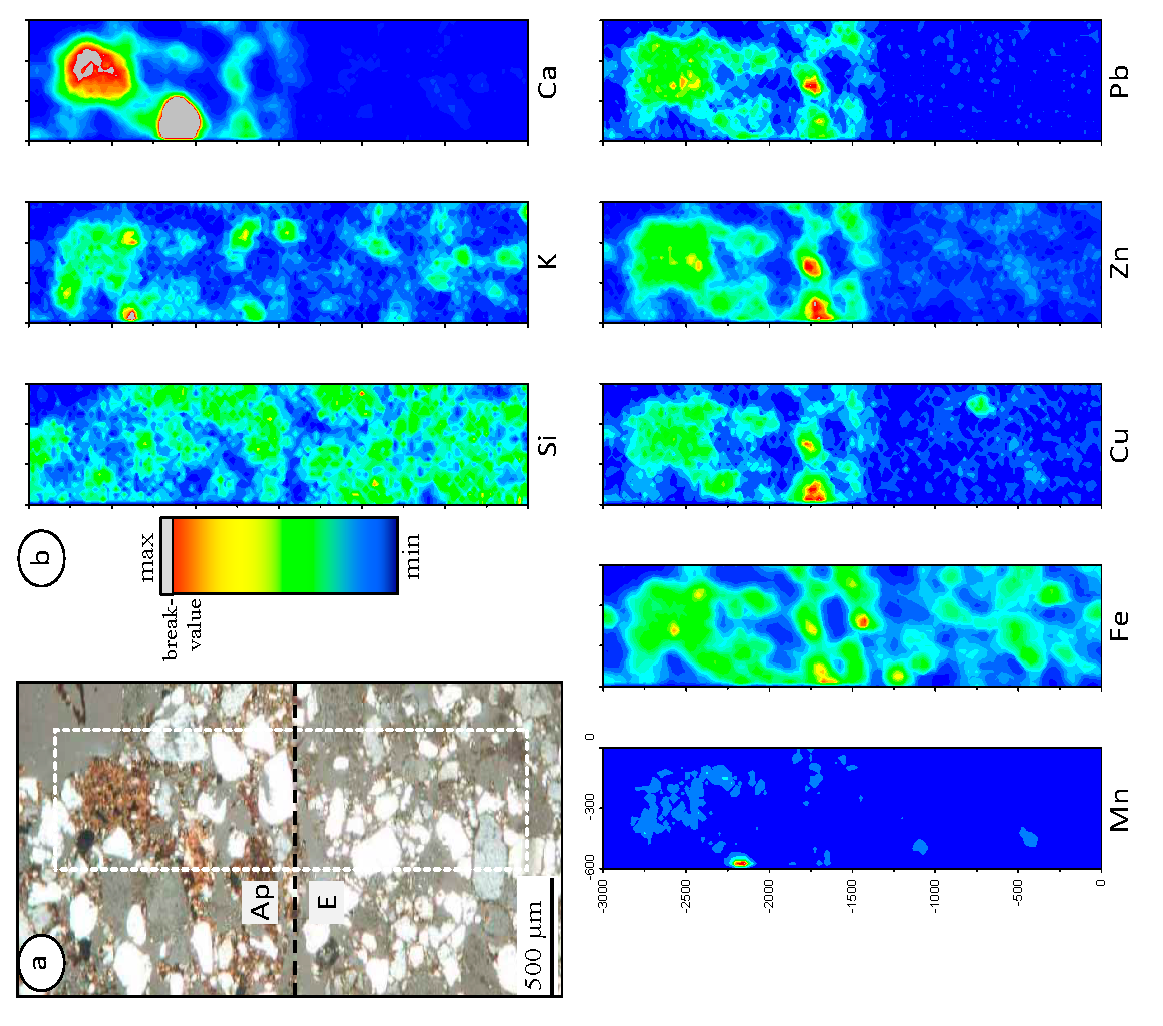
3.2.2. Physicochemical Significance of Horizon Boundaries Regarding Metal Retention
3.2.3. µ-XRF Elemental Correlation Analyses on Selected Features of A, E, B and C Horizons
4. Discussion
4.1. Relevance of Micrometer-Sized Analyses on Undisturbed Soil Samples
4.2. Soil Processes and Metal Accumulations
4.2.1. Neoformation of Metal-Phases
4.2.2. Accumulation of TM by Soil Constituents
4.2.3. Metal Transport via the Soil Solution in the Soil Profile
4.2.4. Biotic Metal Accumulation
4.2.5. Uncommon Soil Processes in Humid Temperate Climatic Conditions
4.3. Heterogeneous TM Distribution Patterns in Soils Call for Adapted Sampling Strategies
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Ettler, V. Soil contamination near non-ferrous metal smelters: A review. Appl. Geochem. 2016, 64, 56–74. [Google Scholar] [CrossRef]
- Adriano, D.C. Biogeochemical processes regulating metal behavior. In Trace Elements in Terrestrial Environments—Biogeochemistry, Bioavailability, and Risks of Metals; Adriano, D.C., Ed.; Springer: New York, NY, USA, 2001; ISBN 978-1-4684-9505-8. [Google Scholar]
- Sobanska, S.; Ricq, N.; Laboudigue, A.; Guillermo, R.; Brémard, C.; Laureyns, J.; Merlin, J.C.; Wignacourt, J.P. Microchemical investigations of dust emitted by a lead smelter. Environ. Sci. Technol. 1999, 33, 1334–1339. [Google Scholar] [CrossRef]
- Lamy, I.; van Oort, F.; Dère, C.; Baize, D. Use of major- and trace-element correlations to assess metal migration in sandy Luvisols irrigated with wastewater. Eur. J. Soil Sci. 2006, 57, 731–740. [Google Scholar] [CrossRef]
- Stoops, G. Guidelines for Analysis and Description of Soil and Regolith Thin Sections; SSSA Inc.: Madison, WI, USA, 2003; ISBN 0-8911-842-8. [Google Scholar]
- Violante, A.; Cozolino, V.; Perelomov, L.; Caporale, A.G.; Pigna, M. Mobility and bioavailability of heavy metals and metalloids in soil environments. J. Soil Sci. Plant Nutr. 2010, 10, 268–292. [Google Scholar] [CrossRef]
- Kretzschmar, R.; Borkovec, M.; Grolimund, D.; Elimelech, M. Mobile subsurface colloids and their role in contaminant transport. Adv. Agron. 1999, 66, 121–193. [Google Scholar] [CrossRef]
- Jacobson, A.R.; Dousset, S.; Andreux, F.; Baveye, P.C. Electron microprobe and synchrotron X-ray fluorescence mapping of the heterogeneous distribution of copper in high-copper vineyard soils. Environ. Sci. Technol. 2007, 41, 6343–6349. [Google Scholar] [CrossRef] [PubMed]
- Scarciglia, F.; Barca, D.; De Rosa, R.; Pulice, I. Application of laser ablation ICP-MS and traditional micromorphological techniques to the study of an Alfisol (Sardinia, Italy) in thin sections: Insights into trace element distribution. Geoderma 2009, 152, 113–126. [Google Scholar] [CrossRef]
- Scarciglia, F.; Barca, D. A powerful tool for assessing distribution and fate of potentially toxic metals (PTMs) in soils: Integration of laser ablation spectrometry (LA-ICP-MS) on thin sections with soil micromorphology and geochemistry. Environ. Sci. Pollut. Res. 2017, 24, 9776–9790. [Google Scholar] [CrossRef] [PubMed]
- Zampella, M.; Adamo, P.; Caner, L.; Petit, S.; Righi, D.; Terribile, F. Chromium and copper in micromorphological features and clay fractions of volcanic soils with andic properties. Geoderma 2010, 157, 185–195. [Google Scholar] [CrossRef]
- Baize, D.; van Oort, F. Potentially Harmful Elements in Forest Soils. A Pedological Viewpoint. In Potential Harmful Elements, Environment and Human Health; Bini, C., Bech, J., Eds.; Springer Science + Business Media: Dordrecht, The Netherlands, 2014; pp. 151–198. ISBN 978-94-017-8964-6. [Google Scholar]
- Stoops, G.; Marcellino, V.; Mees, F. Interpretation of Micromorphological Features of Soils and Regoliths; Elsevier: Amsterdam, The Netherlands, 2010; 720p, ISBN 978-0-444-53156-8. [Google Scholar]
- Leguédois, S.; van Oort, F.; Jongmans, A.G.; Chevallier, P. Morphology, chemistry and distribution of neoformed mineral species in agricultural land affected by metallurgical point-source pollution. Environ. Pollut. 2004, 130, 135–148. [Google Scholar] [CrossRef] [PubMed]
- Van Oort, F.; Jongmans, A.G.; Citeau, L.; Lamy, I.; Chevallier, P. Microscale Zn and Pb distribution patterns in subsurface soil horizons: An indication for metal transport dynamics. Eur. J. Soil Sci. 2006, 57, 154–166. [Google Scholar] [CrossRef]
- Van Oort, F.; Labanowski, J.; Jongmans, T.; Thiry, M. Le devenir des polluants métalliques dans les sols: Révélateur d’impacts de l’activité humaine sur la pédogenèse? Etude Gest. Sols 2007, 14, 287–303. [Google Scholar]
- Van Oort, F.; Jongmans, A.G.; Lamy, I.; Baize, D.; Chevallier, P. Impacts of long-term wastewater irrigation on the development of sandy Luvisols: Consequences for metal pollutant distributions. Eur. J. Soil Sci. 2008, 59, 925–938. [Google Scholar] [CrossRef]
- Van Oort, F.; Thiry, M.; Foy, E.; Fujisaki, K.; Delarue, G.; Dairon, R.; Jongmans, A.G. Impacts of one century of wastewater discharge on soil transformation through ferrolysis and related metal pollutant distributions. Sci. Total Environ. 2017, 590–591, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Neaman, A.; Martínez, C.E.; Trolard, F.; Bourrié, G. Trace element associations with Fe- and Mn-oxides in soil nodules: Comparison of selective dissolution with electron probe microanalysis. Appl. Geochem. 2008, 23, 778–782. [Google Scholar] [CrossRef]
- Huot, H.; Simonnot, M.O.; Watteau, F.; Marion, P.; Yvon, J.; De Donato, P.; Morel, J.L. Early transformation and transfer processes in a Technosol developing on iron industry deposits. Eur. J. Soil Sci. 2014, 65, 470–484. [Google Scholar] [CrossRef]
- Kelly, S.D.; Hesterberg, D.; Ravel, B. Analysis of soils and minerals using X-ray absorption spectroscopy. In Methods of Soil Analysis, Part 5—Mineralogical Methods; Ulery, A.L., Drees, L.R., Eds.; SSSA Inc.: Madison, WI, USA, 2008; pp. 387–463. ISBN 978-0-89118-746-9. [Google Scholar]
- Sterckeman, T.; Douay, F.; Proix, N.; Fourrier, H.; Perdrix, E. Assessment of the contamination of cultivated soils by eighteen trace elements around smelters in the north of France. Water Air Soil Pollut. 2002, 135, 173–194. [Google Scholar] [CrossRef]
- Thiry, M.; Huet-Taillanter, S.; Schmitt, J.M. La friche industrielle de Mortagne-du-Nord (59). I. Prospection du site, composition des scories, hydrologie et estimation des flux. Bull. Soc. Géol. Fr. 2002, 173, 369–381. [Google Scholar] [CrossRef]
- Van Oort, F.; Thiry, M.; Jongmans, A.G.; Bourennane, H.; Cambier, P.; Lamy, I.; Citeau, L.; Nahmani, J. Pollutions métalliques : Distributions hétérogènes du Zn, Pb, Cd, et Cu et relations avec l’usage des sols. In Contaminations Métalliques des Agrosystèmes et Écosystèmes Péri-Urbains; Cambier, P., Schvartz, C., van Oort, F., Eds.; Éditions Quæ: Versailles, France, 2009; pp. 15–44. ISBN 978-2-7592-0275-1. [Google Scholar]
- Fernandez, C.; Labanowski, J.; Cambier, P.; Jongmans, A.G.; van Oort, F. Fate of airborne pollution in soils as related to agricultural management: 1. distributions of Zn and Pb in soil profiles. Eur. J. Soil Sci. 2007, 58, 547–559. [Google Scholar] [CrossRef]
- Fernandez, C.; Labanowski, J.; Jongmans, T.; Bermond, A.; Cambier, P.; Lamy, I.; van Oort, F. Fate of airborne metal pollution in soils as related to agricultural management: 2. Assessing the role of biological activity in micro-scale Zn and Pb distributions in A, B and C horizons. Eur. J. Soil Sci. 2010, 61, 514–524. [Google Scholar] [CrossRef]
- IUSS Working Group WRB. World Reference Base for Soil Resources. In International Soil Classification System for Naming Soils and Creating Legends for Soil Maps; World Soil Resources Reports No. 106; FAO: Rome, Italy, 2014; ISBN 978-92-5-108369-7. [Google Scholar]
- Toze, S. Reuse of effluent water—Benefits and risks. Agric. Water Manag. 2006, 80, 147–159. [Google Scholar] [CrossRef]
- Ternes, T.A.; Bonerz, M.; Herrmann, N.; Teiser, B.; Andersen, H. Irrigation of treated wastewater in Braunschweig, Germany: An option to remove pharmaceuticals and musk fragrances. Chemosphere 2007, 66, 894–904. [Google Scholar] [CrossRef] [PubMed]
- Védry, B.; Gousailles, M.; Affholder, M.; Lefaux, A.; Bontoux, J. From sewage water treatment to wastewater reuse. One century of Paris sewage farms history. Water Sci. Technol. 2001, 43, 101–107. [Google Scholar] [PubMed]
- Tamtam, F.; van Oort, F.; LeBot, B.; Dinh, T.; Mompelat, S.; Chreveuil, M.; Lamy, I.; Thiry, M. Assessing antibiotic contamination in metal contaminated soils four years after cessation of long-term wastewater irrigation. Sci. Total Environ. 2011, 409, 540–547. [Google Scholar] [CrossRef] [PubMed]
- Thiry, M.; van Oort, F.; Thiesson, J.; van Vliet-Lanoë, B. Periglacial morphogenesis in the Paris Basin: Insight from geophysical prospection and impacts for the fate of soil pollution. Geomorphology 2013, 197, 34–44. [Google Scholar] [CrossRef] [Green Version]
- FitzPatrick, E.A. Soil Microscopy and Micromorphology; Wiley: Chichester, UK, 1993; 304p, ISBN 0-471-93859-9. [Google Scholar]
- Canti, M.G. An investigation of microscopic calcareous spherulites from herbivore dungs. J. Archaeol. Sci. 1997, 24, 219–231. [Google Scholar] [CrossRef]
- Bullock, P.N.; Fédoroff, N.; Jongerius, A.; Stoops, G.; Tursina, T. Handbook for Soil Thin Section Description; Waine Research Publications: Albrighton, UK, 1985; 152p, ISBN 09051847-09-2. [Google Scholar]
- Labanowski, J.; Sebastia, J.; Foy, E.; Jongmans, A.G.; Lamy, I.; van Oort, F. Fate of metal-associated POM in a soil under arable land use contaminated by metallurgical fallout in northern France. Environ. Pollut. 2007, 149, 59–69. [Google Scholar] [CrossRef] [PubMed]
- Fendorf, S.; La Force, M.J.; Li, G. Temporal changes in soil partitioning and bioaccessibility of arsenic, chromium and lead. J. Environ. Qual. 2004, 33, 2049–2055. [Google Scholar] [CrossRef] [PubMed]
- Brinkman, R. Ferrolysis, a hydromorphic soil forming process. Geoderma 1970, 3, 199–206. [Google Scholar] [CrossRef]
- Thompson, I.A.; Huber, D.M.; Guest, C.A.; Schulze, D.G. Fungal manganese oxidation in a reduced soil. Environ. Microbiol. 2005, 7, 1480–1487. [Google Scholar] [CrossRef] [PubMed]
- Flores-Velez, L.M.; Ducaroir, J.; Jaunet, A.M.; Robert, M. Study of the distribution of copper in an acid sandy vineyard soil by three different methods. Eur. J. Soil Sci. 1996, 47, 523–532. [Google Scholar] [CrossRef]
- Besnard, E.; Chenu, C.; Robert, M. Influence of organic amendments on copper distribution among particle-size and density fractions in Champagne vineyard soils. Environ. Pollut. 2001, 112, 329–337. [Google Scholar] [CrossRef]
- Citeau, L.; Lamy, I.; van Oort, F.; Elsass, F. Colloidal facilitated transfer of metals in soils under different land use. Colloids Surf. A 2003, 217, 11–19. [Google Scholar] [CrossRef]
- Banfield, J.F.; Nealson, K.H. Geomicrobiology: Intercations between Microbes and Minerals; Reviews in Mineralogy; Mineralogical Society of America: Chantilly, VA, USA, 1997; Volume 35, 448p, ISBN 978-0939950454. [Google Scholar]
- Lund, U.; Fobian, A. Pollution of two soils by arsenic, chromium and copper, Denmark. Geoderma 1991, 49, 83–103. [Google Scholar] [CrossRef]
- Cotter-Howells, J.D.; Caporn, S. Remediation of contaminated land by formation of heavy metal phosphates. App. Geochem. 1996, 11, 335–342. [Google Scholar] [CrossRef]
- Hettiarachchi, G.M.; Pierzynski, G.M. In situ stabilization of soil lead using phosphorus and manganese oxide: Influence of plant growth. J. Environ. Qual. 2002, 31, 564–572. [Google Scholar] [CrossRef] [PubMed]
- Magdi Selim, H. Phosphates in Soil. Interaction with Micronutriments, Radionuclides and Heavy Metals; CRC Press, Taylor & Francis Group: Boca Raton, FL, USA, 2015; 381p, ISBN 978-1-4822-3679-8. [Google Scholar]
- Christensen, B.T. Physical fractionaton of soil and structural and functional complexity in organic matter turnover. Eur. J. Soil Sci. 2001, 52, 345–353. [Google Scholar] [CrossRef]
- Ducaroir, J.; Lamy, I. Evidence of trace metal association with soil organic matter using particle size fractionation after physical dispersion treatment. Analyst 1995, 120, 741–745. [Google Scholar] [CrossRef]
- Lehmann, J.; Kleber, M. The contentious nature of soil organic matter. Nature 2015, 528, 60–68. [Google Scholar] [CrossRef] [PubMed]
- Mikutta, R.; Kleber, M.; Torn, M.S.; Jahn, R. Stabilization of soil organic matter: Association with minerals or chemical recalcitrance? Biogeochemistry 2006, 77, 25–56. [Google Scholar] [CrossRef]
- Bundt, M.; Albrecht, A.; Froidevaux, P.; Blaser, P.; Flühler, H. Impact of preferential flow on radionuclide distribution in soil. Environ. Sci. Technol. 2000, 34, 3895–3899. [Google Scholar] [CrossRef]
- Knechtenhofer, L.A.; Xifra, I.O.; Scheinost, A.C.; Flühler, H.; Kretzschmar, R. Fate of heavy metals in a strongly acidic shooting-range soil: Small-scale metal distribution and its relation to preferential water flow. J. Plant Nutr. Soil Sci. 2003, 166, 84–92. [Google Scholar] [CrossRef]
- Tack, F.M.G. Trace elements: General soil chemistry, principles and processes. In Trace Elements in Soils; Hooda, P.S., Ed.; Wiley: Chichester, UK, 2010; pp. 9–39. ISBN 978-1-045-16037-7. [Google Scholar]
- Citeau, L. Etude des Colloïdes Naturels Circulant dans les eaux Gravitaires de sols Contaminés: Nature des Colloïdes et Réactivité vis-à-vis des Métaux. Ph.D. Thesis, 2004, INAPG, Paris, France.
- Kühn, P.; Aguilar, J.; Miedema, R. Textural pedofeatures and related horizons. In Interpretation of Micromorphological Features of Soils and Regoliths; Stoops, G., Marcelino, V., Mees, F., Eds.; Elsevier: Amsterdam, The Netherlands, 2010; pp. 217–250. ISBN 978-0-444-53156-8. [Google Scholar]
- Ehrlich, H.L. Geomicrobiology: Its significance for geology. Earth Sci. Rev. 1998, 45, 45–60. [Google Scholar] [CrossRef]
- Soltner, D. Les Bases de la Production Végétale 1. Le sol, 12th ed.; Sciences et Techniques Agricoles: Paris, France, 1983. [Google Scholar]

| Hor | Depth | Org. C | BD | Clay | Sand | pH | S/CEC | Ca | Fe | Mn | Zn | Pb | Cu |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| - | cm | g/kg | kg/m3 | g/kg | - | - | g/100g | mg/kg | |||||
| Ap1 | 0–30 | 18 | 1520 | 76 | 688 | 6.8 | sat | 0.30 | 1.05 | 153 | 1046 | 362 | 56 |
| Ap2 | 30–35 | 22 | 1700 | 76 | 699 | 7.2 | sat | 0.22 | 1.40 | 291 | 2635 | 517 | 113 |
| AB | 35–45 | 3 | 1650 | 77 | 673 | 7.3 | sat | 0.14 | 1.03 | 143 | 940 | 21.5 | 11 |
| B1 | 45–60 | 2 | 1680 | 85 | 657 | 7.0 | sat | 0.19 | 1.07 | 406 | 259 | 14.6 | 8.6 |
| B2g | 60–80 | <1 | 1550 | 96 | 631 | 6.7 | sat | 0.17 | 1.25 | 660 | 86 | 11.1 | 6.7 |
| BCg | 80–120 | <1 | 1550 | 166 | 639 | 6.6 | sat | 0.22 | 1.91 | 461 | 53 | 12.3 | 6.7 |
| C1g | 120–150 | <1 | 1650 | 124 | 819 | 6.1 | sat | 0.15 | 1.55 | 171 | 28 | 11.9 | 6.0 |
| Hor | Depth | Org. C | BD | Clay | Sand | pH | CaCO3 | Ca | Fe | Mn | Zn | Pb | Cu |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| - | cm | g/kg | kg/m3 | g/kg | - | % | g/100g | mg/kg | |||||
| Ap | 0−40 | 31.0 | 1250 | 91 | 767 | 7.1 | 43.9 | 2.25 | 1.25 | 161 | 1130 | 607 | 329 |
| E | 40−55 | 1.3 | 1730 | 41 | 877 | 7.3 | <1 | 0.12 | 0.56 | 40 | 246 | 7.9 | 44.6 |
| Bt | 55−75 | 2.4 | 1610 | 206 | 717 | 7.5 | 2.5 | 0.40 | 2.49 | 118 | 463 | 11.8 | 23.3 |
| B/C | 75−90 | 1.5 | 1570 | 39 | 827 | 8.3 | 162 | 5.64 | 1.06 | 118 | 171 | 7.3 | 27.1 |
| Cs | 90−120 | 1.4 | 1710 | 108 | 759 | 8.7 | 382 | 9.68 | 0.75 | 160 | 27 | 4.0 | 5.5 |
| Cc * | >60 | 1.7 | - | 196 | 597 | 8.6 | 573 | 13.9 | 0.56 | 81 | 52 | 3.8 | 10.1 |
| Photo | ρ Major/Major | ρ Trace/Trace | ρ Zn/Major | ρ Pb/Major | ρ Cu/Major | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n° | Fe/Mn | Fe/Ca | Ca/Mn | Zn/Pb | Zn/Cu | Cu/Pb | Zn/Fe | Zn/Mn | Zn/Ca | Pb/Fe | Pb/Mn | Pb/Ca | Cu/Fe | Cu/Mn | Cu/Ca | Additional Remarkable Correlation |
| 3a | 0.98 | 0.94 | 0.95 | 0.98 | 0.80 | 0.82 | 0.98 | 0.96 | 0.91 | 0.98 | 0.98 | 0.93 | 0.80 | 0.84 | 0.80 | Fe/P: 0.57 |
| 3b | - | 0.52 | - | 0.87 | 0.55 | - | 0.80 | - | 0.76 | 0.84 | - | 0.61 | - | - | 0.58 | |
| 3c | 0.73 | 0.55 | - | 0.63 | 0.68 | 0.60 | 0.67 | 0.92 | - | 0.94 | 0.66 | - | 0.54 | 0.64 | - | |
| 3d | - | 0.55 | - | 0.61 | 0.59 | - | 0.73 | 0.59 | - | 0.60 | - | - | - | - | - | |
| 3e | 0.57 | - | - | - | - | - | 0.81 | 0.85 | - | - | - | - | - | - | - | |
| 3f | 0.89 | 0.56 | 0.61 | - | - | - | 0.94 | 0.88 | - | - | - | - | - | - | - | Ti/K: 0.82; Ca/K: 0.74 |
| 3g | 0.70 | - | 0.56 | - | - | - | 0.90 | 0.68 | - | - | - | - | - | - | - | Fe/Ti: 0.84; Zn/Ti: 0.82; Mn/Ti: 0.71; |
| 3h | 0.58 | - | - | - | - | - | 0.59 | 0.78 | - | - | - | - | - | - | - | |
| 3i | - | 0.66 | - | - | - | - | - | - | - | 0.78 | - | - | - | - | - | K/Ti: 0.67 |
| Photo | ρ Major/Major | ρ Trace/Trace | ρ Zn/Major | ρ Pb/Major | ρ Cu/Major | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n° | Fe/Mn | Fe/Ca | Ca/Mn | Zn/Pb | Zn/Cu | Cu/Pb | Zn/Fe | Zn/Mn | Zn/Ca | Pb/Fe | Pb/Mn | Pb/Ca | Cu/Fe | Cu/Mn | Cu/Ca | Other Remarkable Correlation |
| 6a | - | 0.76 | - | 0.76 | 0.86 | 0.68 | 0.95 | - | 0.74 | 0.75 | - | - | 0.85 | - | 0.66 | |
| 6b | - | 0.79 | - | 0.77 | 0.52 | 0.53 | 0.88 | - | 0.76 | 0.80 | - | 0.63 | 0.52 | - | - | |
| 6c1 | 0.84 | 0.77 | 0.62 | 0.94 | 0.91 | 0.86 | 0.98 | 0.81 | 0.80 | 0.92 | 0.73 | 0.76 | 0.88 | 0.65 | 0.79 | Cu/Cr: 0.70; Pb/Cr: 0.64; Zn/Cr: 0.63 |
| 6c2 | 0.70 | 0.86 | 0.74 | 0.95 | 0.95 | 0.93 | 0.91 | 0.71 | 0.92 | 0.91 | 0.71 | 0.90 | 0.90 | 0.75 | 0.90 | Pb/Cr: 0.64; Cu/Cr: 0.62; Zn/Cr: 0.62 |
| 6d | 0.65 | 0.65 | 0.57 | 0.78 | 0.80 | 0.73 | 0.83 | 0.62 | 0.84 | 0.61 | 0.53 | 0.82 | 0.69 | 0.52 | 0.76 | Zn/K: 0.66 |
| 6d1 | 0.69 | 0.80 | 0.65 | 0.88 | 0.86 | 0.81 | 0.92 | 0.69 | 0.84 | 0.84 | 0.65 | 0.80 | 0.82 | 0.61 | 0.74 | Fe/K: 0.75; Zn/K: 0.75, Pb/K: 0.70 |
| 6d2 | - | 0.54 | - | - | - | - | 0.71 | - | - | - | - | - | - | - | - | |
| 6e | 0.63 | - | - | - | 0.75 | - | 0.67 | 0.98 | - | - | - | - | - | 0.77 | - | Ni/Mn: 0.86; Ni/Fe: 0.62 |
| 6f | - | - | - | 0.52 | 0.86 | - | 0.97 | - | - | 0.53 | - | - | 0.88 | - | - | |
| 6g | - | - | - | 0.78 | 0.66 | 0.57 | 0.98 | - | - | 0.76 | - | - | 0.65 | - | - | Fe/Ti: 0.70; Zn/Ti: 0.66 |
| 6h | - | 0.98 | - | - | - | - | 0.94 | 0.60 | 0.94 | - | - | - | - | - | - | Zn/K: 0.87; Zn/Ti: 0.88; Fe/K: 0.70 |
| 6i | 0.79 | - | - | - | - | - | 0.86 | 0.95 | - | - | - | - | - | - | - | Ni/Mn: 0.74; Zn/Ni: 0.71; Fe/K: 0.62 |
| 6j | 0.57 | - | - | - | - | - | 0.66 | 0.82 | - | - | - | - | - | - | - | Ni/Mn: 0.64; Fe/K: 0.58; Zn/K: 0.53 |
| 6k | 0.72 | - | - | 0.65 | 0.72 | 0.66 | - | - | - | - | 0.75 | 0.68 | - | Fe/Ti: 0.70; Zn/Ti: 0.67; Fe/K: 0.58 | ||
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Van OORT, F.; Foy, E.; Labanowski, J.; Leguédois, S.; Jongmans, T. Soil Processes, Pedofeatures and Microscale Metal Distributions: Relevant Study of Contaminant-Dynamics Calls for Pedology-Based Soil-Depth Sampling Strategies. Soil Syst. 2018, 2, 17. https://doi.org/10.3390/soilsystems2010017
Van OORT F, Foy E, Labanowski J, Leguédois S, Jongmans T. Soil Processes, Pedofeatures and Microscale Metal Distributions: Relevant Study of Contaminant-Dynamics Calls for Pedology-Based Soil-Depth Sampling Strategies. Soil Systems. 2018; 2(1):17. https://doi.org/10.3390/soilsystems2010017
Chicago/Turabian StyleVan OORT, Folkert, Eddy Foy, Jérôme Labanowski, Sophie Leguédois, and Toine Jongmans. 2018. "Soil Processes, Pedofeatures and Microscale Metal Distributions: Relevant Study of Contaminant-Dynamics Calls for Pedology-Based Soil-Depth Sampling Strategies" Soil Systems 2, no. 1: 17. https://doi.org/10.3390/soilsystems2010017





