Constraints to Synergistic Fe Mobilization from Calcareous Soil by a Phytosiderophore and a Reductant
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. General Experimental Procedure
2.3. Experiments
2.4. Analysis
3. Results and Discussion
3.1. Ascorbate Residence Time in Soil Solution
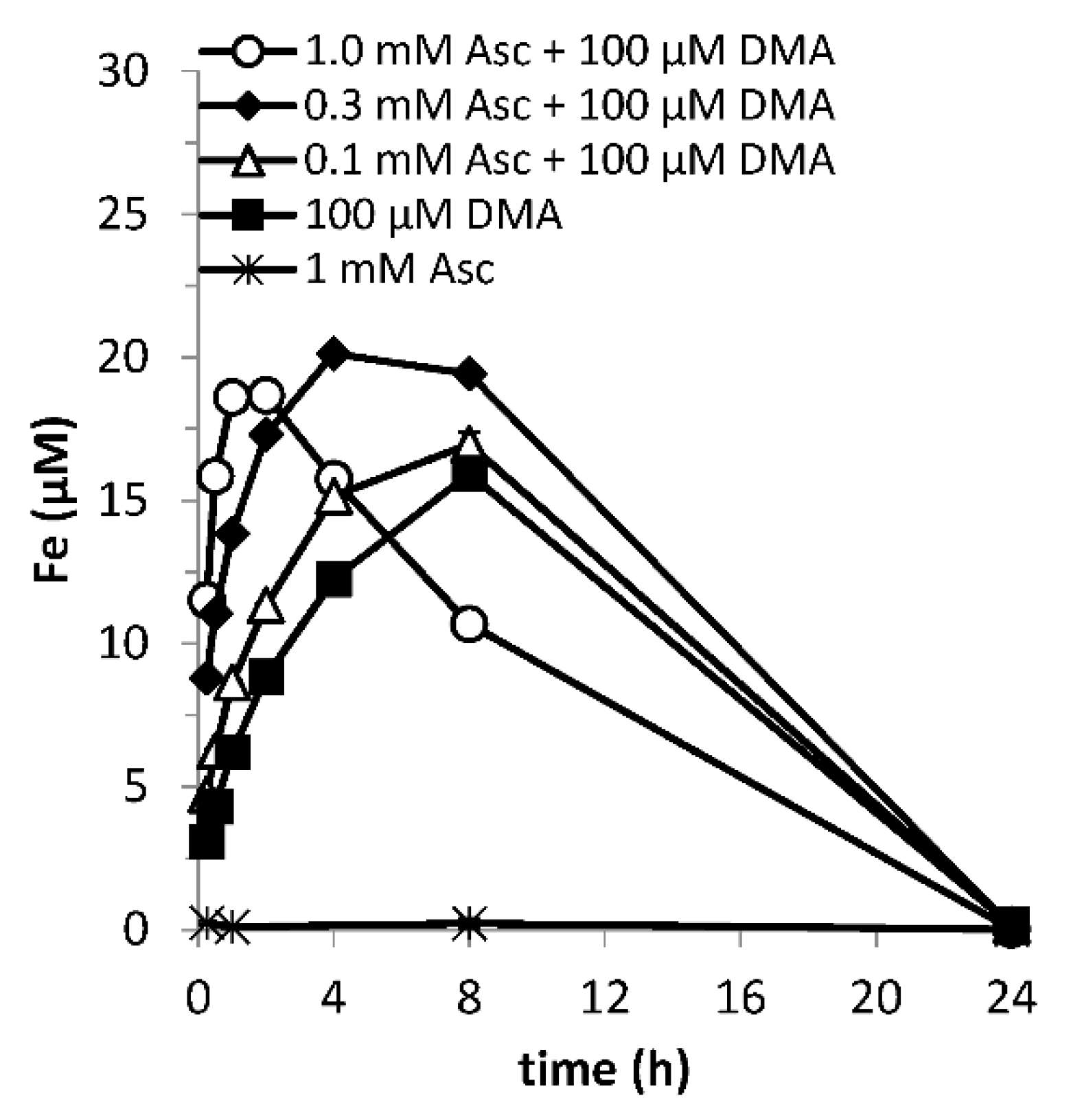
3.2. Lag Time between Addition of Reductant and Chelating Ligand
3.3. Varying Reductant Concentration
3.4. Effect of Microbial Degradation
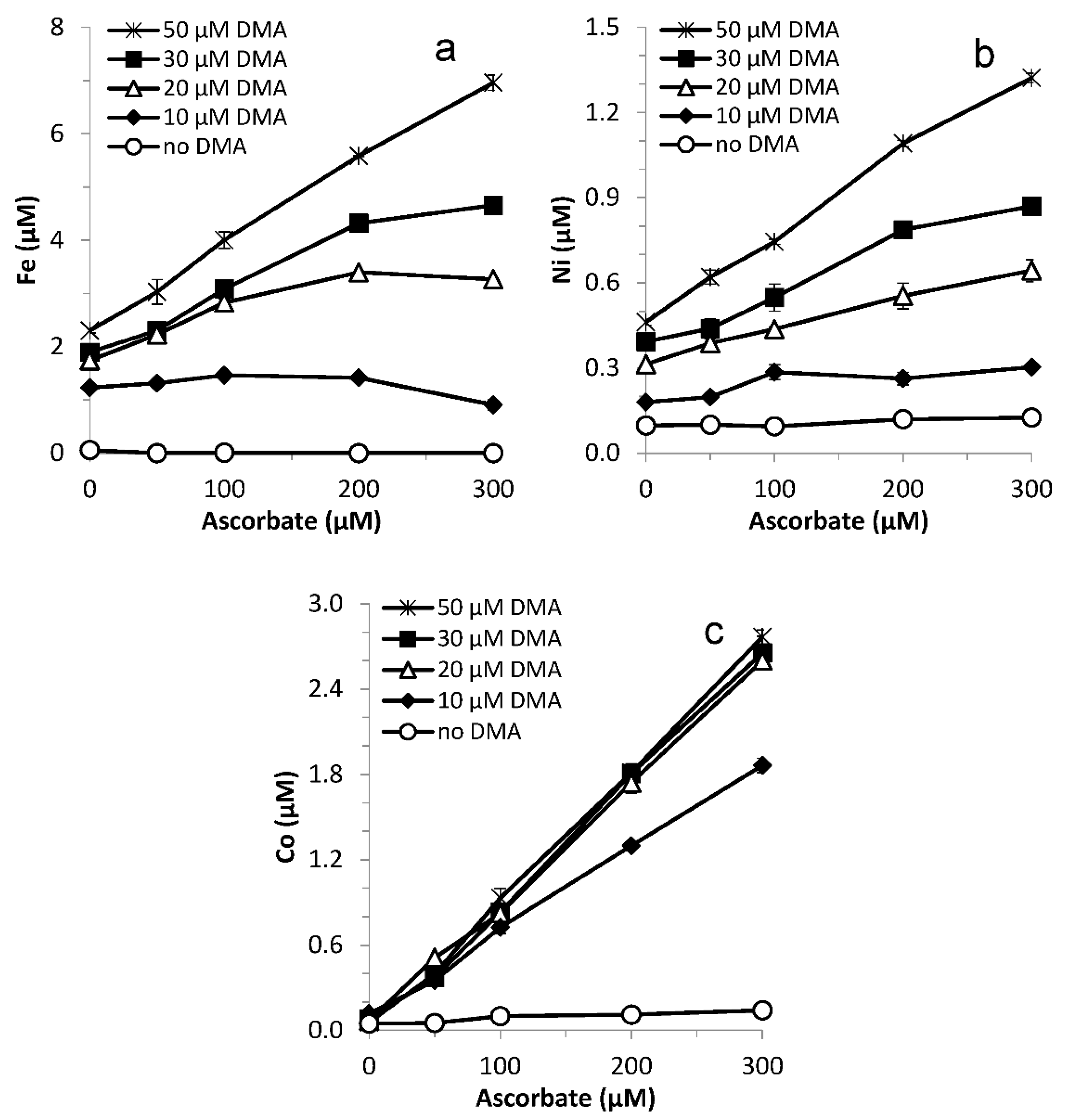
3.5. Varying Ligand Concentration
3.6. Varying Ligand and Reductant Concentration
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
| Extraction | ||||
|---|---|---|---|---|
| Origin/name | Santomera | CDB | Fe (g kg−1) | 10.2 |
| Region | Murcia | AmOx | Fe (g kg−1) | 0.5 |
| Country | Spain | DTPA | Fe (mg kg−1) | 4.9 |
| Soil classification | entisol | Cu (mg kg−1) | 1.6 | |
| pH CaCl2 | 7.8 | Ni (mg kg−1) | 0.3 | |
| EC (mS cm−1) | 0.11 | Zn (mg kg−1) | 0.5 | |
| SOC (g kg−1) | 7.3 | Co (mg kg−1) | 0.0 | |
| Clay (g kg−1) | 300 | Mn (mg kg−1) | 3.1 | |
| CaCO3 (g kg−1) | 500 | |||
Appendix B
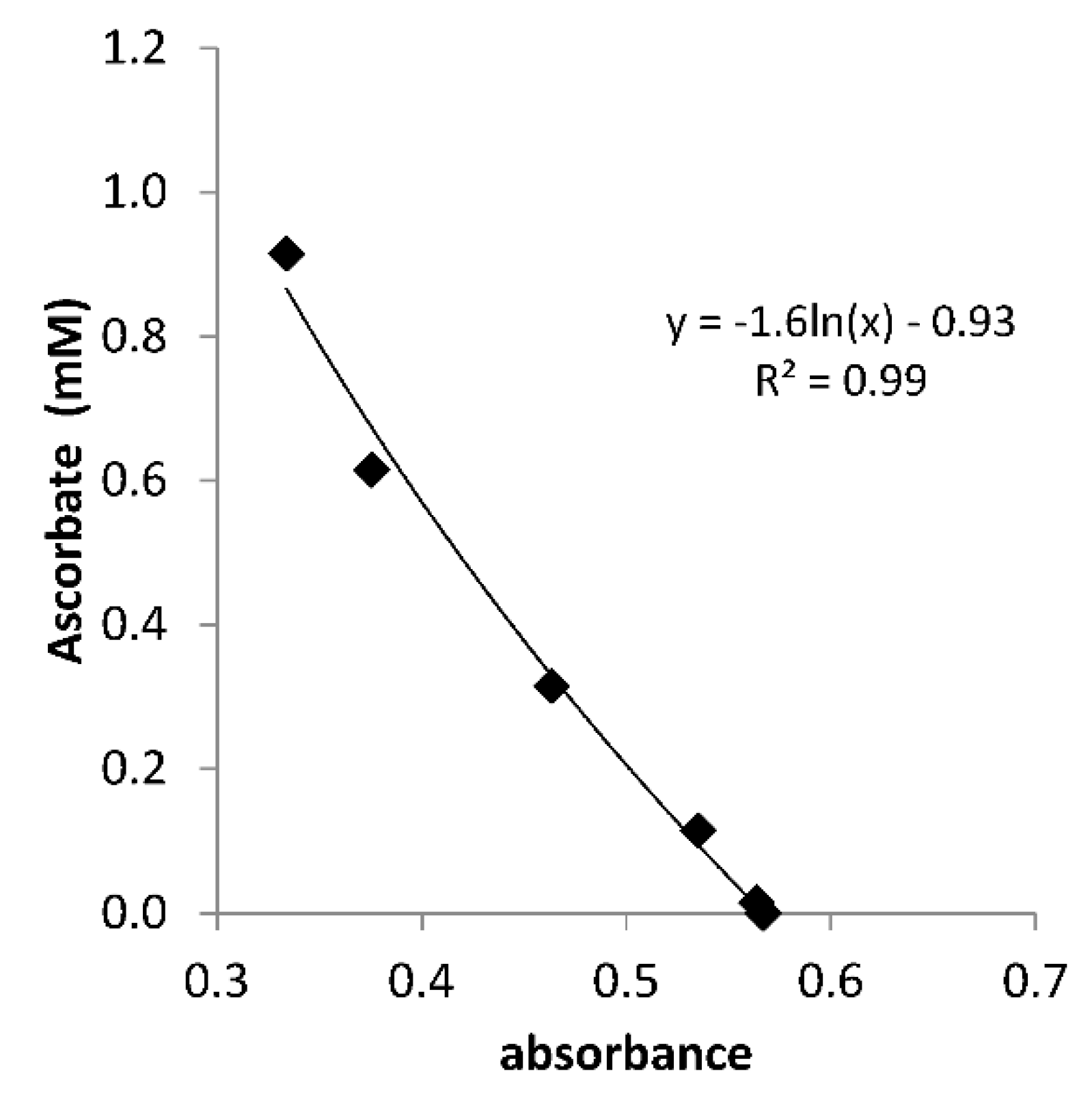
| Interaction Time (h) | Absorbance | Concentration (mM) |
|---|---|---|
| 0.083 | 0.5594 | 0.022 |
| 0.25 | 0.5616 | <0.02 |
| 0.5 | 0.5668 | <0.02 |
| 1 | 0.5646 | <0.02 |
| 4 | 0.5618 | <0.02 |
Appendix C
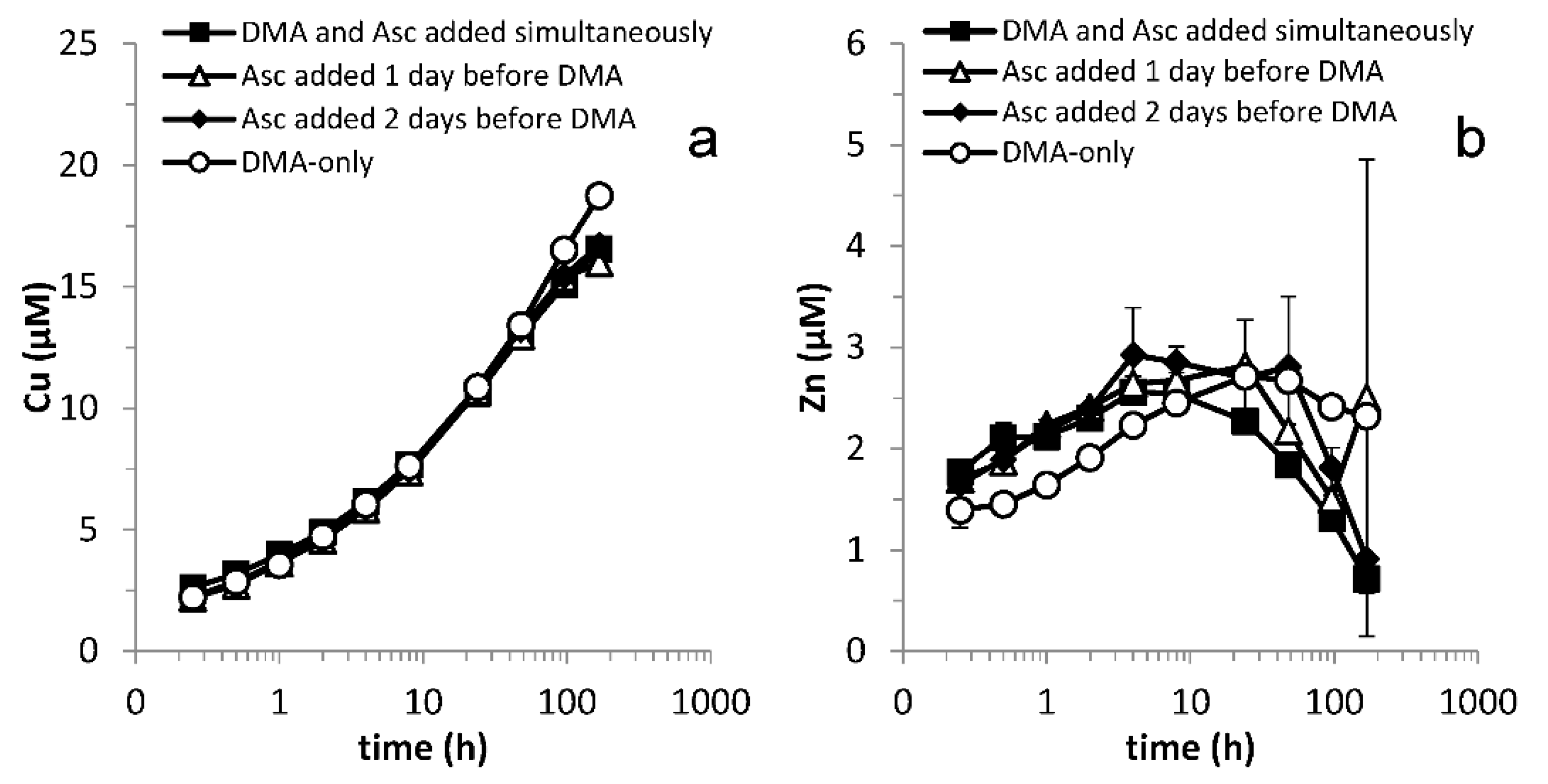
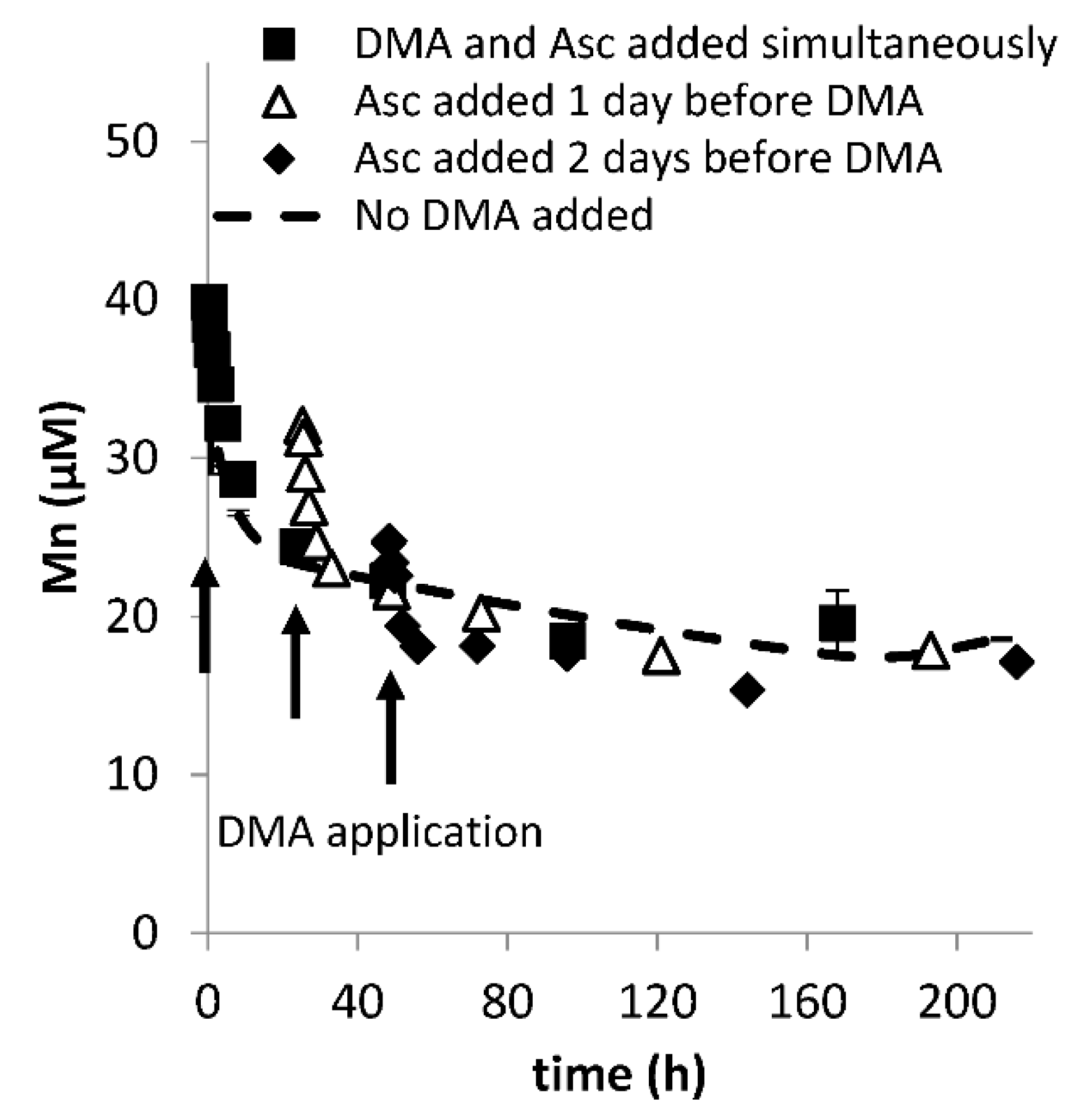
Appendix D
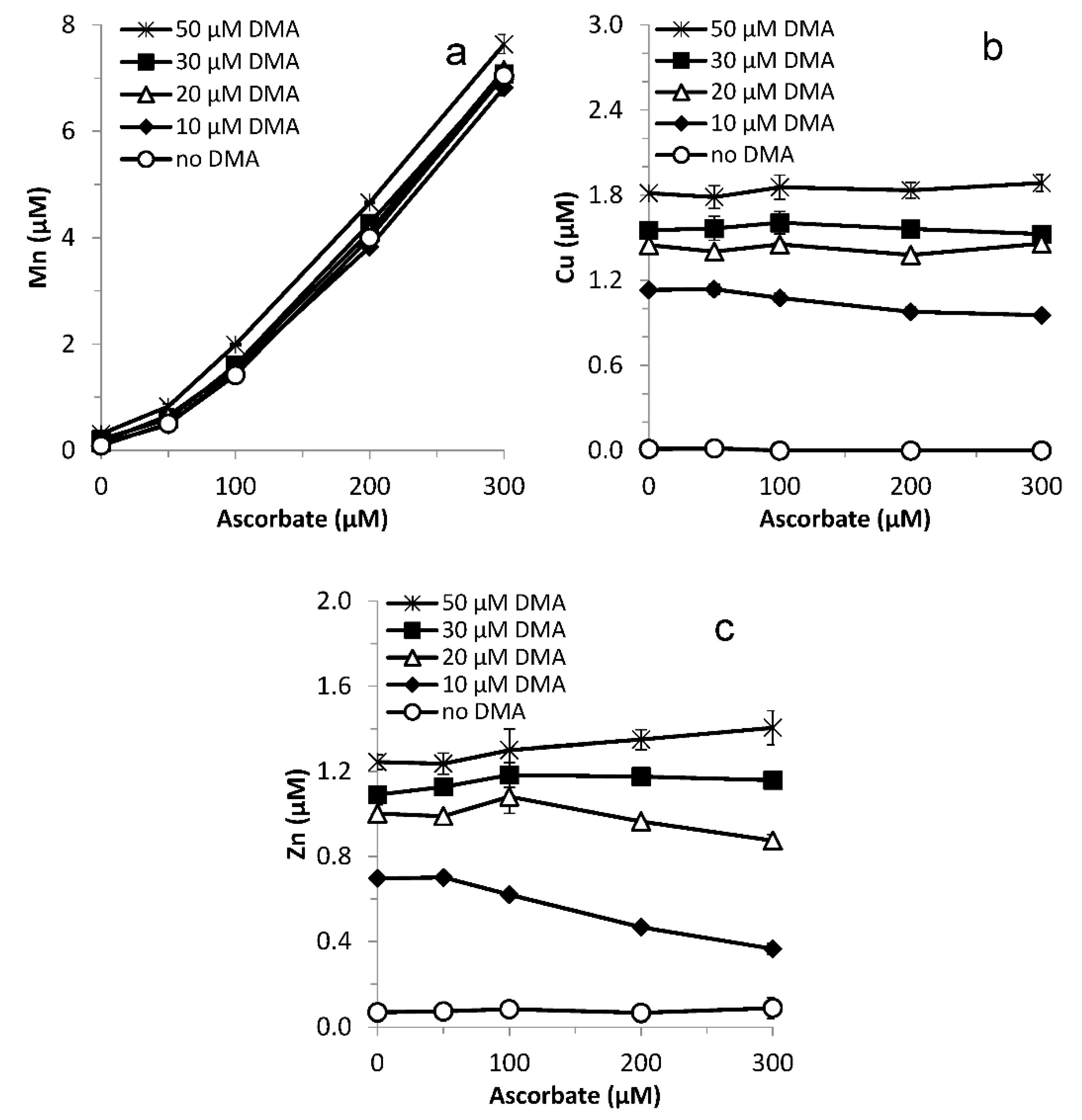
Appendix E
| [ascorbate] (µM) | |||||
|---|---|---|---|---|---|
| [DMA] (µM) | 0 | 50 | 100 | 200 | 300 |
| 10 | ● | ||||
| 20 | ● | ● | |||
| 30 | ● | ● | ● | ● | |
| 50 | ● | ● | ● | ● | ● |
References
- Hersman, L.E.; Huang, A.; Maurice, P.A.; Forsythe, J.E. Siderophore production and iron reduction by Pseudomonas mendocina in response to iron deprivation. Geomicrobiol. J. 2000, 17, 261–273. [Google Scholar] [CrossRef]
- Marschner, H. Mineral Nutrition of Higher Plants, 2nd ed.; Academic Press: London, UK, 1995; p. 889. [Google Scholar]
- Lindsay, W.L. Chemical Equilibria in Soils; John Wiley and Sons: New York, NY, USA, 1979; p. 449. [Google Scholar]
- Mortvedt, J.J. Correcting iron deficiencies in annual and perennial plants: Present technologies and future prospects. Plant Soil 1991, 130, 273–279. [Google Scholar] [CrossRef]
- Martin, J.H.; Fitzwater, S.E. Iron-deficiency limits phytoplankton growth in the northeast pacific subarctic. Nature 1988, 331, 341–343. [Google Scholar] [CrossRef]
- Marschner, H.; Römheld, V.; Kissel, M. Different strategies in higher-plants in mobilization and uptake of iron. J. Plant Nutr. 1986, 9, 695–713. [Google Scholar] [CrossRef]
- Marschner, H.; Römheld, V. Strategies of plants for acquisition of iron. Paper presented at the Seventh International Symposium on Iron Nutrition in Soils and Plants, Zaragoza, Spain, 27 June–2 July 1993; pp. 261–274, 1981 ref. [Google Scholar]
- Zinder, B.; Furrer, G.; Stumm, W. The coordination chemistry of weathering II Dissolution of Fe(III) oxides. Geochim. Cosmochim. Acta 1986, 50, 1861–1869. [Google Scholar] [CrossRef]
- Kraemer, S.M. Iron oxide dissolution and solubility in the presence of siderophores. Aquat. Sci. 2004, 66, 3–18. [Google Scholar] [CrossRef]
- Takagi, S.I. Naturally occurring iron-chelating compounds in oat-root and rice-root washings. I. Activity measurement and prelininary characterization. Soil Sci. Plant Nutr. 1976, 22, 423–433. [Google Scholar] [CrossRef]
- Lemanceau, P.; Bauer, P.; Kraemer, S.; Briat, J.F. Iron dynamics in the rhizosphere as a case study for analyzing interactions between soils, plants and microbes. Plant Soil 2009, 321, 513–535. [Google Scholar] [CrossRef]
- Crowley, D.; Kraemer, S.M. Function of Siderophores in the Plant Rhizosphere. In The Rhizosphere: Biochemistry and Organic Substances at the Soil-Plant Interface; Pinton, R., Varanini, Z., Nannipieri, P., Eds.; CRC Press: Boca Raton, FL, USA, 2008; p. 616. [Google Scholar]
- Kraemer, S.M.; Crowley, D.E.; Kretzschmar, R. Geochemical aspects of phytosiderophore-promoted iron acquisition by plants. In Advances Agronomy; Sparks, D.L., Ed.; Elsevier: Amsterdam, The Netherlands, 2006; Volume 91, pp. 1–46. [Google Scholar]
- Takagi, S.; Nomoto, K.; Takemoto, T. Physiological aspect of mugineic acid, a possible phytosiderophore of graminaceous plants. J. Plant Nutr. 1984, 7, 469–477. [Google Scholar] [CrossRef]
- Oburger, E.; Gruber, B.; Schindlegger, Y.; Schenkeveld, W.D.C.; Hann, S.; Kraemer, S.M.; Wenzel, W.; Puschenreiter, M. Root exudation of phytosiderophores from soil grown wheat. New Phytol. 2014, 203, 1161–1174. [Google Scholar] [CrossRef]
- Schenkeveld, W.D.C.; Schindlegger, Y.; Oburger, E.; Puschenreiter, M.; Hann, S.; Kraemer, S.M. Geochemical processes constraining iron uptake in strategy II Fe acquisition. Environ. Sci. Technol. 2014, 48, 12662–12670. [Google Scholar] [CrossRef] [PubMed]
- Oburger, E.; Dell’mour, M.; Hann, S.; Wieshammer, G.; Puschenreiter, M.; Wenzel, W.W. Evaluation of a novel tool for sampling root exudates from soil-grown plants compared to conventional techniques. Environ. Exp. Bot. 2013, 87, 235–247. [Google Scholar] [CrossRef]
- Fan, T.W.M.; Lane, A.N.; Pedler, J.; Crowley, D.; Higashi, R.M. Comprehensive analysis of organic ligands in whole root exudates using nuclear magnetic resonance and gas chromatography mass spectrometry. Anal. Biochem. 1997, 251, 57–68. [Google Scholar] [CrossRef] [PubMed]
- Reichard, P.U.; Kraemer, S.M.; Frazier, S.W.; Kretzschmar, R. Goethite dissolution in the presence of phytosiderophores: Rates, mechanisms, and the synergistic effect of oxalate. Plant Soil 2005, 276, 115–132. [Google Scholar] [CrossRef]
- Schenkeveld, W.D.C.; Wang, Z.M.; Giammar, D.E.; Kraemer, S.M. Synergistic Effects between Biogenic Ligands and a Reductant in Fe Acquisition from Calcareous Soil. Environ. Sci. Technol. 2016, 50, 6381–6388. [Google Scholar] [CrossRef] [PubMed]
- Reichard, P.U.; Kretzschmar, R.; Kraemer, S.M. Dissolution mechanisms of goethite in the presence of siderophores and organic acids. Geochim. Cosmochim. Acta 2007, 71, 5635–5650. [Google Scholar] [CrossRef]
- Akafia, M.M.; Harrington, J.M.; Bargar, J.R.; Duckworth, O.W. Metal oxyhydroxide dissolution as promoted by structurally diverse siderophores and oxalate. Geochim. Cosmochim. Acta 2014, 141, 258–269. [Google Scholar] [CrossRef]
- Cheah, S.F.; Kraemer, S.M.; Cervini-Silva, J.; Sposito, G. Steady-state dissolution kinetics of goethite in the presence of desferrioxamine B and oxalate ligands: Implications for the microbial acquisition of iron. Chem. Geol. 2003, 198, 63–75. [Google Scholar] [CrossRef]
- Cervini-Silva, J.; Sposito, G. Steady-state dissolution kinetics of aluminum-goethite in the presence of desferrioxamine-B and oxalate ligands. Environ. Sci. Technol. 2002, 36, 337–342. [Google Scholar] [CrossRef]
- Zhong, L.Y.; Yang, J.W.; Liu, L.M.; Li, X.Y. Desferrioxamine-B promoted dissolution of an Oxisol and the effect of low-molecular-weight organic acids. Biol. Fertil. Soils 2013, 49, 1077–1083. [Google Scholar] [CrossRef]
- Banwart, S.; Davies, S.; Stumm, W. The role of oxalate in accelerating the reductive dissolution of hematite (alpha-Fe2O3) by ascorbate. Colloid Surf. 1989, 39, 303–309. [Google Scholar] [CrossRef]
- Wang, Z.M.; Schenkeveld, W.D.C.; Kraemer, S.M.; Giammar, D.E. Synergistic Effect of Reductive and Ligand-Promoted Dissolution of Goethite. Environ. Sci. Technol. 2015, 49, 7236–7244. [Google Scholar] [CrossRef] [PubMed]
- Kang, K.; Schenkeveld, W.D.C.; Biswakarma, J.; Borowski, S.C.; Hug, S.J.; Hering, J.G.; Kraemer, S.M. The catalytic role of low Fe(II) concentrations in ligand-controlled dissolution of Fe(III)(hydr)oxide minerals. Environ. Sci. Technol. 2018. [Google Scholar] [CrossRef] [PubMed]
- Biswakarma, J.; Kang, K.; Borowski, S.C.; Schenkeveld, W.D.C.; Kraemer, S.M.; Hering, J.G.; Hug, S.J. Fe(II)-catalzyed ligand-controlled dissolution of iron(hydr)oxides. Environ. Sci. Technol. 2018. [Google Scholar] [CrossRef]
- Zuo, Y.M.; Zhang, F.S. Effect of peanut mixed cropping with gramineous species on micronutrient concentrations and iron chlorosis of peanut plants grown in a calcareous soil. Plant Soil 2008, 306, 23–36. [Google Scholar] [CrossRef]
- Xiong, H.C.; Kakei, Y.; Kobayashi, T.; Guo, X.T.; Nakazono, M.; Takahashi, H.; Nakanishi, H.; Shen, H.Y.; Zhang, F.S.; Nishizawa, N.K.; et al. Molecular evidence for phytosiderophore-induced improvement of iron nutrition of peanut intercropped with maize in calcareous soil. Plant Cell Environ. 2013, 36, 1888–1902. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marschner, H.; Treeby, M.; Römheld, V. Role of root-induced changes in the rhizosphere for iron acquisition in higher plants. J. Plant Nutr. Soil Sci. 1989, 152, 197–204. [Google Scholar] [CrossRef]
- Dehner, C.; Morales-Soto, N.; Behera, R.K.; Shrout, J.; Theil, E.C.; Maurice, P.A.; Dubois, J.L. Ferritin and ferrihydrite nanoparticles as iron sources for Pseudomonas aeruginosa. J. Biol. Inorg. Chem. 2013, 18, 371–381. [Google Scholar] [CrossRef] [Green Version]
- Vartivarian, S.E.; Cowart, R.E. Extracellular iron reductases: Identification of a new class of enzymes by siderophore-producing microorganisms. Arch. Biochem. Biophys. 1999, 364, 75–82. [Google Scholar] [CrossRef]
- Schmidt, H.; Gunther, C.; Weber, M.; Sporlein, C.; Loscher, S.; Bottcher, C.; Schobert, R.; Clemens, S. Metabolome Analysis of Arabidopsis thaliana Roots Identifies a Key Metabolic Pathway for Iron Acquisition. PLoS ONE 2014, 9, e102444. [Google Scholar] [CrossRef]
- Fourcroy, P.; Siso-Terraza, P.; Sudre, D.; Saviron, M.; Reyt, G.; Gaymard, F.; Abadia, A.; Abadia, J.; Alvarez-Fernandez, A.; Briat, J.F. Involvement of the ABCG37 transporter in secretion of scopoletin and derivatives by Arabidopsis roots in response to iron deficiency. New Phytol. 2014, 201, 155–167. [Google Scholar] [CrossRef] [PubMed]
- Schmid, N.B.; Giehl, R.F.H.; Doll, S.; Mock, H.P.; Strehmel, N.; Scheel, D.; Kong, X.L.; Hider, R.C.; von Wiren, N. Feruloyl-CoA 6′-Hydroxylase1-Dependent Coumarins Mediate Iron Acquisition from Alkaline Substrates in Arabidopsis. Plant Physiol. 2014, 164, 160–172. [Google Scholar] [CrossRef] [PubMed]
- Schenkeveld, W.D.C.; Reichwein, A.M.; Bugter, M.H.J.; Temminghoff, E.J.M.; van Riemsdijk, W.H. Performance of Soil-Applied FeEDDHA Isomers in Delivering Fe to Soybean Plants in Relation to the Moment of Application. J. Agric. Food Chem. 2010, 58, 12833–12839. [Google Scholar] [CrossRef] [PubMed]
- Namba, K.; Murata, Y.; Horikawa, M.; Iwashita, T.; Kusumoto, S. A practical synthesis of the phytosiderophore 2′-deoxymugineic acid: A key to the mechanistic study of iron acquisition by graminaceous plants. Angew. Chem. Int. Ed. 2007, 46, 7060–7063. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Rayo, S.; Di Foggia, M.; Moreira, E.R.; Donnini, S.; Bombai, G.; Filippini, G.; Pisi, A.; Rombola, A.D. Physiological responses in roots of the grapevine rootstock 140 Ruggeri subjected to Fe deficiency and Fe-heme nutrition. Plant Physiol. Biochem. 2015, 96, 171–179. [Google Scholar] [CrossRef] [PubMed]
- Walter, M.; Kraemer, S.M.; Schenkeveld, W.D.C. The effect of pH, electrolytes and temperature on the rhizosphere geochemistry of phytosiderophores. Plant Soil 2017, 418, 5–23. [Google Scholar] [CrossRef] [PubMed]
- Schenkeveld, W.D.C.; Kimber, R.L.; Walter, M.; Oburger, E.; Puschenreiter, M.; Kraemer, S.M. Experimental considerations in metal mobilization from soil by chelating ligands: The influence of soil-solution ratio and pre-equilibration—A case study on Fe acquisition by phytosiderophores. Sci. Total Environ. 2017, 579, 1831–1842. [Google Scholar] [CrossRef] [PubMed]
- Puschenreiter, M.; Gruber, B.; Wenzel, W.W.; Schindlegger, Y.; Hann, S.; Spangl, B.; Schenkeveld, W.D.C.; Kraemer, S.M.; Oburger, E. Phytosiderophore-induced mobilization and uptake of Cd, Cu, Fe, Ni, Pb and Zn by wheat plants grown on metal-enriched soils. Environ. Exp. Bot. 2017, 138, 67–76. [Google Scholar] [CrossRef]
- Manceau, A.; Drits, V.A.; Silvester, E.; Bartoli, C.; Lanson, B. Structural mechanism of Co2+ oxidation by the phyllomanganate buserite. Am. Miner. 1997, 82, 1150–1175. [Google Scholar] [CrossRef]
- Peacock, C.L. Physiochemical controls on the crystal-chemistry of Ni in birnessite: Genetic implications for ferromanganese precipitates. Geochim. Cosmochim. Acta 2009, 73, 3568–3578. [Google Scholar] [CrossRef]
- Post, J.E. Manganese oxide minerals: Crystal structures and economic and environmental significance. Proc. Natl. Acad. Sci. USA 1999, 96, 3447–3454. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morgan, J.J. Kinetics of reaction between O2 and Mn(II) species in aqueous solutions. Geochim. Cosmochim. Acta 2005, 69, 35–48. [Google Scholar] [CrossRef]
- Handler, R.M.; Beard, B.L.; Johnson, C.M.; Scherer, M.M. Atom Exchange between Aqueous Fe(II) and Goethite: An Fe Isotope Tracer Study. Environ. Sci. Technol. 2009, 43, 1102–1107. [Google Scholar] [CrossRef]
- Frierdich, A.J.; Catalano, J.G. Controls on Fe(II)-Activated Trace Element Release from Goethite and Hematite. Environ. Sci. Technol. 2012, 46, 1519–1526. [Google Scholar] [CrossRef] [PubMed]
- Frierdich, A.J.; Catalano, J.G. Fe(II)-Mediated Reduction and Repartitioning of Structurally Incorporated Cu, Co, and Mn in Iron Oxides. Environ. Sci. Technol. 2012, 46, 11070–11077. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.M.; Thompson, A. Ferrous Iron Oxidation under Varying pO(2) Levels: The Effect of Fe(III)/Al(III) Oxide Minerals and Organic Matter. Environ. Sci. Technol. 2018, 52, 597–606. [Google Scholar] [CrossRef]
- Baltpurvins, K.A.; Burns, R.C.; Lawrance, G.A.; Stuart, A.D. Effect of pH and anion type on the aging of freshly precipitated iron(III) hydroxide sludges. Environ. Sci. Technol. 1996, 30, 939–944. [Google Scholar] [CrossRef]
- ThomasArrigo, L.K.; Mikutta, C.; Byrne, J.; Kappler, A.; Kretzschmar, R. Iron(II)-Catalyzed Iron Atom Exchange and Mineralogical Changes in Iron-rich Organic Freshwater Flocs: An Iron Isotope Tracer Study. Environ. Sci. Technol. 2017, 51, 6897–6907. [Google Scholar] [CrossRef] [PubMed]
- Xiao, W.; Jones, A.M.; Collins, R.N.; Waite, T.D. Investigating the effect of ascorbate on the Fe(II)-catalyzed transformation of the poorly crystalline iron mineral ferrihydrite. Biochim. Biophys. Acta-Gen. Subj. 2018, 1862, 1760–1769. [Google Scholar] [CrossRef] [PubMed]
- Tamura, H.; Kawamura, S.; Hagayama, M. Acceleration of the oxidation of Fe2+ ions by Fe(III)-oxyhydroxides. Corros. Sci. 1980, 20, 963–971. [Google Scholar] [CrossRef]
- Oburger, E.; Gruber, B.; Wanek, W.; Watzinger, A.; Stanetty, C.; Schindlegger, Y.; Hann, S.; Schenkeveld, W.D.C.; Kraemer, S.M.; Puschenreiter, M. Microbial decomposition of 13C- labeled phytosiderophores in the rhizosphere of wheat: Mineralization dynamics and key microbial groups involved. Soil Biol. Biochem. 2016, 98, 196–207. [Google Scholar] [CrossRef]
- Schenkeveld, W.D.C.; Oburger, E.; Gruber, B.; Schindlegger, Y.; Hann, S.; Puschenreiter, M.; Kraemer, S.M. Metal mobilization from soils by phytosiderophores—Experiment and equilibrium modeling. Plant Soil 2014, 383, 59–71. [Google Scholar] [CrossRef] [PubMed]
- Simanova, A.A.; Pena, J. Time-Resolved Investigation of Cobalt Oxidation by Mn(III)-Rich delta-MnO2 Using Quick X-ray Absorption Spectroscopy. Environ. Sci. Technol. 2015, 49, 10867–10876. [Google Scholar] [CrossRef] [PubMed]
- Walter, M.; Oburger, E.; Schindlegger, Y.; Hann, S.; Puschenreiter, M.; Kraemer, S.M.; Schenkeveld, W.D.C. Retention of phytosiderophores by the soil solid phase—Adsorption and desorption. Plant Soil 2016. [Google Scholar] [CrossRef] [PubMed]
- Römheld, V. The role of phytosiderophores in acquisition of iron and other micronutrients in graminaceous species—An ecological approach. Plant Soil 1991, 130, 127–134. [Google Scholar] [CrossRef]
- Matocha, C.J.; Sparks, D.L.; Amonette, J.E.; Kukkadapu, R.K. Kinetics and mechanism of birnessite reduction by catechol. Soil Sci. Soc. Am. J. 2001, 65, 58–66. [Google Scholar] [CrossRef]
- Stone, A.T.; Morgan, J.J. Reduction and dissolution of manganese(III) and manganese(IV) oxides by organics 1. reaction with hydroquinone. Environ. Sci. Technol. 1984, 18, 450–456. [Google Scholar] [CrossRef]





| Metal | kDMA | a | kasc | b | ksyn | c | d | R2 | Tangent |
|---|---|---|---|---|---|---|---|---|---|
| (s−1) | (s−1) | (L s−1 mol−1) | |||||||
| Fe | 9.8 × 10−8 | 0.37 | n.d. | n.d. | 4.4 × 10−3 | 0.56 | 1 | 0.997 | 1.00 |
| Ni | 3.8 × 10−6 | 0.91 | n.d. | n.d. | 1.4 × 10−4 | 0.39 | 1 | 0.993 | 0.99 |
| Co | n.d. | n.d. | n.d. | n.d. | 4.9 × 10−5 | 0.16 | 1 | 0.995 | 1.00 |
| Mn | n.d. | n.d. | 5.6 × 10−4 (3.3 × 10−5) # | 1.37 (1) # | n.d. | n.d. | 1 | 0.996 (0.97) | 1.03 (1.20) |
| Cu | 4.9 × 10−8 | 0.32 | n.d. | n.d. | n.d. | n.d. | n.d. | 0.98 | 1.06 |
| Zn | 5.2 × 10−8 | 0.36 | n.d. | n.d. | n.d. | n.d. | n.d. | 0.91 | 0.97 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schenkeveld, W.D.C.; Kraemer, S.M. Constraints to Synergistic Fe Mobilization from Calcareous Soil by a Phytosiderophore and a Reductant. Soil Syst. 2018, 2, 67. https://doi.org/10.3390/soilsystems2040067
Schenkeveld WDC, Kraemer SM. Constraints to Synergistic Fe Mobilization from Calcareous Soil by a Phytosiderophore and a Reductant. Soil Systems. 2018; 2(4):67. https://doi.org/10.3390/soilsystems2040067
Chicago/Turabian StyleSchenkeveld, Walter D. C., and Stephan M. Kraemer. 2018. "Constraints to Synergistic Fe Mobilization from Calcareous Soil by a Phytosiderophore and a Reductant" Soil Systems 2, no. 4: 67. https://doi.org/10.3390/soilsystems2040067






