Potential of Released Essential Oils from Active Packaging to Reduce Refrigeration Needs of Fruit and Vegetables
Abstract
:1. Impact of Produce Heat Generation during Respiration on the Refrigeration Needs
2. Ethylene Production and Needed Energy Consumption for Its Removal in the Plant Product Facilities
- Accelerated senescence and maturation
- Induction of physiological disorders (e.g., foliar disorders in leafy vegetables)
- Formation of isocoumarins (bitter flavor)
- Sprouting of tubercules
- Abscission of leaves, flowers, etc.
- Other quality degradation (e.g., asparagus hardening, etc.)
- Other effects in plants: stimulates the germination of dormant seeds, changes the direction of seedling growth, can stimulate flowering, etc.
3. Refrigerated Storage as a Conventional Postharvest Technology to Extend the Shelf Life of Plant Products
4. Ethylene Reduction as an Alternative to Reduce the Energy Needs in Refrigeration Facilities
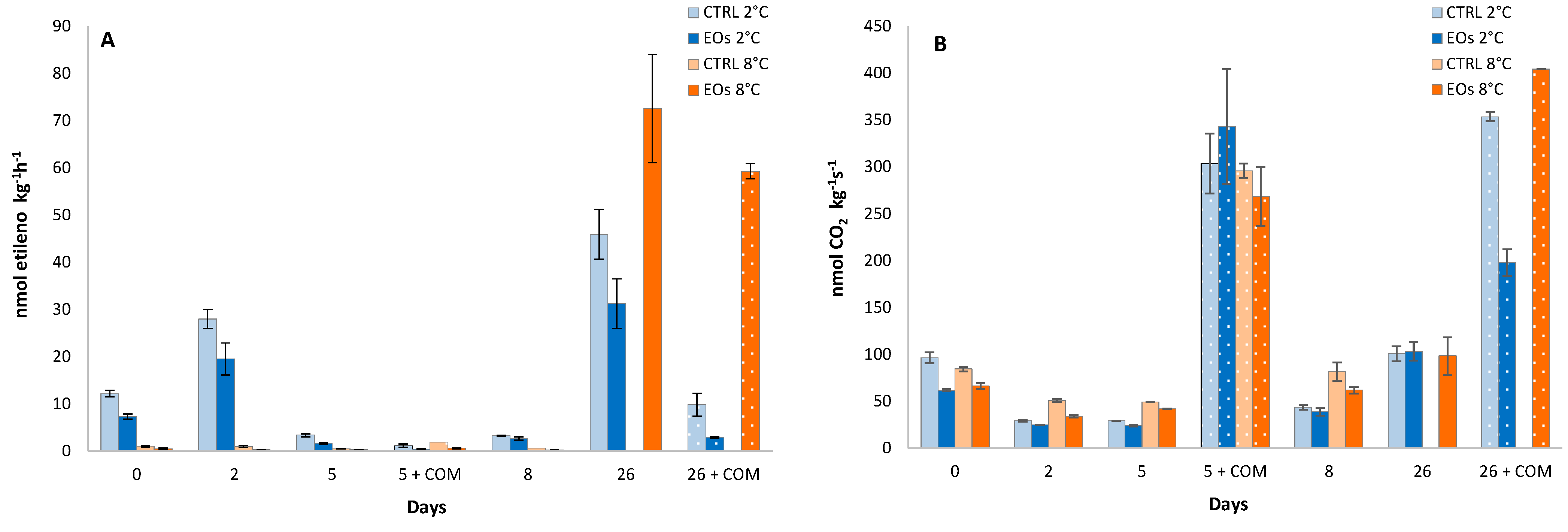
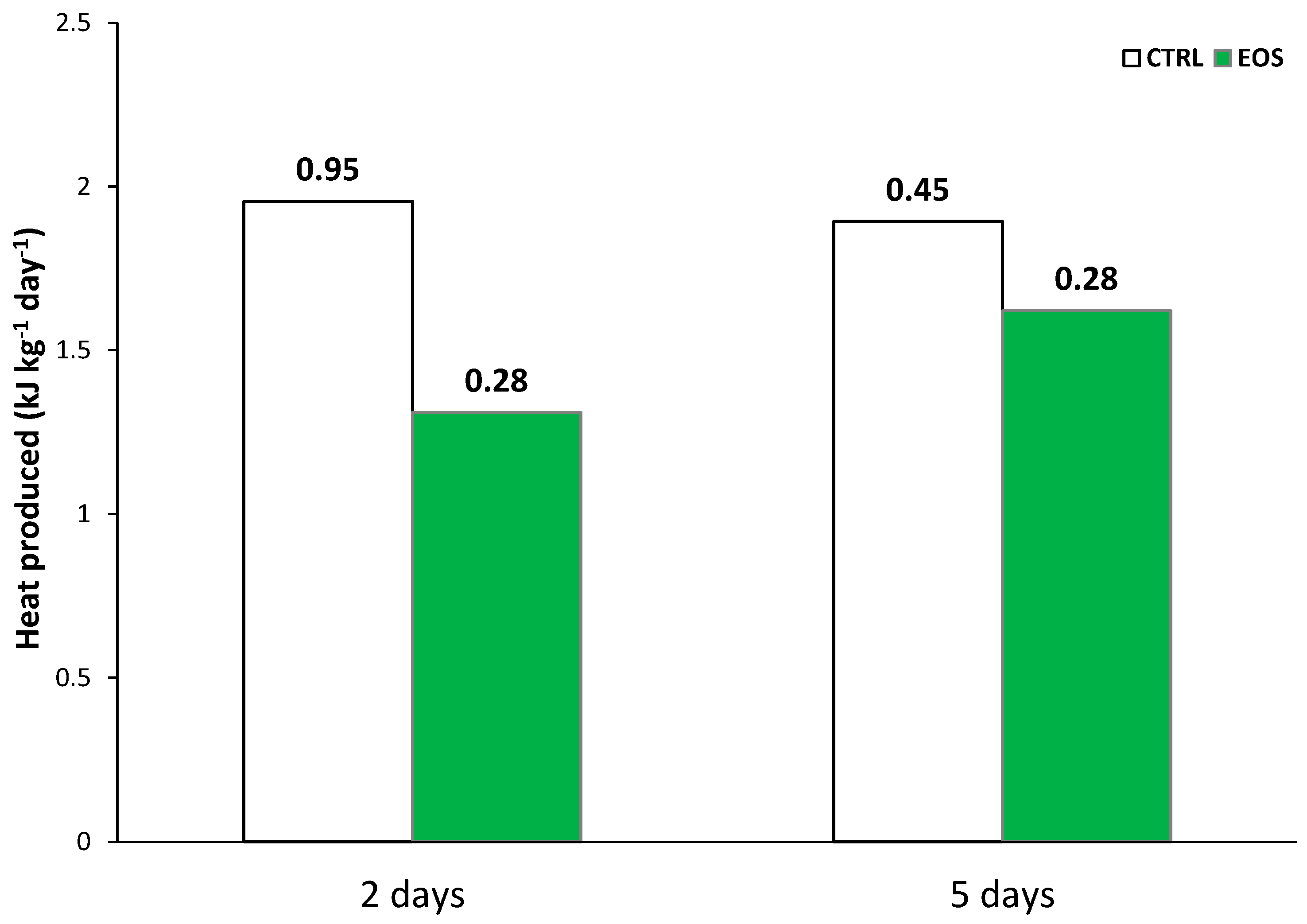
5. Active Packaging with Encapsulated Essential Oils: A Clean Technology for Reducing Energy Needs in Refrigeration Facilities
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kader, A.A. Postharvest biology and technology: An overview. In Postharvest Technology of Horticultural Crops; Kader, A.A., Ed.; University of California: Davis, CA, USA, 2002; pp. 39–48. [Google Scholar]
- Gross, K.C.; Wang, Y.; Saltveit, M. The Commercial Storage of Fruits, Vegetables, and Florist and Nursery Stocks; Gross, K.C., Wang, Y., Saltveit, M., Eds.; Agricultural Research Service of USDA: Washington, DC, USA, 2016; Volume 66. [Google Scholar]
- Kader, A.A. (Ed.) Postharvest Technology of Horticultural Crops, 3rd ed.; University of California, Agriculture and Natural Resources: Richmond, CA, USA, 2002. [Google Scholar]
- Becker, B.R.; Fricke, B.A. Transpiration and respiration of fruits and vegetables. In Proceedings of the International Institute for Refrigeration Meeting, Commissions C2 with B2, D1 and D2-3; International Institute for Refrigeration: Lexington, KY, USA, 1996; pp. 110–121. [Google Scholar]
- Ryall, A.L.; Lipton, J.W. (Eds.) Handling, Transportation, and Storage of Fruits and Vegetables, 3rd ed.; Medtech: Westport, CO, USA, 2014. [Google Scholar]
- Singh, R.P.; Chakraverty, A. Postharvest Technology: Cereals, Pulses, Fruits and Vegetables; Science Publishers Inc.: Enfield, NH, USA, 2001. [Google Scholar]
- Reid, M.S. Ethylene in postharvest technology. In Postharvest Technology of Horticultural Crops; Kader, A.A., Ed.; University of California, Agriculture and Natural Resources: Richmond, CA, USA, 2002; p. 149. ISBN 9781879906518. [Google Scholar]
- Álvarez-Hernández, M.H.; Martínez-Hernández, G.B.; Avalos-Belmontes, F.; Castillo-Campohermoso, M.A.; Contreras-Esquivel, J.C.; Artés-Hernández, F. Potassium permanganate-based ethylene scavengers for fresh horticultural produce as an active packaging. Food Eng. Rev. 2019, 11, 159–183. [Google Scholar] [CrossRef]
- Wills, R.B.H. Potential for more sustainable energy usage in the postharvest handling of horticultural produce through management of ethylene. Climate 2021, 9, 147. [Google Scholar] [CrossRef]
- Álvarez-Hernández, M.H.; Artés-Hernández, F.; Ávalos-Belmontes, F.; Castillo-Campohermoso, M.A.; Contreras-Esquivel, J.C.; Ventura-Sobrevilla, J.M.; Martínez-Hernández, G.B. Current scenario of adsorbent materials used in ethylene scavenging systems to extend fruit and vegetable postharvest life. Food Bioprocess Technol. 2018, 11, 511–525. [Google Scholar] [CrossRef]
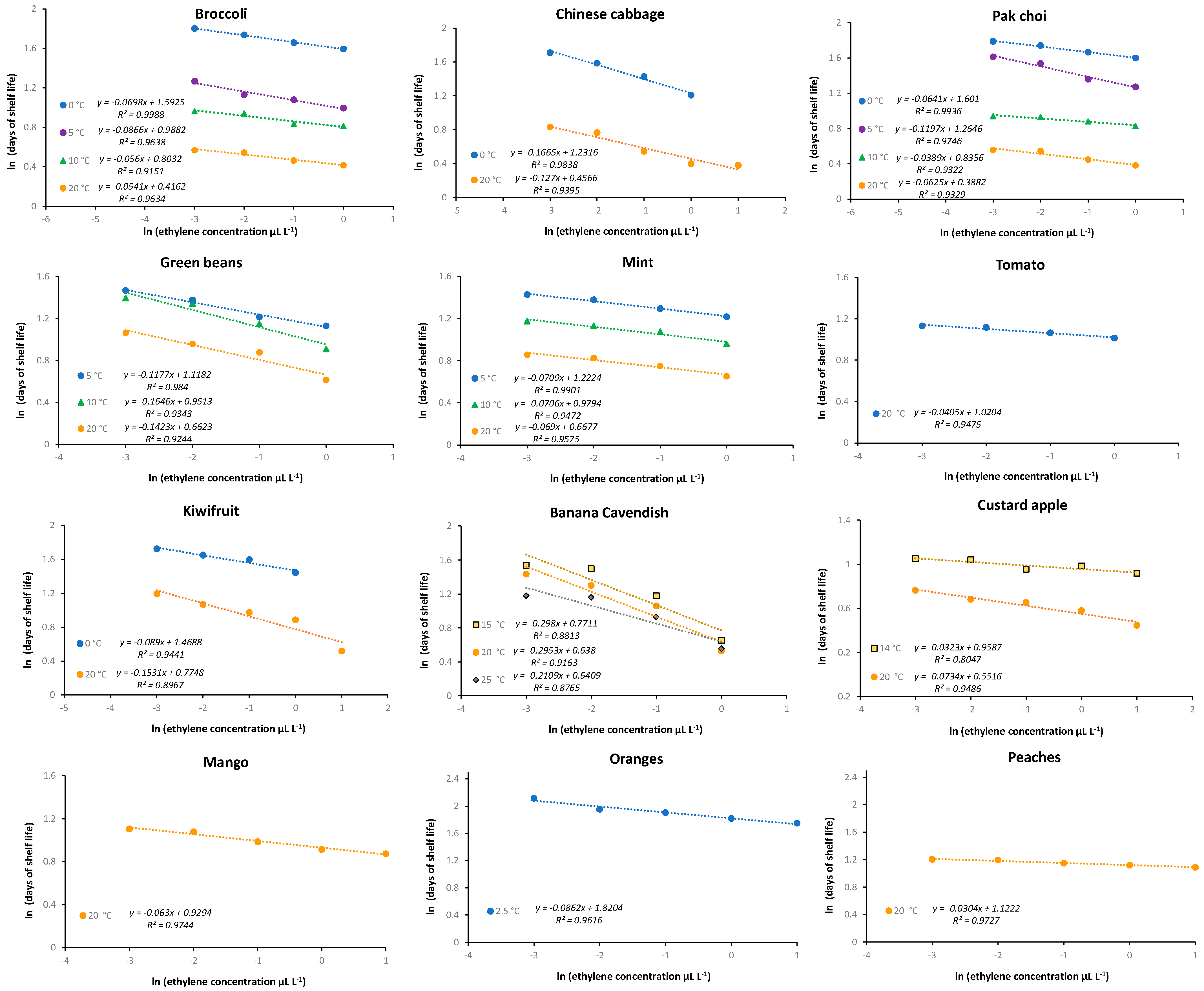
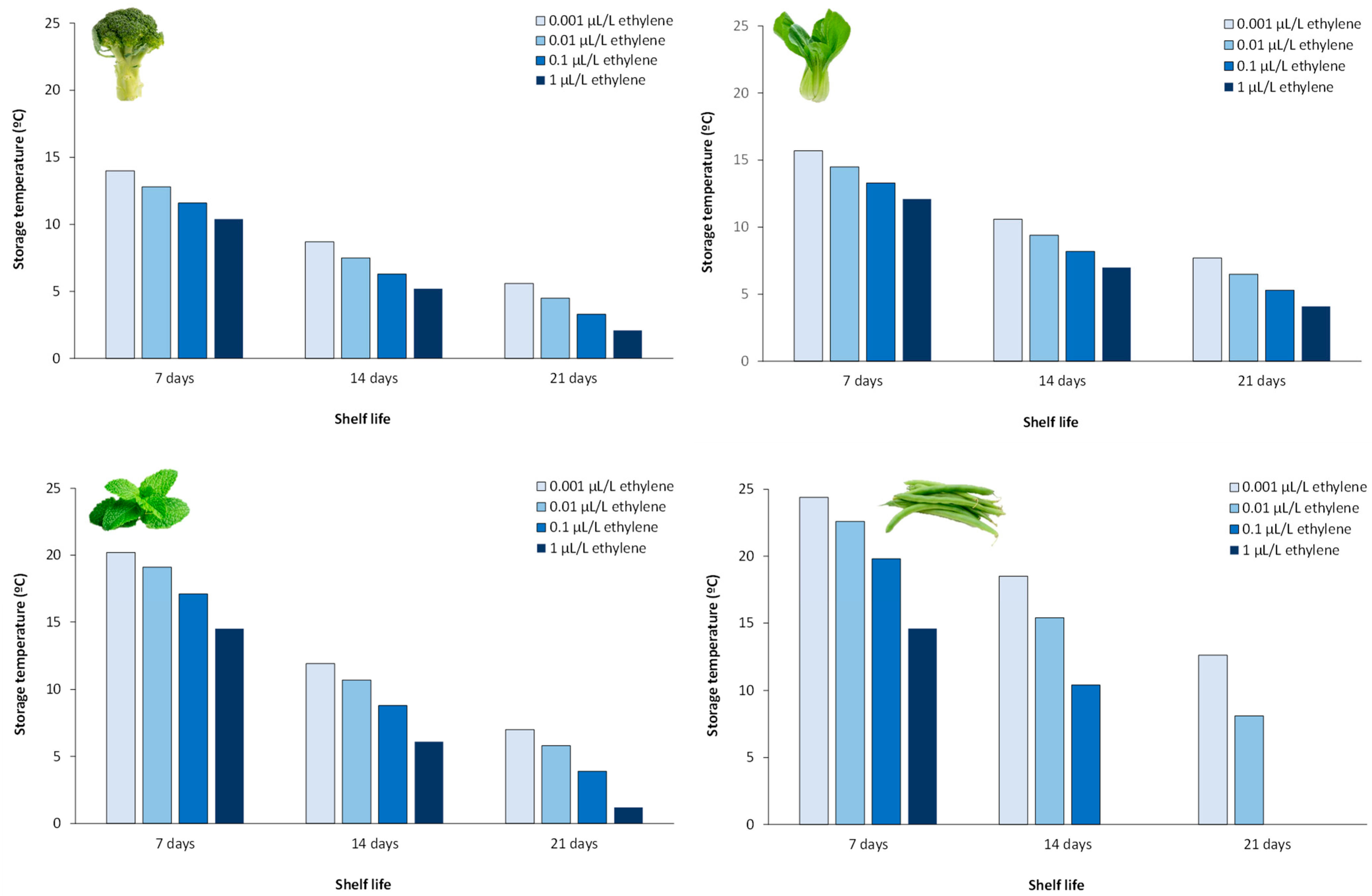
- Li, Y.; Wills, R.B.H.; Golding, J.B. Interaction of ethylene concentration and storage temperature on postharvest life of the green vegetables pak choi, broccoli, mint, and green bean. J. Hortic. Sci. Biotechnol. 2017, 92, 288–293. [Google Scholar] [CrossRef]
- Kim, G.H.; Wills, R.B.H. Effect of ethylene on storage life of lettuce. J. Sci. Food Agric. 1995, 69, 197–201. [Google Scholar] [CrossRef]
- Wills, R.B.H.; Warton, M.A.; Ku, V.V.V. Ethylene levels associated with fruit and vegetables during marketing. Aust. J. Exp. Agric. 2000, 40, 465–470. [Google Scholar] [CrossRef]
- Morris, L.L.; Kader, A.A.; Klaustermeyer, J.A.; Cheney, C.C. Avoiding ethylene concentrations in harvested lettuce. Calif. Agric. 1978, 32, 14–15. [Google Scholar]
- Schouten, S.P. Significance of ethylene in postharvest technology. In Ethylene and Plant Development; Roberts, J.A., Tucker, G.A., Eds.; Butterworths: London, UK, 1985; pp. 353–362. [Google Scholar]
- Reid, M.; Dodge, L. New ethylene absorbents: No miracle cure. Perishables Handl. Newsl. 1995, 83, 8. [Google Scholar]
- Sisler, E.C.; Blankenship, S.M. Method of Counteracting an Ethylene Response in Plants. U.S. Patent 5518988A, 3 June 1996. [Google Scholar]
- Saunders, C.; Barber, A.; Taylor, G. (Eds.) Food Miles-Comparative Energy/Emissions Performance of New Zealand’s Agriculture Industry; Agribusiness and Economics Research Unit (Lincoln University): Lincoln, New Zealand, 2006; ISBN 0909042713. [Google Scholar]
- Pearson, S.F. How to improve energy efficiency in refrigerating equipment. 17th Informatory Note on refrigerating technologies. IIR Inf. Notes Refrig. Technol. 2003, 83, 16–22. [Google Scholar]
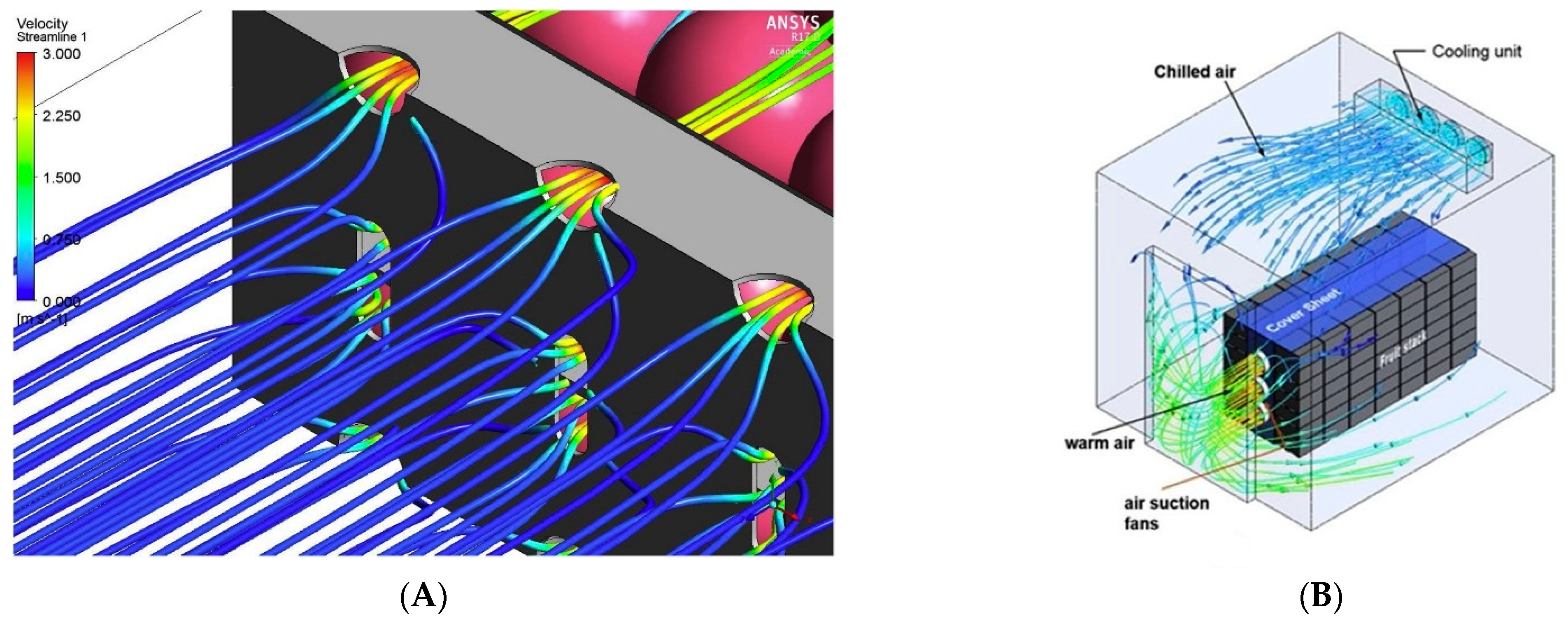
- Wu, W.; Defraeye, T. Identifying heterogeneities in cooling and quality evolution for a pallet of packed fresh fruit by using virtual cold chains. Appl. Therm. Eng. 2018, 133, 407–417. [Google Scholar] [CrossRef]
- Brosnan, T.; Sun, D.W. Precooling techniques and applications for horticultural products—A review. Int. J. Refrig. 2001, 24, 154–170. [Google Scholar] [CrossRef]
- Duan, Y.; Wang, G.B.; Fawole, O.A.; Verboven, P.; Zhang, X.R.; Wu, D.; Opara, U.L.; Nicolai, B.; Chen, K. Postharvest precooling of fruit and vegetables: A review. Trends Food Sci. Technol. 2020, 100, 278–291. [Google Scholar] [CrossRef]
- Delele, M.A.; Verboven, P.; Ho, Q.T.; Nicolaï, B.M. Advances in mathematical modelling of postharvest refrigeration processes. Stewart Postharvest Rev. 2010, 6, 1–8. [Google Scholar] [CrossRef]
- Castro, L.R.; Vigneault, C.; Cortez, L.A.B. Container opening design for horticultural produce cooling efficiency. J. food, Agric. Environ. 2004, 2, 135–140. [Google Scholar]
- Mukama, M.; Ambaw, A.; Opara, U.L. Advances in design and performance evaluation of fresh fruit ventilated distribution packaging: A review. Food Packag. Shelf Life 2020, 24, 100472. [Google Scholar] [CrossRef]
- Albornoz, K.; Cantwell, M.I.; Zhang, L.; Beckles, D.M. Integrative analysis of postharvest chilling injury in cherry tomato fruit reveals contrapuntal spatio-temporal responses to ripening and cold stress. Sci. Rep. 2019, 9, 2795. [Google Scholar] [CrossRef] [Green Version]
- Fawole, O.A.; Riva, S.C.; Opara, U.L. Efficacy of edible coatings in alleviating shrivel and maintaining quality of japanese plum (Prunus salicina lindl.) during export and shelf life conditions. Agronomy 2020, 10, 1023. [Google Scholar] [CrossRef]
- Robertson, G.L. Food Packaging: Principles and Practice, 3rd ed.; CRC Press: Boca Raton, FL, USA, 2016; ISBN 9780429105401. [Google Scholar]
- Pathare, P.B.; Opara, U.L.; Vigneault, C.; Delele, M.A.; Al-Said, F.A.J. Design of packaging vents for cooling fresh horticultural produce. Food Bioprocess Technol. 2012, 5, 2031–2045. [Google Scholar] [CrossRef]
- McCormick, R.; Neuwald, D.A.; Streif, J. A case study: Potential energy savings using 1-MCP with “Gala” apples in commercial CA storage. Acta Hortic. 2010, 877, 323–326. [Google Scholar] [CrossRef]
- Kader, A.; Zagory, D.; Kerbel, E. Modified atmosphere packaging of fruits and vegetables. Crit. Rev. Food Sci. Nutr. 1989, 28, 1. [Google Scholar] [CrossRef]
- Yousuf, B.; Qadri, O.S.; Srivastava, A.K. Recent developments in shelf-life extension of fresh-cut fruits and vegetables by application of different edible coatings: A review. LWT 2018, 89, 198–209. [Google Scholar] [CrossRef]
- Urban, L.; Charles, F.; de Miranda, M.R.A.; Aarrouf, J. Understanding the physiological effects of UV-C light and exploiting its agronomic potential before and after harvest. Plant Physiol. Biochem. 2016, 105, 149–161. [Google Scholar] [CrossRef] [PubMed]
- Serrano, M.; Martínez-Romero, D.; Guillén, F.; Valverde, J.M.; Zapata, P.J.; Castillo, S.; Valero, D. The addition of essential oils to MAP as a tool to maintain the overall quality of fruits. Trends Food Sci. Technol. 2008, 19, 464–471. [Google Scholar] [CrossRef]
- Watkins, C.B. The use of 1-methylcyclopropene (1-MCP) on fruits and vegetables. Biotechnol. Adv. 2006, 24, 389–409. [Google Scholar] [CrossRef] [PubMed]
- Kfoury, M.; Auezova, L.; Greige-Gerges, H.; Fourmentin, S. Promising applications of cyclodextrins in food: Improvement of essential oils retention, controlled release and antiradical activity. Carbohydr. Polym. 2015, 131, 264–272. [Google Scholar] [CrossRef]
- Chung, K.-T.; Wong, T.Y.; Wei, C.-I.; Huang, Y.-W.; Lin, Y. Tannins and human health: A review. Crit. Rev. Food Sci. Nutr. 1998, 38, 421–464. [Google Scholar] [CrossRef]
- European Union (EU). Regulation (EC) No 1334/2008 of the European Parliament and of the Council of 16 December 2008 on flavourings and certain food ingredients with flavouring properties for use in and on foods and amending Council Regulation (EEC) No 1601/91, Regulations (EC) No 2232/96 and (EC) No 110/2008 and Directive 2000/13/EC, Regulations (EC). Off. J. Eur. Union 2008, 354, 34–50. [Google Scholar]
- European Union (EU). Commission Implementing Regulation (EU) No 872/2012 of 1 October 2012 adopting the list of flavouring substances provided for by Regulation (EC) No 2232/96 of the European Parliament and of the Council, introducing it in Annex I to Regulation (EC) No 1334/2008 of the European Parliament and of the Council and repealing Commission Regulation (EC) No 1565/2000 and Commission Decision 1999/217/EC Text with EEA relevance, Regulation (EC) No 1334. Off. J. Eur. Union 2012, 267, 1–161. [Google Scholar]
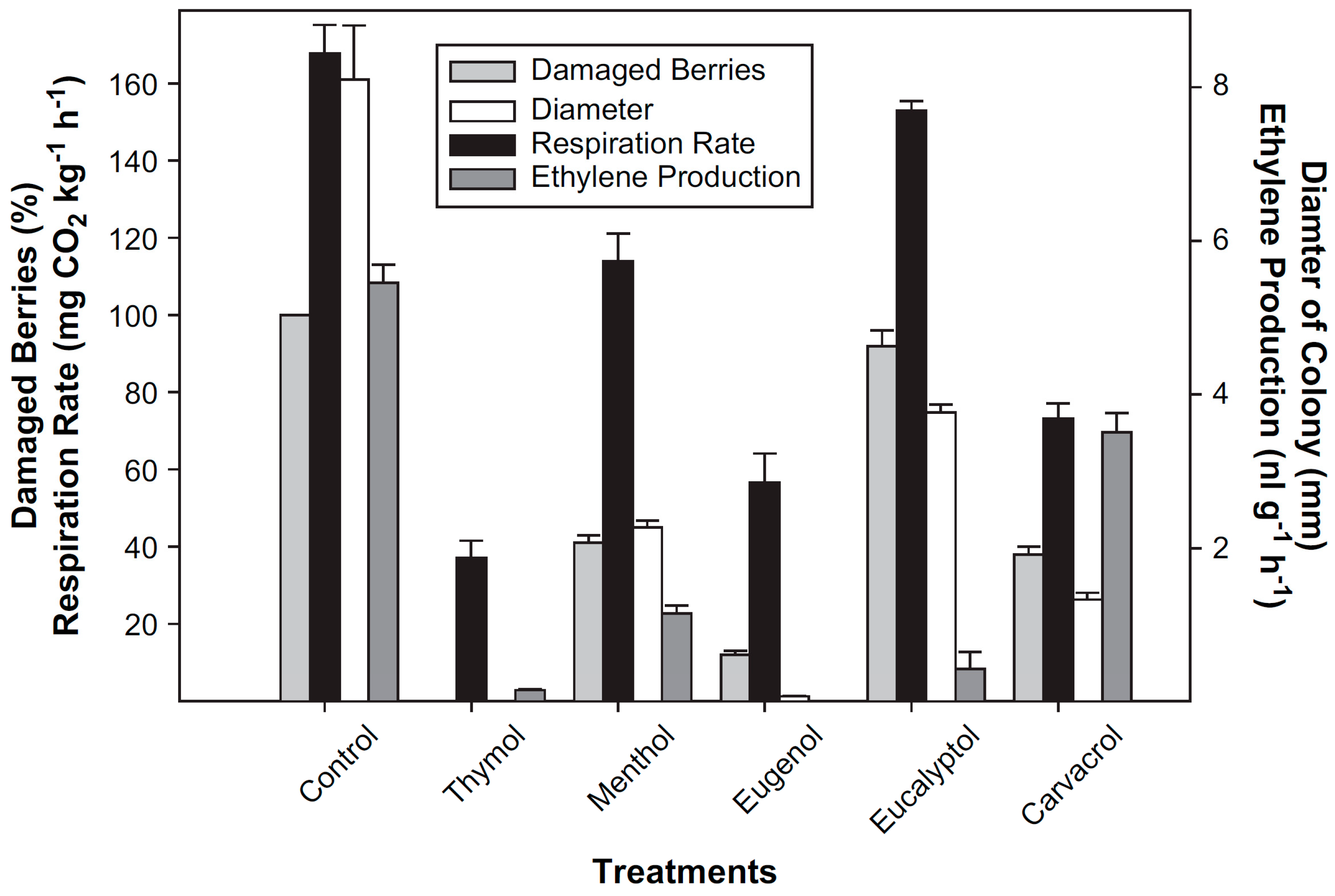
- Martínez-Romero, D.; Guillén, F.; Valverde, J.M.; Bailén, G.; Zapata, P.; Serrano, M.; Castillo, S.; Valero, D. Influence of carvacrol on survival of Botrytis cinerea inoculated in table grapes. Int. J. Food Microbiol. 2007, 115, 144–148. [Google Scholar] [CrossRef]
- Valverde, J.M. New Non-Polluting Technologies to Preserve the Quality of Table Grapes during Post-Harvest Conservation; Universidad Miguel Hernández: Elche, Spain, 2005. [Google Scholar]
- Rabbany, A.B.M.G.; Mizutani, F. Effect of essential oils on ethylene production and ACC content in apple fruit and peach seed tissues. J. Jpn. Soc. Hortic. Sci. 1996, 65, 7–13. [Google Scholar] [CrossRef] [Green Version]
- López-Gómez, A.; Navarro-Martínez, A.; Martínez-Hernández, G.B. Active paper sheets including nanoencapsulated essential oils: A green packaging technique to control ethylene production and maintain quality in fresh horticultural products. A case study in flat peaches. Foods 2020, 9, 1904. [Google Scholar] [CrossRef]
- Navarro-Martínez, A.; López-Gómez, A.; Martínez-Hernández, G.B. Potential of essential oils from active packaging to highly reduce ethylene biosynthesis in broccoli and apples. ACS Food Sci. Technol. 2021, 1, 1050–1058. [Google Scholar] [CrossRef]





| Respiration Coefficients | ||
|---|---|---|
| f (×10−4) | g | |
| Apples | 5.687 | 2.598 |
| Blueberries | 0.725 | 3.258 |
| Brussel sprouts | 27.24 | 2.573 |
| Cabbage | 6.080 | 2.618 |
| Carrots | 500.2 | 1.793 |
| Grapefruit | 35.83 | 1.998 |
| Grapes | 0.7056 | 3.033 |
| Green peppers | 3.510 | 2.741 |
| Lemons | 111.9 | 1.774 |
| Lima beans | 9.105 | 2.848 |
| Limes | 2.983 × 10−4 | 4.733 |
| Onions | 3.668 | 2.538 |
| Oranges | 2.805 | 2.684 |
| Peaches | 0.1300 | 3.642 |
| Pears | 6.361 | 3.204 |
| Plums | 0.8608 | 2.972 |
| Potatoes | 170.9 | 1.769 |
| Snap beans | 32.83 | 2.508 |
| Sugar beets | 85.91 | 1.888 |
| Strawberries | 3.668 | 3.033 |
| Tomatoes | 2.007 | 2.835 |
| Temperature (°C) | Assumed Q10 | Relative Velocity of Deterioration | Relative Shelf Life | Loss per Day (%) |
|---|---|---|---|---|
| 0 | - | 1.5 | 100 | 1 |
| 10 | 3.0 | 3.0 | 33 | 3 |
| 20 | 2.5 | 7.5 | 13 | 8 |
| 30 | 2.0 | 15.0 | 7 | 14 |
| 40 | 1.5 | 22.5 | 14 | 25 |
| Commodity | Respiratory Heat Generated per Unit Mass (mW kg−1) | |||
|---|---|---|---|---|
| 0 °C | 5 °C | 10 °C | 15 °C | |
| Apples | 10–12 | 15–21 | 41–61 | 41–92 |
| Apricot | 15–17 | 19–27 | 33–56 | 63–101 |
| Blackberries | 46–68 | 85–135 | 154–280 | 208–431 |
| Broccoli | 55–63 | 102–474 | - | 514–1000 |
| Cabbage | 12–40 | 28–63 | 36–86 | 66–169 |
| Celery | 21 | 32 | 58–81 | 110 |
| Sweet corn | 125 | 230 | 331 | 482 |
| Leeks | 28–48 | 58–86 | 158–201 | 245–346 |
| Lettuce (head) | 27–50 | 39–59 | 64–118 | 114–121 |
| Onions | 7–9 | 10–20 | 21 | 33 |
| Oranges | 9 | 14–19 | 35–40 | 38–67 |
| Peaches | 11–19 | 19–27 | 46 | 98–125 |
| Potatoes | - | 17–20 | 20–30 | 20–35 |
| Strawberries | 36–52 | 48–98 | 145–280 | 210–273 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martínez-Hernández, G.B.; López-Gómez, A. Potential of Released Essential Oils from Active Packaging to Reduce Refrigeration Needs of Fruit and Vegetables. Clean Technol. 2022, 4, 1255-1268. https://doi.org/10.3390/cleantechnol4040077
Martínez-Hernández GB, López-Gómez A. Potential of Released Essential Oils from Active Packaging to Reduce Refrigeration Needs of Fruit and Vegetables. Clean Technologies. 2022; 4(4):1255-1268. https://doi.org/10.3390/cleantechnol4040077
Chicago/Turabian StyleMartínez-Hernández, Ginés Benito, and Antonio López-Gómez. 2022. "Potential of Released Essential Oils from Active Packaging to Reduce Refrigeration Needs of Fruit and Vegetables" Clean Technologies 4, no. 4: 1255-1268. https://doi.org/10.3390/cleantechnol4040077







