Adsorption Cooler Design, Dynamic Modeling, and Performance Analyses
Abstract
:1. Introduction
2. Materials and Methods
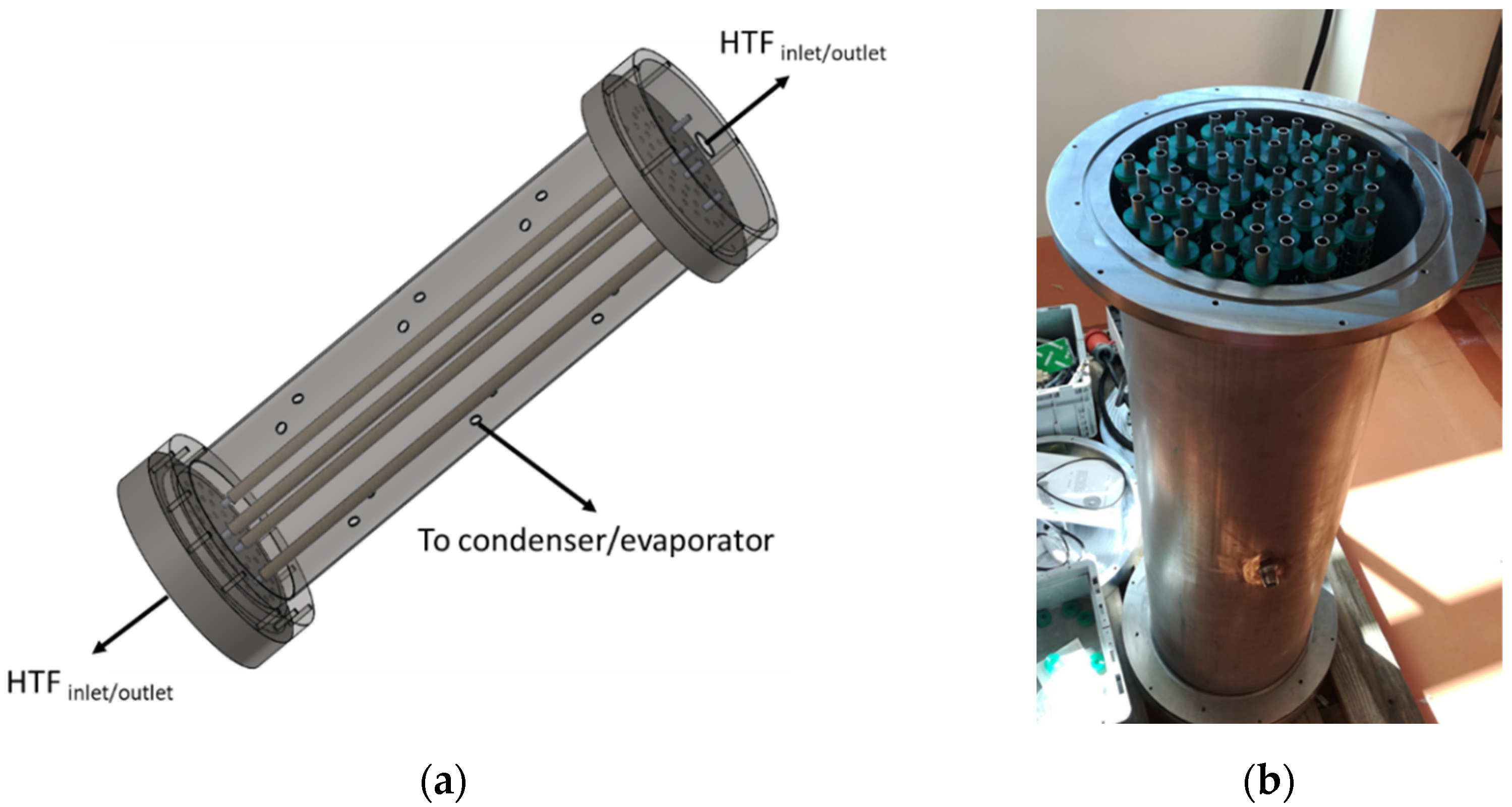
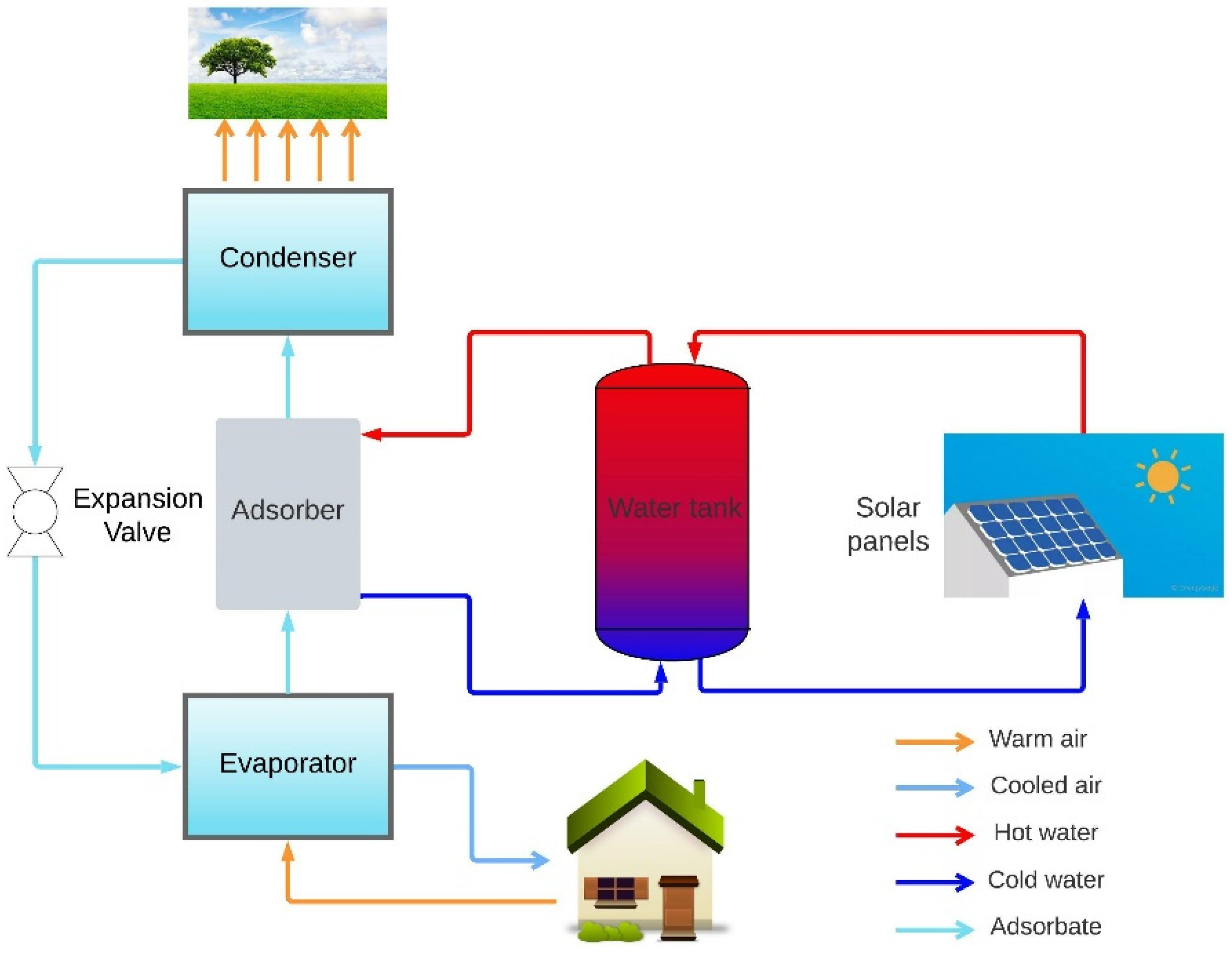
2.1. Design
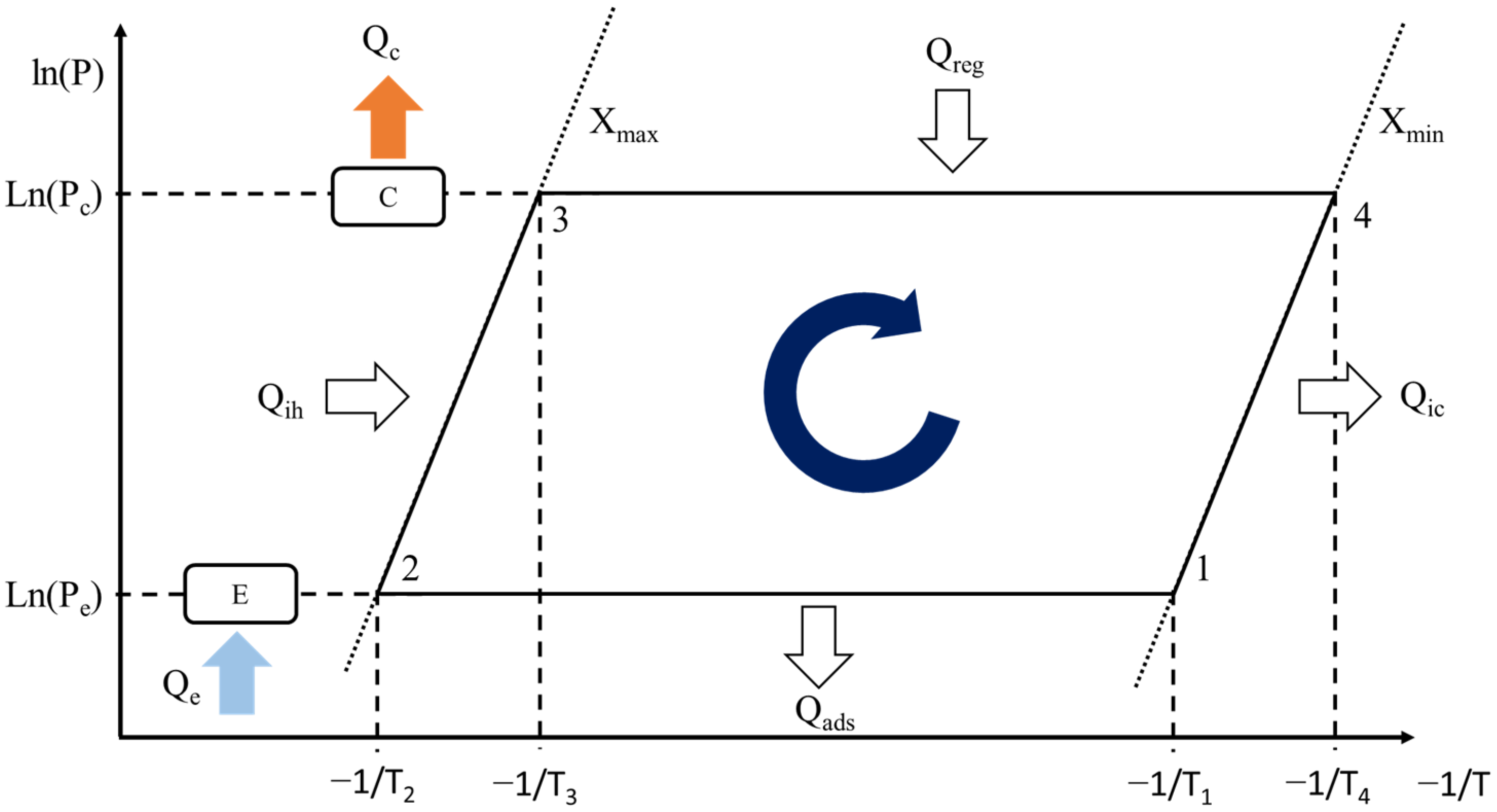
2.2. Modeling
- The adsorbent bed is homogenous;
- The evaporator and the condenser are ideal heat exchangers with uniform pressures;
- The adsorbate vapour phase behaves as an ideal gas and the adsorbed phase is in the liquid state;
- The specific heats of the adsorbate vapour and liquid phases are constants;
- The adsorbate vapour around the adsorbent is saturated vapour;
- The thermophysical properties of solid materials do not change with temperature;
- The temperature, adsorbent content, and pressure in the adsorbent bed do not vary along the angular direction.
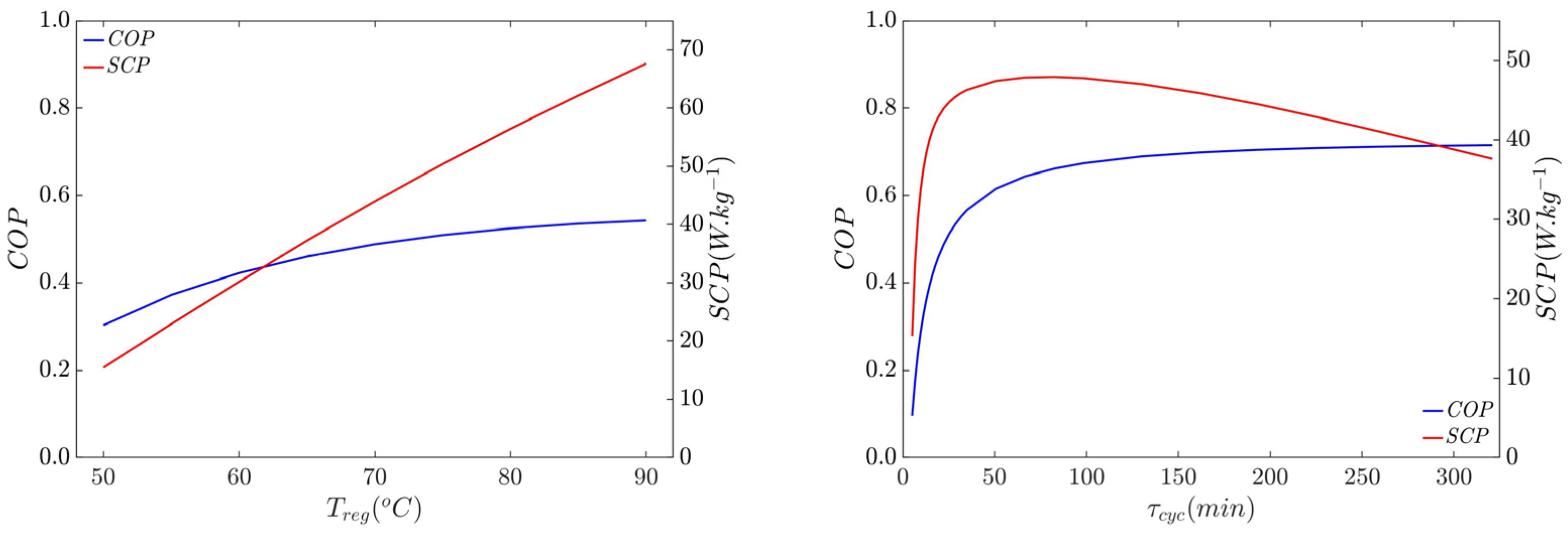
3. Thermally Driven Cooling Performance and Dynamics
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Nomenclature
| AC | Adsorption cooling |
| C | Specific heat (J·kg−1·K−1) |
| Cp | Constant pressure specific heat (J·kg−1·K−1) |
| COPc | Cooling coefficient of performance |
| d | Diameter (m) |
| Def0 | Effective diffusion coefficient (m2·s−1) |
| Ea | Activation energy (J·kg−1) |
| HTF | Heat transfer fluid |
| hf→m | Fluid–metal convective heat transfer coefficient (W·m−2·K−1) |
| hm→s | Adsorbent–metal heat transfer coefficient (W·m−2·K−1) |
| k | Thermal conductivity (W·m−1·K−1) |
| k0 | Pre-exponential coefficient (kg·kg−1·Pa−1) |
| kD | Blake–Kozeny permeability (m2) |
| KLDF | LDF constant (s−1) |
| L | Tube length (m) |
| m | Mass (kg) |
| P | Pressure (Pa) |
| Q | Heat (J) |
| qm | Monolayer specific capacity (kg·kg−1) |
| r | Radial coordinate (m) |
| Rp | Particle radius (m) |
| R′ | Adsorbate specific gas constant (J·kg−1·K−1) |
| SCP | Specific cooling power (W·kg−1) |
| t | Time (s) |
| tSG | Non-dimensional Toth constant |
| T | Temperature (K) |
| u | Adsorbate velocity (m·s−1) |
| v | Velocity (m·s−1) |
| X | Adsorbate concentration in the adsorbent (kga·kgs−1) |
| z | Axial longitudinal coordinate (m) |
| Subscripts | |
| a | Adsorbate |
| ads | Adsorption |
| bed | Adsorbent bed |
| c | Condenser/cooling |
| cyc | Cycle |
| e | Evaporator |
| eq | Equilibrium |
| f | Fluid |
| h | Heating |
| ic | Isosteric cooling |
| ih | Isosteric heating |
| in | Inner |
| ini | Initial |
| m | Metal |
| max | Maximum |
| min | Minimum |
| out | Outer |
| p | Particle |
| reg | Regeneration |
| s | Adsorbent |
| v | Vapour/vaporization |
| Greek letters | |
| ΔHads | Adsorption heat (J·kg−1) |
| ΔHv | Heat of vaporization (J·kg−1) |
| ε | Adsorbent bed porosity |
| μ | Dynamic viscosity (Pa·s) |
| ρ | Adsorbate density (kg·m−3) |
| σ | Thickness (m) |
| τ | Cycle time (s) |
References
- REN21. Renewables 2020 Global Status Report; REN21: Paris, France, 2020. [Google Scholar]
- Boruta, P.; Bujok, T.; Sztekler, M.K. Adsorbents, Working Pairs and Coated Beds for Natural Refrigerants in Adsorption Chillers—State of the Art. Energies 2021, 14, 4707. [Google Scholar] [CrossRef]
- Boman, D.B.; Hoysall, D.C.; Pahinkar, D.G.; Ponkala, M.J.; Garimella, S. Screening of working pairs for adsorption heat pumps based on thermodynamic and transport characteristics. Appl. Therm. Eng. 2017, 123, 422–434. [Google Scholar] [CrossRef]
- Freni, A.; Maggio, G.; Sapienza, A.; Frazzica, A.; Restuccia, G.; Vasta, S. Comparative analysis of promising adsorbent/adsorbate pairs for adsorptive heat pumping, air conditioning and refrigeration. Appl. Therm. Eng. 2016, 104, 85–95. [Google Scholar] [CrossRef]
- Calabrese, L.; Bonaccorsi, L.; Freni, A.; Proverbio, E. Silicone composite foams for adsorption heat pump applications. Sustain. Mater. Technol. 2017, 12, 27–34. [Google Scholar] [CrossRef]
- Calabrese, L.; Brancato, V.; Bonaccorsi, L.; Frazzica, A.; Caprì, A.; Freni, A.; Proverbio, E. Development and characterization of silane-zeolite adsorbent coatings for adsorption heat pump applications. Appl. Therm. Eng. 2017, 116, 364–371. [Google Scholar] [CrossRef]
- Henninger, S.K.; Ernst, S.-J.; Gordeeva, L.G.; Bendix, P.; Fröhlich, D.; Grekova, A.D.; Bonaccorsi, L.; Aristov, Y.; Jaenchen, J. New materials for adsorption heat transformation and storage. Renew. Energy 2017, 110, 59–68. [Google Scholar] [CrossRef]
- Kim, C.; Cho, K.; Kim, S.K.; Lee, E.K.; Kim, J.-N.; Choi, M. Alumina-coated ordered mesoporous silica as an efficient and stable water adsorbent for adsorption heat pump. Microporous Mesoporous Mater. 2017, 239, 310–315. [Google Scholar] [CrossRef]
- Pinheiro, J.M.; Valente, A.A.; Salústio, S.; Ferreira, N.; Rocha, J.; Silva, C.M. Application of the novel ETS-10/water pair in cyclic adsorption heating processes: Measurement of equilibrium and kinetics properties and simulation studies. Appl. Therm. Eng. 2015, 87, 412–423. [Google Scholar] [CrossRef]
- Zhang, L.Z.; Wang, L. Effects of coupled heat and mass transfers in adsorbent on the performance of a waste heat adsorption cooling unit. Appl. Therm. Eng. 1999, 19, 195–215. [Google Scholar] [CrossRef]
- Poyelle, F.; Guilleminot, J.-J.; Meunier, F. Experimental Tests and Predictive Model of an Adsorptive Air Conditioning Unit. Ind. Eng. Chem. Res. 1999, 38, 298–309. [Google Scholar] [CrossRef]
- Freni, A.; Frazzica, A.; Dawoud, B.; Chmielewski, S.; Calabrese, L.; Bonaccorsi, L. Adsorbent coatings for heat pumping applications: Verification of hydrothermal and mechanical stabilities. Appl. Therm. Eng. 2013, 50, 1658–1663. [Google Scholar] [CrossRef]
- Girnik, I.S.; Grekova, A.D.; Gordeeva, L.G.; Aristov, Y.I. Dynamic optimization of adsorptive chillers: Compact layer vs. bed of loose grains. Appl. Therm. Eng. 2017, 125, 823–829. [Google Scholar] [CrossRef]
- Kowsari, M.M.; Niazmand, H.; Tokarev, M. Bed configuration effects on the finned flat-tube adsorption heat exchanger performance: Numerical modeling and experimental validation. Appl. Energy 2018, 213, 540–554. [Google Scholar] [CrossRef]
- Rivero-Pacho, A.M.; Critoph, R.E.; Metcalf, S.J. Modelling and development of a generator for a domestic gas-fired carbon-ammonia adsorption heat pump. Renew. Energy 2017, 110, 180–185. [Google Scholar] [CrossRef] [Green Version]
- Wittstadt, U.; Füldner, G.; Laurenz, E.; Warlo, A.; Große, A.; Herrmann, R.; Schnabel, L.; Mittelbach, W. A novel adsorption module with fiber heat exchangers: Performance analysis based on driving temperature differences. Renew. Energy 2017, 110, 154–161. [Google Scholar] [CrossRef]
- Sapienza, A.; Gullì, G.; Calabrese, L.; Palomba, V.; Frazzica, A.; Brancato, V.; La Rosa, D.; Vasta, S.; Freni, A.; Bonaccorsi, L.; et al. An innovative adsorptive chiller prototype based on 3 hybrid coated/granular adsorbers. Appl. Energy 2016, 179, 929–938. [Google Scholar] [CrossRef]
- Palomba, V.; Vasta, S.; Freni, A. Experimental testing of AQSOA FAM Z02/water adsorption system for heat and cold storage. Appl. Therm. Eng. 2017, 124, 967–974. [Google Scholar] [CrossRef]
- Dawoud, B. On the development of an innovative gas-fired heating appliance based on a zeolite-water adsorption heat pump; system description and seasonal gas utilization efficiency. Appl. Therm. Eng. 2014, 72, 323–330. [Google Scholar] [CrossRef]
- Fernandes, M.S.; Brites, G.J.V.N.; Costa, J.J.; Gaspar, A.R.; Costa, V.A.F. Review and future trends of solar adsorption refrigeration systems. Renew. Sustain. Energy Rev. 2014, 39, 102–123. [Google Scholar] [CrossRef]
- Dias, J.M.S.; Costa, V.A.F. Which dimensional model for the analysis of a coated tube adsorber for adsorption heat pumps? Energy 2019, 174, 1110–1120. [Google Scholar] [CrossRef]
- Dias, J.M.S.; Costa, V.A.F. Modeling and Analysis of a Coated Tube Adsorber for Adsorption Heat Pumps. Energies 2021, 14, 6878. [Google Scholar] [CrossRef]
- Dias, J.M.S.; Costa, V.A.F. Evaluating the performance of a coated tube adsorber for adsorption cooling. [Évaluation de la per-formance d’un adsorbeur à tube enrobé pour le refroidissement par adsorption]. Int. J. Refrig. 2020, 118, 21–30. [Google Scholar] [CrossRef]
- Dias, J.M.S.; Costa, V.A.F. Adsorption heat pumps for heating applications: A review of current state, literature gaps and development challenges. Renew. Sustain. Energy Rev. 2018, 98, 317–327. [Google Scholar] [CrossRef]
- Li, X.H.; Hou, X.H.; Zhang, X.; Yuan, Z.X. A review on development of adsorption cooling—Novel beds and advanced cycles. Energy Convers. Manag. 2015, 94, 221–232. [Google Scholar] [CrossRef]
- Pesaran, A.; Lee, H.; Hwang, Y.; Radermacher, R.; Chun, H.-H. Review article: Numerical simulation of adsorption heat pumps. Energy 2016, 100, 310–320. [Google Scholar] [CrossRef]
- Wilkes, J.O.; Birmingham, S.G. Fluid Mechanics for Chemical Engineers with Microfluidics and CFD; Pearson Education: London, UK, 2006. [Google Scholar]
- Sircar, S. Linear-driving-force model for non-isothermal gas adsorption kinetics. J. Chem. Soc. Faraday Trans. 1 Phys. Chem. Condens. Phases 1983, 79, 785–796. [Google Scholar] [CrossRef]
- Shampine, L.F.; Reichelt, M.W. The MATLAB ODE Suite. SIAM J. Sci. Comput. 1997, 18, 1–22. [Google Scholar] [CrossRef] [Green Version]
- Muttakin, M.; Islam, M.A.; Malik, K.S.; Pahwa, D.; Saha, B.B. Study on optimized adsorption chiller employing various heat and mass recovery schemes. Int. J. Refrig. 2021, 126, 222–237. [Google Scholar] [CrossRef]
- Wang, D.; Zhang, J.; Tian, X.; Liu, D.; Sumathy, K. Progress in silica gel–water adsorption refrigeration technology. Renew. Sustain. Energy Rev. 2014, 30, 85–104. [Google Scholar] [CrossRef]
- Alahmer, A.; Ajib, S.; Wang, X. Comprehensive strategies for performance improvement of adsorption air conditioning systems: A review. Renew. Sustain. Energy Rev. 2019, 99, 138–158. [Google Scholar] [CrossRef]

| Parameter | Value | Unit |
|---|---|---|
| Cs | 921 | J·kg−1·K−1 |
| din,tube | 0.01 | m |
| hm→s | 30 | W·m−2·K−1 |
| ks | 0.198 | W·m−1·K−1 |
| Ltube | 1 | m |
| ms | 5.35 | kg |
| tads | 600 | s |
| Tc | 30 | °C |
| Te | 15 | °C |
| Tf,ads | 18 | °C |
| Tf,reg | 70 | °C |
| vHTF | 0.05 | m·s−1 |
| ρs | 2027 | kg·m−3 |
| σs | 0.002 | m |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dias, J.M.S.; Costa, V.A.F. Adsorption Cooler Design, Dynamic Modeling, and Performance Analyses. Clean Technol. 2022, 4, 1152-1161. https://doi.org/10.3390/cleantechnol4040070
Dias JMS, Costa VAF. Adsorption Cooler Design, Dynamic Modeling, and Performance Analyses. Clean Technologies. 2022; 4(4):1152-1161. https://doi.org/10.3390/cleantechnol4040070
Chicago/Turabian StyleDias, João M. S., and Vítor A. F. Costa. 2022. "Adsorption Cooler Design, Dynamic Modeling, and Performance Analyses" Clean Technologies 4, no. 4: 1152-1161. https://doi.org/10.3390/cleantechnol4040070







