Advances in Carbon Capture and Use (CCU) Technologies: A Comprehensive Review and CO2 Mitigation Potential Analysis
Abstract
:1. Introduction
2. CCU Technologies
2.1. Framework
2.2. CO2 Capture
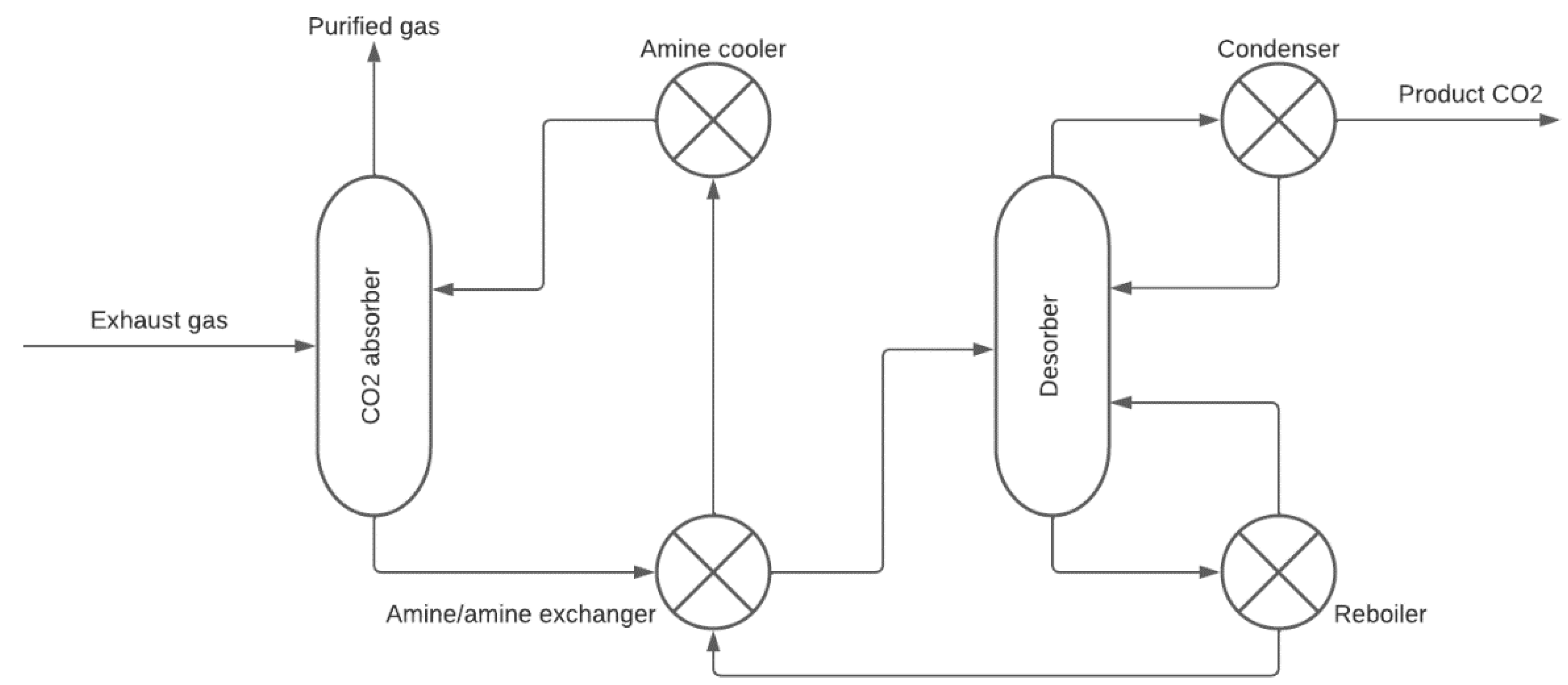
2.2.1. Absorption-Based CO2 Capture
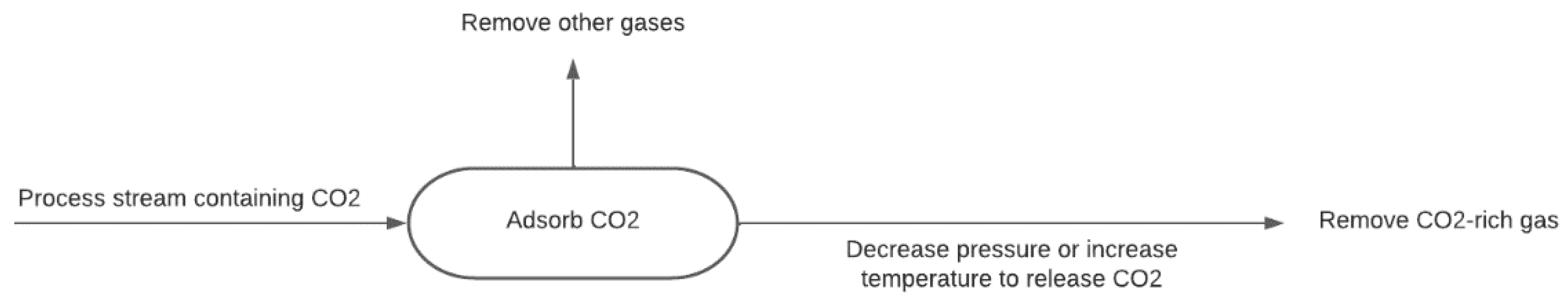
2.2.2. Adsorption-Based CO2 Capture
2.2.3. Membrane-Based CO2 Separation
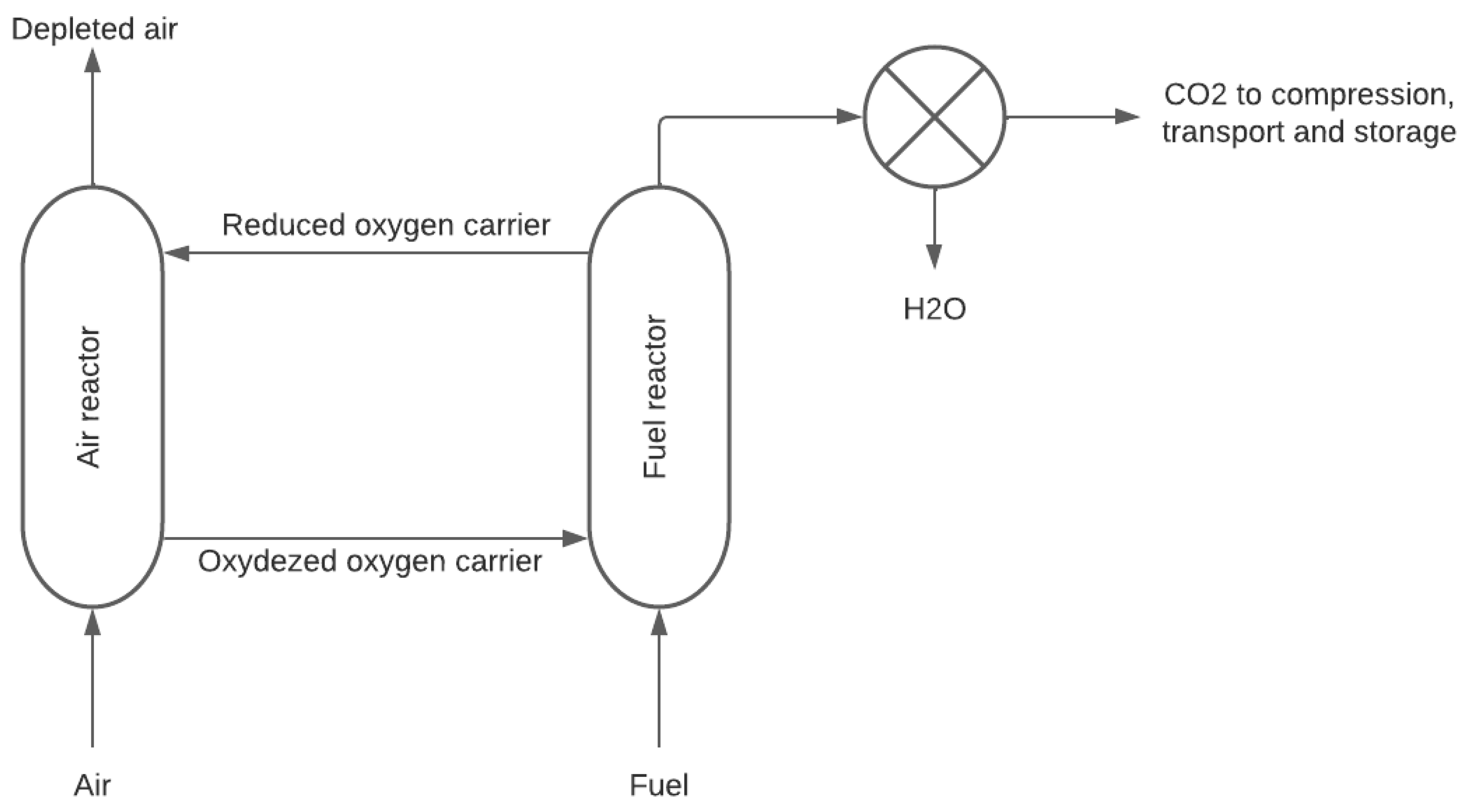
2.2.4. Chemical Looping-Based CO2 Capture
3. Recent Developments and Potential Uses
4. Future Opportunities
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Peres, C.B.; Resende, P.R.; Nunes, L.J.R.; de Morais, L.C. Circular economy: A comprehensive review of eco-friendly wollastonite applications. Sustainability 2022, 14, 3070. [Google Scholar] [CrossRef]
- International Energy Agency. Global CO2 Emissions Rebounded to Their Highest Level in History in 2021. Available online: https://www.iea.org/news/global-co2-emissions-rebounded-to-their-highest-level-in-history-in-2021 (accessed on 31 July 2022).
- Perathoner, S.; van Geem, K.M.; Marin, G.B.; Centi, G. Reuse of CO2 in energy intensive process industries. Chem. Commun. 2021, 57, 10967. [Google Scholar] [CrossRef] [PubMed]
- Renforth, P.; Sick, V.; Sheehan, S.W.; Pace, G. Scaling CO2 capture with downstream flow CO2 conversion to ethanol. Front. Clim. 2021, 1, 656108. [Google Scholar] [CrossRef]
- Al-Mamoori, A.; Krishnamurthy, A.; Rownaghi, A.A.; Ezaei, F. Carbon capture and utilization update. Energy Technol. 2017, 5, 834–849. [Google Scholar] [CrossRef] [Green Version]
- Gao, W.; Liang, S.; Wang, R.; Jiang, Q.; Zhang, Y.; Zheng, Q.; Xie, B.; Ying Toe, C.; Zhu, X.; Wang, J.; et al. Industrial carbon dioxide capture and utilization: State of the art and future challenges. Chem. Soc. Rev. 2020, 49, 8584. [Google Scholar] [CrossRef]
- Challa, P.; Paleti, G.; Madduluri, V.R.; Gadamani, S.B.; Pothu, R.; Burri, D.R.; Boddula, R.; Perugopu, V.; Kamaraju, S.R.R. Trends in emission and utilization of CO2: Sustainable feedstock in the synthesis of value-added fine chemicals. Catal. Surv. Asia 2022, 26, 80–91. [Google Scholar] [CrossRef]
- Ding, M.; Liu, X.; Ma, P.; Yao, J. Porous materials for capture and catalytic conversion of CO2 at low concentration. Coord. Chem. Rev. 2022, 465, 214576. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, Q.; Li, H.; Chen, S.; Teng, F. Investment decision on carbon capture and utilization (CCU) technologies—A real option model based on technology learning effect. Appl. Energy 2022, 322, 119514. [Google Scholar] [CrossRef]
- Wich, T.; Lueke, W.; Deerberg, G.; Oles, M. Carbon2Chem®-CCU as a step toward a circular economy. Front. Energy Res. 2020, 7, 162. [Google Scholar] [CrossRef] [Green Version]
- Costa, L.P.D.M.; de Miranda, D.M.V.; de Oliveira, A.C.; Falcon, L.; Pimenta, M.S.S.; Bessa, I.G.; Wouters, S.J.; Andrade, M.; Pinto, J. Capture and reuse of carbon dioxide (CO2) for a plastics circular economy: A review. Processes 2021, 9, 759. [Google Scholar] [CrossRef]
- Dimitriou, I.; García-Gutiérrez, P.; Elder, R.H.; Cuéllar-Franca, R.M.; Azapagic, A.; Allen, R.W. Carbon dioxide utilisation for production of transport fuels: Process and economic analysis. Energy Environ. Sci. 2015, 8, 1775–1789. [Google Scholar] [CrossRef] [Green Version]
- Garcia-Garcia, G.; Fernandez, M.C.; Armstrong, K.; Woolass, S.; Styring, P. Analytical review of life-cycle environmental impacts of carbon capture and utilization technologies. ChemSusChem 2021, 14, 995–1015. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Liu, J.; Zhang, Q.; Teng, F.; McLellan, B.C. A critical review on deployment planning and risk analysis of carbon capture, utilization, and storage (CCUS) toward carbon neutrality. Renew. Sustain. Energy Rev. 2022, 167, 112537. [Google Scholar] [CrossRef]
- Kamkeng, A.D.N.; Wang, M.; Hu, J.; Du, W.; Qian, F. Transformation technologies for CO2 utilisation: Current status, challenges and future prospects. Chem. Eng. J. 2021, 409, 128138. [Google Scholar] [CrossRef]
- Pahija, E.; Golshan, S.; Blais, B.; Boffito, D.C. Perspectives on the process intensification of CO2 capture and utilization. Chem. Eng. Process. Process Intensif. 2022, 176, 108958. [Google Scholar] [CrossRef]
- Mikulčić, H.; Skov, I.R.; Dominković, D.F.; Wan Alwi, S.R.; Manan, Z.A.; Tan, R.; Duić, N.; Mohamad, S.N.H.; Wang, X. Flexible carbon capture and utilization technologies in future energy systems and the utilization pathways of captured CO2. Renew. Sustain. Energy Rev. 2019, 114, 109338. [Google Scholar] [CrossRef]
- Ravikumar, D.; Zhang, D.; Keoleian, G.; Miller, S.; Sick, V.; Li, V. Carbon dioxide utilization in concrete curing or mixing might not produce a net climate benefit. Nat. Commun. 2021, 12, 1–13. [Google Scholar] [CrossRef]
- Zhang, S.; Liu, L.; Zhang, L.; Zhuang, Y.; Du, J. An optimization model for carbon capture utilization and storage supply chain: A case study in Northeastern China. Appl. Energy 2018, 231, 194–206. [Google Scholar] [CrossRef]
- Gupta, S.; Li, L. The potential of machine learning for enhancing CO2 sequestration, storage, transportation, and utilization-based processes: A brief perspective. Miner. Met. Mater. Soc. 2022, 74, 414–428. [Google Scholar] [CrossRef]
- Cao, L.; Lei, S.; Guan, Y.; Wang, Y.; Zhang, Y.; Tian, J.; Wang, T.; Luo, B.; Ren, T. CCUS industry under target of carbon-peak and carbon-neutrality: Progress and challenges. Front. Energy Res. 2022, 10, 860665. [Google Scholar] [CrossRef]
- Lin, Q.; Zhang, X.; Wang, T.; Zheng, C.; Gao, X. Technical perspective of carbon capture, utilization, and storage. Engineering 2022, 14, 27–32. [Google Scholar] [CrossRef]
- Wienchol, P.; Korus, A.; Szlęk, A.; Ditaranto, M. Thermogravimetric and Kinetic study of thermal degradation of various types of municipal solid waste (MSW) under N2, CO2 and oxy-fuel conditions. Energy 2022, 248, 123573. [Google Scholar] [CrossRef]
- Yew, S.; Chai, W.; How, B.S. Review of carbon capture absorbents for CO2 utilization. Greenh. Gases Sci. Technol. 2022, 12, 1–34. [Google Scholar] [CrossRef]
- Ozkan, M.; Akhavi, A.A.; Coley, W.C.; Shang, R.; Ma, Y. Progress in carbon dioxide capture materials for deep decarbonization. Chem 2022, 8, 141–173. [Google Scholar] [CrossRef]
- Ribeiro, M.G.; Hisse, D.; Prado, M.L.; dos Santos, T.C.; Ronconi, C.M. Sustainable technologies of CO2 capture: A brief review. Rev. Virtual De Química 2022, 14, 517–528. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, S.; Feng, D.; Gao, J.; Dong, L.; Zhao, Y.; Sun, S.; Huang, Y.; Qin, Y. Functional biochar synergistic solid/liquid-phase CO2 capture: A review. Energy Fuels 2022, 36, 2945–2970. [Google Scholar] [CrossRef]
- Aghel, B.; Janati, S.; Wongwises, S.; Shadloo, M.S. Review on CO2 capture by blended amine solutions. Int. J. Greenh. Gas Control 2022, 119, 103715. [Google Scholar] [CrossRef]
- Hu, G.; Smith, K.H.; Wu, Y.; Mumford, K.A.; Kentish, S.E.; Stevens, G.W. Carbon Dioxide capture by solvent absorption using amino acids: A review. Chin. J. Chem. Eng. 2018, 26, 2229–2237. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhu, C.; Chu, C.; Fu, T.; Ma, Y. Mass transfer and capture of carbon dioxide using amino acids sodium aqueous solution in microchannel. Chem. Eng. Process. Process Intensif. 2022, 173, 108831. [Google Scholar] [CrossRef]
- Øi, L.; Procedia, S.K.-E. Comparison of energy consumption for different CO2 absorption configurations using different simulation tools. Energy Procedia 2014, 63, 1186–1195. [Google Scholar] [CrossRef]
- Akinola, T.E.; Bonilla Prado, P.L.; Wang, M. Experimental studies, molecular simulation and process modelling\simulation of adsorption-based post-combustion carbon capture for power plants: A state-of-the-art review. Appl. Energy 2022, 317, 119156. [Google Scholar] [CrossRef]
- Gunawardene, O.H.P.; Gunathilake, C.A.; Vikrant, K.; Amaraweera, S.M. Carbon Dioxide capture through physical and chemical adsorption using porous carbon materials: A review. Atmosphere 2022, 13, 397. [Google Scholar] [CrossRef]
- Farmahini, A.H.; Krishnamurthy, S.; Friedrich, D.; Brandani, S.; Sarkisov, L. Performance-Based screening of porous materials for carbon capture. Chem. Rev. 2021, 121, 10666–10741. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.; Bai, J.; Hu, X.; Jiang, Z.; Wang, L. Nitrogen-doped porous carbons from polyacrylonitrile fiber as effective CO2 adsorbents. J. Environ. Sci. 2023, 125, 533–543. [Google Scholar] [CrossRef] [PubMed]
- Peres, C.B.; Rosa, A.H.; de Morais, L.C. CO2 adsorption of bagasse waste feedstock using thermogravimetric analyses. J. Therm. Anal. Calorim. 2021, 147, 5973–5984. [Google Scholar] [CrossRef]
- Zheng, Y.; Wu, J.; Zhang, L.; Guo, Y.; Xu, Z.; Huang, Y.; Huang, P.; Zhang, J.; Zhao, C. Unravelling the pore templating effect on CO2 adsorption performance of alkali metal nitrates promoted MgO pellets. Chem. Eng. J. 2022, 450, 137944. [Google Scholar] [CrossRef]
- Wang, J.; Zhou, Y.; Hu, X. Adsorption of CO2 by a novel zeolite doped amine modified ternary aerogels. Environ. Res. 2022, 214, 113855. [Google Scholar] [CrossRef]
- Maniarasu, R.; Rathore, S.K.; Murugan, S. Biomass-based activated carbon for CO2 adsorption—A review. Energy Environ. 2022, 0, 0958305X2210934. [Google Scholar] [CrossRef]
- Lu, T.; Li, Q.; Shao, J.; Wang, L.; Pang, R.; Wu, X.; Hu, X. Nitrogen and sulfur co-doped porous carbons from polyacrylonitrile fibers for CO2 adsorption. J. Taiwan Inst. Chem. Eng. 2021, 128, 148–155. [Google Scholar] [CrossRef]
- Cabriga, C.K.C.; Clarete, K.V.B.; Zhang, J.A.T.; Pacia, R.M.P.; Ko, Y.S.; Castro, J.C. Evaluation of biochar derived from the slow pyrolysis of rice straw as a potential adsorbent for carbon dioxide. Biomass Convers. Biorefinery 2021, 1, 3. [Google Scholar] [CrossRef]
- Berger, A.H.; Bhown, A.S. Comparing physisorption and chemisorption solid sorbents for use separating CO2 from flue gas using temperature swing adsorption. Energy Procedia 2011, 4, 562–567. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Yuan, X.; Deng, S.; Zeng, X.; Yu, Z.; Li, S.; Li, K. Waste polyethylene terephthalate (PET) plastics-derived activated carbon for CO2 capture: A route to a closed carbon loop. Green Chem. 2020, 22, 6836–6845. [Google Scholar] [CrossRef]
- Nandi, M.; Uyama, H. Exceptional CO2 adsorbing materials under different conditions. Chem. Rec. 2014, 14, 1134–1148. [Google Scholar] [CrossRef] [PubMed]
- Gunathilake, C.; Manchanda, A.S.; Ghimire, P.; Kruk, M.; Jaroniec, M. Amine-modified silica nanotubes and nanospheres: Synthesis and CO2 sorption properties. Environ. Sci. Nano 2016, 3, 806–817. [Google Scholar] [CrossRef]
- Yao, M.; Wang, L.; Hu, X.; Hu, G.; Luo, M.; Fan, M. Synthesis of Nitrogen-doped carbon with three-dimensional mesostructures for CO2 capture. J. Mater. Sci. 2015, 50, 1221–1227. [Google Scholar] [CrossRef]
- Benedetti, V.; Cordioli, E.; Patuzzi, F.; Baratieri, M. CO2 Adsorption study on pure and chemically activated chars derived from commercial biomass gasifiers. J. CO2 Util. 2019, 33, 46–54. [Google Scholar] [CrossRef]
- Ochedi, F.O.; Liu, Y.; Adewuyi, Y.G. State-of-the-art review on capture of CO2 using adsorbents prepared from waste materials. Process Saf. Environ. Prot. 2020, 139, 1–25. [Google Scholar] [CrossRef]
- Chen, C.; Kim, J.; Ahn, W.S. CO2 Capture by amine-functionalized nanoporous materials: A review. Korean J. Chem. Eng. 2014, 31, 1919–1934. [Google Scholar] [CrossRef]
- Zhao, R.; Liu, L.; Zhao, L.; Deng, S.; Li, S.; Zhang, Y. A comprehensive performance evaluation of temperature swing adsorption for post-combustion carbon dioxide capture. Renew. Sustain. Energy Rev. 2019, 114, 109285. [Google Scholar] [CrossRef]
- Vinodh, R.; Babu, C.M.; Abidov, A.; Palanichamy, M.; Jang, H.T. Facile synthesis of amine modified silica/reduced graphene oxide composite sorbent for CO2 adsorption. Mater. Lett. 2019, 247, 44–47. [Google Scholar] [CrossRef]
- Sullivan, J.M.; Sivak, M. Carbon Capture in Vehicles: A Review of General Support, Available Mechanisms, and Consumer Acceptance Issues; University of Michigan, Transportation Research Institute: Ann Arbor, MI, USA, 2012. [Google Scholar]
- Chen, T.Y.; Deng, X.; Lin, L.C.; Ho, W.S.W. New sterically hindered polyvinylamine-containing membranes for CO2 capture from flue gas. J. Membr. Sci. 2022, 645, 120195. [Google Scholar] [CrossRef]
- Demir, H.; Aksu, G.O.; Gulbalkan, H.C.; Keskin, S. MOF membranes for CO2 capture: Past, present and future. Carbon Capture Sci. Technol. 2022, 2, 100026. [Google Scholar] [CrossRef]
- Amiri, A.; Shahbazian-Yassar, R. Recent progress of high-entropy materials for energy storage and conversion. J. Mater. Chem. 2021, 9, 782–823. [Google Scholar] [CrossRef]
- Gomes, P.; Madejski, P.; Chmiel, K.; Subramanian, N.; Ku’s, T.K. Methods and techniques for CO2 capture: Review of potential solutions and applications in modern energy technologies. Energies 2022, 15, 887. [Google Scholar] [CrossRef]
- Araújo, R.; Barros Do Nascimento, A.; Pimenta De Macedo, H.; Melo, D.M.A.; Santiago, R.C.; Rodrigues De, T.; Araújo, A.; Luiz, R.; Medeiros, B.A.; Adánez, J. Structure and Reactivity of brazilian iron ores as low-cost oxygen carriers for chemical looping combustion. Eng. Chem. Res. 2022, 61, 2469–2482. [Google Scholar] [CrossRef]
- Adánez-Rubio, I.; Samprón, I.; Izquierdo, M.T.; Abad, A.; Gayán, P.; Adánez, J. Coal and biomass combustion with CO2 capture by CLOU process using a magnetic Fe-Mn-supported CuO oxygen carrier. Fuel 2022, 314, 122742. [Google Scholar] [CrossRef]
- Li, J.; Zhang, H.; Gao, Z.; Fu, J.; Ao, W.; Dai, J. CO2 Capture with chemical looping combustion of gaseous fuels: An overview. Energy Fuels 2017, 31, 3475–3524. [Google Scholar] [CrossRef]
- Koytsoumpa, E.I.; Bergins, C.; Kakaras, E. The CO2 economy: Review of CO2 capture and reuse technologies. J. Supercrit. Fluids 2018, 132, 3–16. [Google Scholar] [CrossRef]
- Zhang, K.; Li, S.; Liu, L. Optimized foam-assisted CO2 enhanced oil recovery technology in tight oil reservoirs. Fuel 2020, 267, 117099. [Google Scholar] [CrossRef]
- Jiang, J.; Rui, Z.; Hazlett, R.; Lu, J. An integrated technical-economic model for evaluating CO2 Enhanced oil recovery development. Appl. Energy 2019, 247, 190–211. [Google Scholar] [CrossRef]
- Valluri, S.; Claremboux, V.; Kawatra, S. Opportunities and challenges in CO2 utilization. J. Environ. Sci. 2022, 113, 322–344. [Google Scholar] [CrossRef] [PubMed]
- Norhasyima, R.S.; Mahlia, T.M.I. Advances in CO2 utilization technology: A patent landscape review. J. CO2 Util. 2018, 26, 323–335. [Google Scholar] [CrossRef]
- Reiter, G.; Lindorfer, J. Evaluating CO2 sources for power-to-gas applications—A case study for Austria. J. CO2 Util. 2015, 10, 40–49. [Google Scholar] [CrossRef]
- Huang, C.H.; Tan, C.S. A review: CO2 utilization. Aerosol Air Qual. Res. 2014, 14, 480–499. [Google Scholar] [CrossRef] [Green Version]
- Centi, G.; Perathoner, S.; Salladini, A.; Iaquaniello, G. Economics of CO2 utilization: A critical analysis. Front. Energy Res. 2020, 8, 567986. [Google Scholar] [CrossRef]
- Zhu, Q. Developments on CO2-utilization technologies. Clean Energy 2019, 3, 85–100. [Google Scholar] [CrossRef] [Green Version]
- Dongliang, W.; Wenliang, M.; Huairong, Z.; Guixian, L.; Yong, Y.; Hongwei, L. Green hydrogen coupling with CO2 utilization of coal-to-methanol for high methanol productivity and low CO2 emission. Energy 2021, 231, 120970. [Google Scholar] [CrossRef]
- Aresta, M. Do bio-ethanol and synthetic ethanol produced from air-captured CO2 have the same degree of “Greenness” and relevance to “Fossil C”? Molecules 2022, 27, 2223. [Google Scholar] [CrossRef]
- Peng, W.; Nguyen, T.H.C.; Nguyen, D.L.T.; Wang, T.; Tran, T.V.T.; Le, T.H.; Le, H.K.; Grace, A.N.; Singh, P.; Raizadaa, P.; et al. A roadmap towards the development of superior photocatalysts for solar-driven CO2-to-fuels production. Renew. Sustain. Energy Rev. 2021, 148, 111298. [Google Scholar] [CrossRef]
- Dibenedetto, A.; Chen, Y.; Wu, J.; Wang, X.; Liu, M.; Liu, Y. Synthesis, characterization and application of amine-functionalized hierarchically micro-mesoporous silicon composites for CO2 capture in flue gas. Molecules 2022, 27, 3429. [Google Scholar] [CrossRef]
- Cho, S.; Lim, J.; Cho, H.; Yoo, Y.; Kang, D.; Kim, J. Novel process design of desalination wastewater recovery for CO2 and SOX utilization. Chem. Eng. J. 2022, 433, 133602. [Google Scholar] [CrossRef]
- Baena-Moreno, F.M.; Vega, F.; Pastor-Pérez, L.; Reina, T.R.; Navarrete, B.; Zhang, Z. Novel Process for carbon capture and utilization and saline wastes valorization. J. Nat. Gas Sci. Eng. 2020, 73, 103071. [Google Scholar] [CrossRef]
- Hernández, E.; Santiago, R.; Moya, C.; Vela, S.; Navarro, P.; Palomar, J. Close-cycle process to produce CO2-derived propylene carbonate based on amino acid catalyst and water. J. CO2 Util. 2021, 52, 101656. [Google Scholar] [CrossRef]
- Yao, X.; Yuan, X.; Yu, S.; Lei, M. Economic feasibility analysis of carbon capture technology in steelworks based on system dynamics. J. Clean Prod. 2021, 322, 129046. [Google Scholar] [CrossRef]
- Celaya, C.A.; Méndez-Galván, M.; Castro-Ocampo, O.; Torres-Martínez, L.M.; Luévano-Hipólito, E.; de León, J.N.D.; Lara-García, H.A.; Díaz, G.; Muñiz, J. Exploring the CO2 conversion into hydrocarbons via a Photocatalytic process onto M-Doped Titanate Nanotubes (M = Ni and Cu). Fuel 2022, 324, 124440. [Google Scholar] [CrossRef]
- Ma, Y.; Yi, X.; Wang, S.; Li, T.; Tan, B.; Chen, C.; Majima, T.; Waclawik, E.R.; Zhu, H.; Wang, J. Selective photocatalytic CO2 reduction in aerobic environment by microporous Pd-Porphyrin-based polymers coated hollow TiO2. Nat. Commun. 2022, 13, 1–10. [Google Scholar] [CrossRef]
- Sun, K.; Zhang, Z.; Shen, C.; Rui, N.; Liu, C.-J. The Feasibility study of the indium oxide supported silver catalyst for selective hydrogenation of CO2 to methanol. Green Energy Environ. 2021, 7, 807–817. [Google Scholar] [CrossRef]
- Gomes, M.Z.D.V.; Masdeu, G.; Eiring, P.; Kuhlemann, A.; Sauer, M.; Åkerman, B.; Palmqvist, A.E.C. Improved biocatalytic cascade conversion of CO2 to methanol by enzymes co-immobilized in tailored siliceous mesostructured cellular foams. Catal. Sci. Technol. 2021, 11, 6952. [Google Scholar] [CrossRef]
- Rasouli, H.; Iliuta, I.; Bougie, F.; Garnier, A.; Iliuta, M.C. Hybrid enzymatic CO2 capture process in intensified flat sheet membrane contactors with immobilized carbonic anhydrase. Sep. Purif. Technol. 2022, 287, 120505. [Google Scholar] [CrossRef]
- Hemmatifar, A.; Kang, J.S.; Ozbek, N.; Tan, K.-J.; Hatton, T.A. Electrochemically mediated direct CO2 capture by a stackable bipolar cell. ChemSusChem 2022, 22, e202102533. [Google Scholar] [CrossRef]
- Orlov, A.A.; Valtz, A.; Coquelet, C.; Rozanska, X.; Wimmer, E.; Marcou, G.; Horvath, D.; Poulain, B.; Varnek, A.; de Meyer, F. Computational screening methodology identifieseffective solvents for CO2 capture. Commun. Chem. 2022, 5, 1–7. [Google Scholar] [CrossRef]
- Jin, H.; Andalib, V.; Yasin, G.; Bokov, D.O.; Kamal, M.; Alashwal, M.; Ghazali, S.; Algarni, M.; Mamdouh, A. Computational simulation using machine learning models in prediction of CO2 absorption in environmental applications. J. Mol. Liq. 2022, 358, 119159. [Google Scholar] [CrossRef]
- Zhu, X.; Tsang, D.C.W.; Wang, L.; Su, Z.; Hou, D.; Li, L.; Shang, J. Machine learning exploration of the critical factors for CO2 adsorption capacity on porous carbon materials at different pressures. J. Clean. Prod. 2020, 273, 122915. [Google Scholar] [CrossRef]
- He, Y.; Zhu, L.; Fan, J.; Li, L.; Liu, G. Life cycle assessment of CO2 emission reduction potential of carbon capture and utilization for liquid fuel and power cogeneration. Fuel Process. Technol. 2021, 221, 106924. [Google Scholar] [CrossRef]
- Batuecas, E.; Liendo, F.; Tommasi, T.; Bensaid, S.; Deorsola, F.A.; Fino, D. Recycling CO2 from flue gas for CaCO3 nanoparticles production as cement filler: A life cycle assessment. J. CO2 Util. 2021, 45, 101446. [Google Scholar] [CrossRef]
- Rosental, M.; Fröhlich, T.; Liebich, A. Life cycle assessment of carbon capture and utilization for the production of large volume organic chemicals. Front. Clim. 2020, 2, 586199. [Google Scholar] [CrossRef]
- Khoo, Z.Y.; Ho, E.H.Z.; Li, Y.; Yeo, Z.; Low, J.S.C.; Bu, J.; Chia, L.S.O. Life cycle assessment of a CO2 mineralisation technology for carbon capture and utilisation in Singapore. J. CO2 Util. 2021, 44, 101378. [Google Scholar] [CrossRef]
- Yaïci, W.; Entchev, E.; Longo, M. Recent Advances in Small-Scale Carbon Capture Systems for Micro-Combined Heat and Power Applications. Energies 2022, 15, 2938. [Google Scholar] [CrossRef]
- Arena, N.; Lee, J.; Clift, R. Life cycle assessment of activated carbon production from coconut shells. J. Clean. Prod. 2016, 125, 68–77. [Google Scholar] [CrossRef] [Green Version]
- Gu, H.; Bergman, R.; Anderson, N.; Alanya-Rosenbaum, S. Life cycle assessment of activated carbon from woody biomass. Wood Fiber Sci. 2018, 50, 229–243. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peres, C.B.; Resende, P.M.R.; Nunes, L.J.R.; Morais, L.C.d. Advances in Carbon Capture and Use (CCU) Technologies: A Comprehensive Review and CO2 Mitigation Potential Analysis. Clean Technol. 2022, 4, 1193-1207. https://doi.org/10.3390/cleantechnol4040073
Peres CB, Resende PMR, Nunes LJR, Morais LCd. Advances in Carbon Capture and Use (CCU) Technologies: A Comprehensive Review and CO2 Mitigation Potential Analysis. Clean Technologies. 2022; 4(4):1193-1207. https://doi.org/10.3390/cleantechnol4040073
Chicago/Turabian StylePeres, Christiano B., Pedro M. R. Resende, Leonel J. R. Nunes, and Leandro C. de Morais. 2022. "Advances in Carbon Capture and Use (CCU) Technologies: A Comprehensive Review and CO2 Mitigation Potential Analysis" Clean Technologies 4, no. 4: 1193-1207. https://doi.org/10.3390/cleantechnol4040073






